Abstract
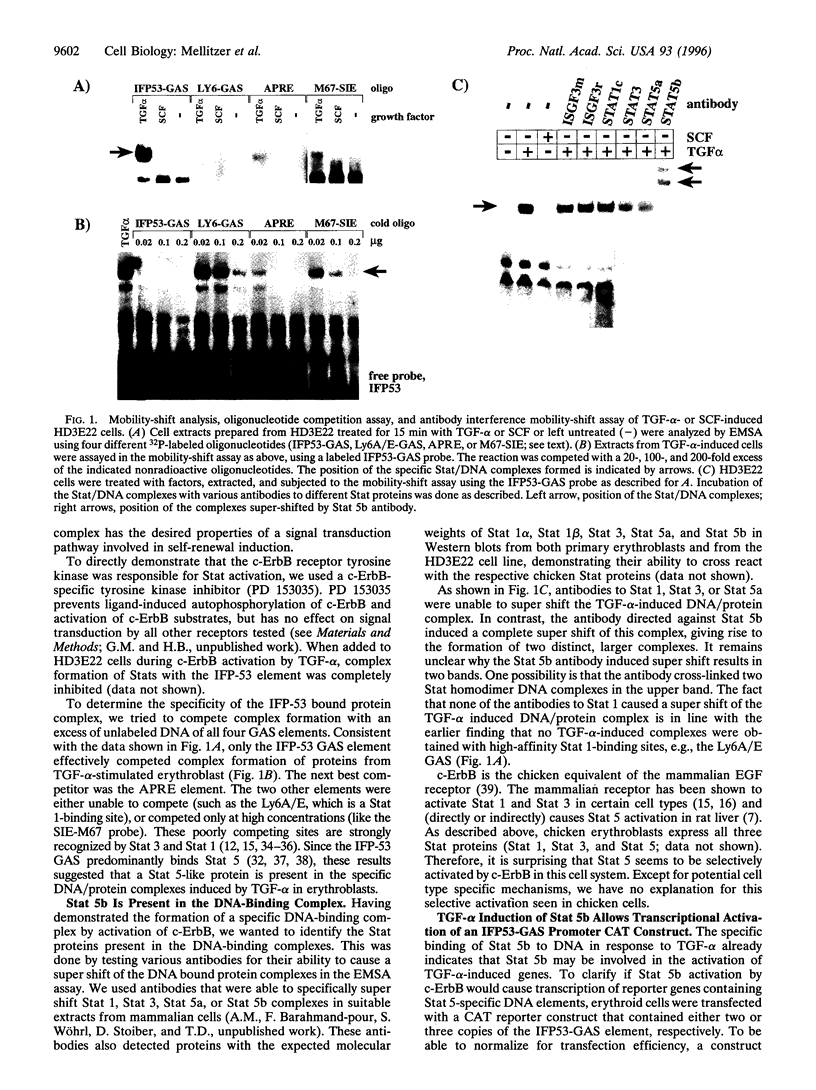
Self renewal of normal erythroid progenitors is induced by the receptor tyrosine kinase c-ErbB, whereas other receptors (c-Kit/Epo-R) regulate erythroid differentiation. To address possible mechanisms that could explain this selective activity of c-ErbB, we analyzed the ability of these receptors to activate the different members of the Stat transcription factor family. Ligand activation of c-ErbB induced the tyrosine phosphorylation, DNA-binding, and reporter gene transcription of Stat 5b in erythroblasts. In contrast, ligand activation of c-Kit was unable to induce any of these effects in the same cells. Activation of the erythropoietin receptor caused specific DNA-binding of Stat 5b, but failed to induce reporter gene transcription. These biochemical findings correlate perfectly with the selective ability of c-ErbB to cause sustained self renewal in erythroid progenitors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Azam M., Erdjument-Bromage H., Kreider B. L., Xia M., Quelle F., Basu R., Saris C., Tempst P., Ihle J. N., Schindler C. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995 Apr 3;14(7):1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahmand-pour F., Meinke A., Eilers A., Gouilleux F., Groner B., Decker T. Colony-stimulating factors and interferon-gamma activate a protein related to MGF-Stat 5 to cause formation of the differentiation-induced factor in myeloid cells. FEBS Lett. 1995 Feb 20;360(1):29–33. doi: 10.1016/0014-5793(95)00072-h. [DOI] [PubMed] [Google Scholar]
- Beadling C., Ng J., Babbage J. W., Cantrell D. A. Interleukin-2 activation of STAT5 requires the convergent action of tyrosine kinases and a serine/threonine kinase pathway distinct from the Raf1/ERK2 MAP kinase pathway. EMBO J. 1996 Apr 15;15(8):1902–1913. [PMC free article] [PubMed] [Google Scholar]
- Beug H., Doederlein G., Freudenstein C., Graf T. Erythroblast cell lines transformed by a temperature-sensitive mutant of avian erythroblastosis virus: a model system to study erythroid differentiation in vitro. J Cell Physiol Suppl. 1982;1:195–207. doi: 10.1002/jcp.1041130427. [DOI] [PubMed] [Google Scholar]
- Beug H., Müllner E. W., Hayman M. J. Insights into erythroid differentiation obtained from studies on avian erythroblastosis virus. Curr Opin Cell Biol. 1994 Dec;6(6):816–824. doi: 10.1016/0955-0674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Decker T., Lew D. J., Darnell J. E., Jr Two distinct alpha-interferon-dependent signal transduction pathways may contribute to activation of transcription of the guanylate-binding protein gene. Mol Cell Biol. 1991 Oct;11(10):5147–5153. doi: 10.1128/mcb.11.10.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers A., Baccarini M., Horn F., Hipskind R. A., Schindler C., Decker T. A factor induced by differentiation signals in cells of the macrophage lineage binds to the gamma interferon activation site. Mol Cell Biol. 1994 Feb;14(2):1364–1373. doi: 10.1128/mcb.14.2.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. W., Kraker A. J., McMichael A., Ambroso L. A., Nelson J. M., Leopold W. R., Connors R. W., Bridges A. J. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science. 1994 Aug 19;265(5175):1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- Gilmour K. C., Pine R., Reich N. C. Interleukin 2 activates STAT5 transcription factor (mammary gland factor) and specific gene expression in T lymphocytes. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10772–10776. doi: 10.1073/pnas.92.23.10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F., Pallard C., Dusanter-Fourt I., Wakao H., Haldosen L. A., Norstedt G., Levy D., Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995 May 1;14(9):2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Gouilleux F. Prolactin-mediated gene activation in mammary epithelial cells. Curr Opin Genet Dev. 1995 Oct;5(5):587–594. doi: 10.1016/0959-437x(95)80027-1. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Meyer S., Martin F., Steinlein P., Beug H. Self-renewal and differentiation of normal avian erythroid progenitor cells: regulatory roles of the TGF alpha/c-ErbB and SCF/c-kit receptors. Cell. 1993 Jul 16;74(1):157–169. doi: 10.1016/0092-8674(93)90303-8. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B., Tang B., Yi T., Quelle F. W. Cytokine receptors and signal transduction. Baillieres Clin Haematol. 1994 Mar;7(1):17–48. doi: 10.1016/s0950-3536(05)80005-8. [DOI] [PubMed] [Google Scholar]
- Lax I., Johnson A., Howk R., Sap J., Bellot F., Winkler M., Ullrich A., Vennstrom B., Schlessinger J., Givol D. Chicken epidermal growth factor (EGF) receptor: cDNA cloning, expression in mouse cells, and differential binding of EGF and transforming growth factor alpha. Mol Cell Biol. 1988 May;8(5):1970–1978. doi: 10.1128/mcb.8.5.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Robinson G. W., Gouilleux F., Groner B., Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8831–8835. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto A. M., Hayman M. J. Role of oligomerization of the S13 Env-Sea oncoprotein in cell transformation. J Virol. 1994 Mar;68(3):1837–1842. doi: 10.1128/jvi.68.3.1837-1842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui A. L., Wakao H., O'Farrell A. M., Harada N., Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995 Mar 15;14(6):1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallard C., Gouilleux F., Bénit L., Cocault L., Souyri M., Levy D., Groner B., Gisselbrecht S., Dusanter-Fourt I. Thrombopoietin activates a STAT5-like factor in hematopoietic cells. EMBO J. 1995 Jun 15;14(12):2847–2856. doi: 10.1002/j.1460-2075.1995.tb07284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R., Durbin J. E., Levy D. E. Acute phase response factor and additional members of the interferon-stimulated gene factor 3 family integrate diverse signals from cytokines, interferons, and growth factors. J Biol Chem. 1994 Sep 30;269(39):24391–24395. [PubMed] [Google Scholar]
- Rothman P., Kreider B., Azam M., Levy D., Wegenka U., Eilers A., Decker T., Horn F., Kashleva H., Ihle J. Cytokines and growth factors signal through tyrosine phosphorylation of a family of related transcription factors. Immunity. 1994 Sep;1(6):457–468. doi: 10.1016/1074-7613(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Epidermal growth factor induces the tyrosine phosphorylation and nuclear translocation of Stat 5 in mouse liver. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4215–4218. doi: 10.1073/pnas.92.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski H. B., Gilman M. Z. Cell-free activation of a DNA-binding protein by epidermal growth factor. Nature. 1993 Mar 4;362(6415):79–83. doi: 10.1038/362079a0. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., Denny C. T., Witte O. N. Leukemia and the disruption of normal hematopoiesis. Cell. 1991 Jan 25;64(2):337–350. doi: 10.1016/0092-8674(91)90643-d. [DOI] [PubMed] [Google Scholar]
- Schroeder C., Gibson L., Nordström C., Beug H. The estrogen receptor cooperates with the TGF alpha receptor (c-erbB) in regulation of chicken erythroid progenitor self-renewal. EMBO J. 1993 Mar;12(3):951–960. doi: 10.1002/j.1460-2075.1993.tb05736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Steinlein P., Deiner E., Leutz A., Beug H. Recombinant murine erythropoietin receptor expressed in avian erythroid progenitors mediates terminal erythroid differentiation in vitro. Growth Factors. 1994;10(1):1–16. doi: 10.3109/08977199409019599. [DOI] [PubMed] [Google Scholar]
- Steinlein P., Wessely O., Meyer S., Deiner E. M., Hayman M. J., Beug H. Primary, self-renewing erythroid progenitors develop through activation of both tyrosine kinase and steroid hormone receptors. Curr Biol. 1995 Feb 1;5(2):191–204. doi: 10.1016/s0960-9822(95)00040-6. [DOI] [PubMed] [Google Scholar]
- Strehlow I., Seegert D., Frick C., Bange F. C., Schindler C., Böttger E. C., Decker T. The gene encoding IFP 53/tryptophanyl-tRNA synthetase is regulated by the gamma-interferon activation factor. J Biol Chem. 1993 Aug 5;268(22):16590–16595. [PubMed] [Google Scholar]
- Wagner B. J., Hayes T. E., Hoban C. J., Cochran B. H. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990 Dec;9(13):4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Harada N., Kitamura T., Mui A. L., Miyajima A. Interleukin 2 and erythropoietin activate STAT5/MGF via distinct pathways. EMBO J. 1995 Jun 1;14(11):2527–2535. doi: 10.1002/j.1460-2075.1995.tb07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegenka U. M., Lütticken C., Buschmann J., Yuan J., Lottspeich F., Müller-Esterl W., Schindler C., Roeb E., Heinrich P. C., Horn F. The interleukin-6-activated acute-phase response factor is antigenically and functionally related to members of the signal transducer and activator of transcription (STAT) family. Mol Cell Biol. 1994 May;14(5):3186–3196. doi: 10.1128/mcb.14.5.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Wegenka U. M., Lütticken C., Buschmann J., Decker T., Schindler C., Heinrich P. C., Horn F. The signalling pathways of interleukin-6 and gamma interferon converge by the activation of different transcription factors which bind to common responsive DNA elements. Mol Cell Biol. 1994 Mar;14(3):1657–1668. doi: 10.1128/mcb.14.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994 Apr 1;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]