Abstract
This research was mainly to study the optimisation of extraction process of Ligusticum chuanxiong Hort alcohol extracts. Stable passage pancreatic cancer HS 766 T cell lines were created by cell culture, and the cell proliferation data were measured by MTT assay. The determination of HS766T cell cycle and apoptosis case was done by PI staining and flow cytometry analysis. The results displayed that Ligusticum chuanxiong Hort can inhibit the proliferation of pancreatic cancer HS 766 T cells. The cell cycle was blocked in G0/G1 phase, and the cell membrane was damaged on observation with microscope. At the same time, the generation of apoptosis was promoted by reduced intracellular Ca2+ concentration. All in all, the HS 766 T cells could be effectively suppressed by Ligusticum chuanxiong Hort alcohol extracts.
Keywords: Ligusticum chuanxiong Hort, HS766T, calcium concentration, cell cycle
Introduction
The incidence of pancreatic cancer was rising, the rate of surgical treatment of early diagnosis was difficult, clinical outcomes was poor, chemotherapy and radiotherapy were insensitive to the cancer cell. All of this told that currently pancreatic cancer is the worst prognosis of malignant tumours. Studies confirmed that adjuvant chemotherapy was better than optimum supportive care, and the survival time of advanced pancreatic cancer could be prolonged. Traditional Chinese medicines were used to check a variety of diseases and bring balance to the body. The comprehensive treatment of a variety of active ingredients could play a good anti-cancer effect. In the treatment of pancreatic cancer, Ligusticum chuanxiong Hort(L.C.H.), Garcinia, and the arsenic trioxide (Jun Xu et al., 2013; Fuyao Liu et al., 2010) are the main traditional chinese medicines which are often prescribed by the clinicians.
The main mechanism of action was to destroy the cell cycle, and damage the cell structure to promote apoptosis. Traditional Chinese medicine preparation included Bufalin, HCPT, Qingyi Huaji and so on. Among them, the Qingyi Huaji could significantly prolong the survival of patients with advanced pancreatic cancer, by way of improving the quality of patient life. However, Bufalin mainly block the human pancreatic cancer cells BxPC-3 cells in G2/M phase (Yanli Xu et al., 2010). Researches about the activity of L.C.H. aqueous extracts on the HS766T cell have been few. This paper mainly focused on the anti-cancer activity and cell cycle, and cell morphology analysis of L. C.H extracts.
Materials and Methods
Materials
Ligusticum chuanxiong Hort (L.C.H.) was purchased from Beijing Tong Ren Tang pharmacy, examined and identified by microscopic and thin-layer chromatography in Beijing University of Chinese Medicine laboratory, indicating that the products are qualified and belong to Apiales umbellales plants. DMEM was purchased from GIBCO. Newborn calf serum was purchased from the Shanghai Branch Hing Trading Co., Ltd.; thiazole nitrogen blue and trypsin were purchased from Sigma, USA; human pancreatic cancer cell lines HS766T from ATCC (American type Culture collection). Calcium fluorescent indicator Fura-2PAM was purchased from the Chinese Academy of Medical Sciences. Other materials used for the study included rotary evaporator RE-52 (Shanghai Ya Rong Biochemical Instrument Factory), Autoclave MLS-3030CH (Shanghai Heng Qin equipment, Co., Ltd.), Clean Bench (100 Code Equipment Co., Ltd.), drain the incubator (Shanghai Yuejin Medical Apparatus Factory), and Micropipettes (Eppendorf).
Preparation
L.C.H. was pulverised and sieved, weighed 80g, added to 1000mL 75% aqueous ethanol, immersed for 40 min at room temperature, and then boiled for 1.5 h. It was filtered after it cooled for a moment and early decoction was obtained. 850 mL of different concentrations of ethanol was added repeatedly, boiled for 1.5 h, combined decoctions, and concentrated to 100 mL. The last extracts were obtained. Extracts were centrifuged at 8000 R/min for 4 min, and then the supernatant was put in 5mL antibiotics special glass bottles, which was 1g/mL medicine supernatant, an equal amount of NMP to dissolve the precipitate. 1g/mL medicine precipitation solution was made and then put in 5 mL glass bottle antibiotics. The supernatant and precipitate solution was sterilised for 25 min at 121 °C, stored in a 4°C refrigerator spare.
Culture of HS766T cell (Shengguang Yuan et al., 2003; Min Zhao et al., 2002)
HS766T single-cell suspension was prepared. The rate of the active cell was 95% by trypan blue detection. 72 h passaged cell wall was digested, and then cell suspension in DMEM culture (2×105/ml) was made by FBS containing 10% foetal calf serum, seeded in each hole of 96-well culture plates (0.11 ml). The cell number was 5×104.
There were six experimental groups, control and blank control groups in total. Final concentration of L.C.H. extracts solution was regulated to 1.5mg/mL, 0.75 mg/mL, 0.375 mg/mL, 0.183 g/mL, 0.09 g/mL, 0.04 g/mL, control group and blank control group. The control group was added only with the culture medium. Each group contains five wells. The experimental cells were cultured at 37□ with 5% CO2 saturated humidity conditions cultured for 24 h, 48 h, 72 h. 20µl MTT was added after cultured for 4 h. The supernatant was discarded and 100 mL of dimethyl sulfoxide was added. After about 15 min, the plate was measured on a microplate reader and the optical density (OD) values detected at 490nm. So, inhibition rate was calculated at last.
The detection of the cell cycle and apoptosis of pancreatic cancer HS766T (Xielin Feng et al., 2004; An Liu et al., 2011)
L.C.H. extracts preparation was added to the HS766T single-cell suspension in the experimental group. The final concentration was adjusted to 0.75 mg/mL, 0.375 mg/mL, 0.18g/mL, incubated at 37□, 5% CO2 saturated humidity conditions for 24 h, 48 h, 72h, and then propidium iodide staining by FACS Calibur flow cytometer was carried out with the cell Quest program. 0.5×105 cells were sampled and measured. Thus with Modifit program, proportion analysis of cell cycle and apoptosis was conducted.
Determination of intracellular free Ca2+ concentration (Yingling Tang, 2013; Huancheng Xu et al., 2013)
Cell suspension in the logarithmic growth phase was taken (concentration at 5 × 105 cells / ml), incubated at 37°C for 5 min, and added to a final concentration of 5µl Fura-2/AM. It was oscillated for 45 min at 37°C constant temperature. The system had to be replenished with 95 % O2 and 5% CO2 to keep itself going. The reaction was terminated with the 0°C culture broth of a certain amount, and finally the cell concentration was adjusted to 5×105/ml. Before Ca2+ concentration was detected, the cell we used was washed 3 times with culture medium, then re-warmed at 37°C for 5 min, and detected by spectrophotometer detector with two wavelengths. The maximum fluorescence was measured after the addition of a final concentration of 0.2% Triton-100. The minimum fluorescence was measured after 10mM EGTA had been added. As for calcium concentrations value, only the auto fluorescence value of the cell suspension and Fura-2 Solution dissociation constant input were considered. The concentration of intracellular calcium was automatically calculated by the computer software.
Statistical analysis
Experimental data were calculated with SPSS 11 5 software, ANOVA (analysis of variance), pair wise comparisons q test method; P <0.05 as a significant difference in standards.
Results
Anti-cancer effect of Ligusticum chuanxiong Hort (L.C.H.)
The most obvious inhibition effect of L.C.H. on the cancer cells was at concentration of 1.5mg/ml and 48h. When L.C.H extracts concentration was 0.183∼1.5mg/ml, the inhibitory effect was relatively high, ranging from 39% to 67%. At the period of 48h∼72h, the viability of the cancer cell itself was reduced; the inhibition rate was significantly decreased compared with the previous 48h value, as shown in Table 1. Observed with microscope, low concentration extracts resulted in cell shrinkage, contents exudate; high concentration extracts would lead to cell contents exudation, cell shrinkage and cell decomposition.
Table 1.
Inhibition rate of L.C.H. Extracts on HS766T cell
| Group | Hole number |
L.C.H. concentration mg/ml |
24h | 48h Inhibition rate (%) |
72h |
| Control group | 20 | 0 | 0 | 0 | 0 |
| Test group | 20 | 1.500 | 62.3 | 66.5 | 58.1 |
| 20 | 0.750 | 50 | 52.4 | 43.2 | |
| 20 | 0.375 | 46.8 | 41.2 | 36.5 | |
| 20 | 0.18 | 38.7 | 39.1 | 30.6 | |
| 20 | 0.090 | 20.7 | 23.5 | 20.1 | |
| 20 | 0.050 | 12.4 | 15.1 | 10.8 | |
| Blank group | 20 | 0 | - | - | - |
Apoptosis and cell cycle of HS766T cell acted on by L.C.H extracts
HS766T cells G0/G1 phase ratio were increased by the effect of L.C.H extracts. The proportion of S-phase cells increased; G2/M of cell ratio decreased. The proportion of apoptosis was significantly greater than that of the control group (Table 2).
Table 2.
The effect of L.C.H extracts on the cell cycle of HS766T
| Groups | L.C.H | Times | Cell cycles | |||
| Concentration | of test | |||||
| (mg/ml) | G0/G1 period | S(%)period | G2/M period | Apoptosis rate (%) |
||
| Control | 10 | 42.15±0.43 | 38.21±0.23 | 18.34±0.41 | 2.1±0.2 | |
| 0.75 | 10 | 44.18±0.27* | 37.38±0.55 | 12.07±0.16 | 4.5±0.4* | |
| 12h group | 0.375 | 10 | 48.52±0.33* | 40.41±0.64 | 15.31±0.28 | 13.2±0.7* |
| 0.183 | 10 | 52.26±0.51 | 47.36±0.72 | 24.05±0.12 | 19.4±0.5 | |
| Control | 10 | 42.15±0.43 | 38.21±0.23 | 18.34±0.41 | 3.7±0.8 | |
| 0.75 | 10 | 45.28±0.52* | 40.15±0.54 | 19.35±0.57 | 7.15±0.7* | |
| 24h group | 0.375 | 10 | 49.01±0.37* | 36.15±0.19 | 16.44±0.62 | 15.4±0.5* |
| 0.183 | 10 | 54.34±0.25 | 48.46±0.27 | 23.08±0.24 | 24.6±0.6 | |
| Control | 10 | 42.15±0.43 | 38.21±0.23 | 18.34±0.41 | 5.3±0.6 | |
| 0.75 | 10 | 47.25±0.61* | 42.11±0.09 | 15.11±0.14 | 9.2±0.32* | |
| 48h group | 0.375 | 10 | 52.14±0.73 | 42.05±0.17 | 19.47±0.48 | 17.3±0.9 |
| 0.183 | 10 | 62.31±0.35 | 56.44±0.75 | 38.08±0.16 | 8.2±0.1 |
Notes: Experimental group compared with control group, *p<0.05.
After giving different concentrations of L.C.H extracts, intracellular Ca2+ levels in HS766T cell rapidly decreased (Table 3); only 3 min later, it reached the lowest value in a dose-dependent manner (r = −0.957, P <0.01) (See Table 3).
Table 3.
The effect of L.C.H extracts on the Ca2+ concentration in HS766T (n=5, x±s, nmol/L)
| Group | Sample concentration | Ca2+ concentration |
| Control | — | 58.36±0.27 |
| 1.5 | 30.21±0.52 | |
| 0.75 | 35.17±0.45 | |
| Samples | 0.375 | 38.51±0.83 |
| 0.183 | 42.38±0.58 | |
| 0.09 | 49.47±0.73 | |
| 0.05 | 51.06±0.59 |
Notes: Compared with control group, * P<0.05, ** P<0.01
Discussion
The present experiment mainly studied the inhibitory activity of L.C.H. extracts on HS766T cell, as well as part of their anti-cancer mechanisms. Inhibitory activity from L.C.H could reflect the obvious anti-pancreatic cancer activity within the first 48h, and it could still maintain a certain anti-cancer activity within 72h. It also could significantly reduce the intracellular calcium levels. This result also means that its reduction of the ability of HS766T cell signal transduction may be due to the variety of calcium ion channel that had been inhibited.
TMP (Tetramethylprazine, TMP) is one of the active ingredients from the L.C.H extracts. Its structure has been confirmed to reveal a wealth of pharmacological effects. Platelet aggregation and thrombosis could be prevented by it, and at the same, it has significant effect on the treatment of various diseases of the respiratory system, heart, brain and blood vessels, etc. Researches have found that TMP has a certain role in the treatment of neuropathic pain. Most studies told that this may be related with the P2X3 receptor in pain conduction dorsal root ganglion (Yun Gao et al., 2007).
TMP also has a therapeutic effect on the peripheral neurotoxicity induced by certain antibiotics (ototoxicity), and retinal degeneration induced by hydrogen peroxide. Given the instance of hydrogen peroxide in cultured rat retinal cells (rat retinal cell), TMP produced a series of changes: lipid peroxidation increased, mitochondrial ROS increased with membrane potential disappear, nerve microtubule protein-2 (MAP-2) lowered and neuroprotective peptide (rattin peptide) changed. Ferulic acid extracted from Ligusticum chuanxiong Hort exhibits a neuroprotective effect for brain injury by regulating the Akt/GSK-3β/CRMP-2, signalling pathway in focal cerebral ischemic injury (Gim et al., 2013).
In the prospect of anticancer activity, TMP could significantly inhibit the proliferation of the Moser cells from colorectal cancer, and in combination with other five chemotherapy drugs would have a synergistic effect on Moser cells. This has nothing to do with transforming growth factor signal transduction pathway (Baorui Liu et al., 2002). The essential oils in L. chuanxiong may exert inhibitory effects on DNA damage and apoptosis induced by UVB. At the same time, they can decrease p21 expression and increase cyclin D1 expression as apoptosis-regulatory genes (Jeong et al., 2009).
Keratinocyte growth factor (KGF) is a member of the fibroblast growth factor (FGF) group of heparin-binding polypeptides. KGF may participate in aberrant paracrine and autocrine pathways in human pancreatic cancer (Siddiqi et al., 1995). p21WAF1-a tumour suppressor gene acts as a downstream effector of p53 function, and mediates G1 cell cycle arrest by inhibiting cyclin-dependent kinases. Hs766T cell showed a significant dose-dependent increase in p21 protein expression when infected with rAd-p21 (Joshi US et al., 1998).
As for other pancreatic cancer cells, TL (Triptolide TL) could inhibit 5-LOX expression in SW1990. Cell proliferation and apoptosis induced 5-lipoxygenase metabolic pathway play an important role in the growth of pancreatic cancer cells. Ginsenoside Rg3 could down Pim-3 and phosphorylated bad protein expression in pancreatic cancer PANC-1 cells, thereby inhibiting the proliferation of PANC-1 cells, and inducing apoptosis (Jie Jian et al., 2009). Ranunculin could significantly inhibit the proliferation of MGC803 and PaTu8988, and its mechanism may be related to apoptosis induction and has nothing to do with cell cycle arrest.
Figure 1.
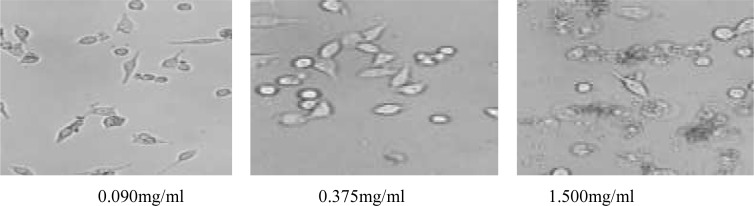
Stimulated cell observed with microscope
References
- 1.Liu An, Deng Zifeng, Hu Jinxi, Han Shaowei, Huang Lili, Lin Shengzhang. Effect of emodin on cell proliferation and apoptosis of human pancreatic cancer cell line Panc-1. Chin Tradit Herbal Drugs. 2011;42(04):756–759. [Google Scholar]
- 2.Liu Baorui, Qian Xiaoping, Zhou Zhengyun. Effects of Four Extracts of Traditional Chinese Medicinal Materials on The Proliferation of Human Colon Cancer Moser Cell Line. Nanjing Univ-Nat Sci. 2002;38(05):667–671. [Google Scholar]
- 3.Liu Fuyao, Cui Naiqiang. The mechanism treatment of pancreatic cancer by TCM. Jilin J Trad Chin Med. 2010;30(11):1011–1012. [Google Scholar]
- 4.Gim S A, Sung J H, Shah F A, Kim M O, Koh P O. Ferulic acid regulates the AKT/GSK-3β/CRMP-2 signalling pathway in a middle cerebral artery occlusion animal model. Lab Anim Res. 2013;29(2):63–69. doi: 10.5625/lar.2013.29.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Huancheng, Zheng Mingxue, Gu Shaopeng, Bai Rui. Effect of Calcium Signal Transduction on Apoptosis. Prog in Veteris. 2013;34(01):112–115. [Google Scholar]
- 6.Siddiqi I, Funatomi H, Kobrin M S, Friess H, Buchler MW, Korc M. Increased Expression of Keratinocyte Growth Factor in Human Pancreatic Cancer. Biochem Bioph Res Co. 1995;215(1):309–315. doi: 10.1006/bbrc.1995.2467. [DOI] [PubMed] [Google Scholar]
- 7.Jeong J B, Ju S Y, Park J H, Lee J R, Yun K W, Kwon S T, Lim J H, Chung G Y, Jeong H J. Antioxidant activity in essential oils of Cnidium officinale Makino and Ligusticum chuanxiong Hort and their inhibitory effects on DNA damage and apoptosis induced by ultraviolet B in mammalian cell. Cancer Epidemiol. 2009;33(1):41–46. doi: 10.1016/j.canep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Jian Jie, Hu Zhifang, Huang Yuan. Effect of ginsenoside Rg3 on Pim-3 and Bad proteins in human pancreatic cancer cell line PANC-1. Chin J Cancer. 2009;28(05):461–465. [PubMed] [Google Scholar]
- 9.Joshi U S, Dergham S T, Chen Y Q, Dugan M C, Crissman J D, Vaitkevicius V K, Sarkar F H. Inhibition of pancreatic tumour cell growth in culture by p21WAF1 recombinant adenovirus. Pancreas. 1998;16(2):107–113. doi: 10.1097/00006676-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Xu Jun, Wang Shuang. The research progress of tumour microenvironment. Cancer Research on Prevention and Treatment. 2013;40(01):111–114. [Google Scholar]
- 11.Zhao Min, Jia Qing, Jiang Ruolan. The growth modulation of somatostatin on human pancreas carcinoma cell lines HS 766T. Chin J Gene Surg. 2002;17(03):39–41. [Google Scholar]
- 12.Yuan Shengguang, Feng Xielin, Tian Ling, Wei Yuquan, Zhang Zhaoda. Inhibitive effect of Smad4 gene on the growth of human pancreatic adenocarcinoma cell line HS766T. Acta Acade Med Milita Tertiae. 2003;25(09):795–798. [Google Scholar]
- 13.Feng Xielin, Zhang Zhaoda, Tian Ling, Wei Yuquan. the Expression of DPC4 of the Pc-3.HS766T Cell Lines and the Sequence Determination. West Chin Med J. 2004;02:206–208. [Google Scholar]
- 14.Xu Yanli, Liu Luming, Zhu Feiye. Anti-pancreatic cancer medicine Research; The 12th national academic conference paper combine traditional Chinese and western medicine tumour assembly; 2010. p. 6. [Google Scholar]
- 15.Tang Yingling. The research progress of intracellular calcium ions. Chin Health Care & Nutri. 2013;2(03):569–570. [Google Scholar]
- 16.Gao Yun, Liang Shangdong, Shao Lijian, Mu Songniu, Xu Changshui, Zhang Chunping. Effect of ginsenoside Rg3 on Pim-3 and Bad proteins in human pancreatic cancer cell line PANC-1. Chin J Cancer. 2007;28(04):458–462. [PubMed] [Google Scholar]


