Abstract
Wu-Ling-San (WLS) formula has been proved to prevent calcium oxalate nephrolithiasis both in vitro and in vivo. This is the first prospective, randomized and placebo-controlled clinical trial of WLS in calcium oxalate nephrolithiasis prevention. All patients who enrolled were asked to drink enough fluid to urinate at least 2 L daily during the study period. A 24-hour urine collection was performed to establish the baseline levels of multiple urinary parameters before taking the medicine. The patients were randomized and divided into two groups. The medication group took 2 gm WLS formula three times daily for 1 month. The control group took 2 gm placebo three times daily for 1 month. A 24-hour urine collection was performed to evaluate multiple urinary and serum parameters from all patients during the study period. A total of 39 patients were enrolled and 28 patients completed the study. Fourteen patients were allocated to WLS group and 14 patients to placebo group. After treatment, the mean urine output level increased to 2796.4 ± 525.7 ml/day (percentage of change, 13.9 %) in the WLS formula group. With placebo therapy, the mean decreased slightly to 2521.4 ± 762.7ml/day (percentage of change, −5.7 %). The percentage of change was significantly different between the two groups (independent t-test, P=0.02). No patient complained of side effects, such as fatigue, dizziness, musculoskeletal symptoms, or gastrointestinal disturbance. WLS formula is a promising adjunct to surgical and medical management of kidney stones. Active therapy with WLS formula has a positive effect on diuresis without leading to electrolyte imbalance.
Keywords: Calcium oxalate, Urinary Stone, Traditional Chinese Medicine, Wu-Ling-San Formula, Nephrolithiasis
Introduction
Urinary stone disease affects 9.6% of the total population in Taiwan, including 4.3% of females and 14.5% of males (Lee et al., 2002). The recurrence rate ranges from 35% within 5 years and after first treatment to 74% within 10 years (Uribarri et al., 1989). A number of medical treatments have been reported to prevent the recurrence of urinary stone, including encouraging patients to take more fluid, nutrient supplements and diet control (Lewandowski et al., 2004).
In recent years, the most popular medication for preventing calcium oxalate nephrolithiasis has been potassium citrate (Barcelo et al., 1993; Ettinger et al., 1997; Hofbauer et al., 1994; Whalley et al., 1996). Potassium citrate effectively reduces the recurrence rate of calcium oxalate nephrolithiasis (Hofbauer et al., 1994; Whalley et al., 1996). However, potassium citrate irritates the gastric mucosa, and this problem may limit patient acceptability. In published clinical trials of potassium citrate, the incidence of gastrointestinal adverse events ranged from 9 to 17% (Barcelo et al., 1993; Ettinger et al., 1997).
Medicinal herbs are widely accepted by people in Taiwan and in Chinese societies elsewhere. We previously reported that Wu-Ling-San (WLS) formula effectively inhibited the process of calcium oxalate nucleation, crystallization and aggregation in vitro and in vivo (Chen et al., 2007; Tsai et al., 2008). These findings inspired us to clarify the nephrolithiasis prevention effects of WLS in a clinical trial. In this study, we examined the efficacy of WLS formula prophylaxis for preventing recurrent calcium oxalate nephrolithiasis. We analyzed the changes in urine output and biochemical parameters of urine and serum.
Materials and Methods
Preparation of Wu-Ling-San Formula Extracts
The WLS formula consists of five herbs including Rhizoma alismatis, Poria cocos Wolf, Polyporus umbellatus Fries, Rhizoma Atractylodis Macrocephalae and Ramulus Cinnamomi Cassiae; the weight of each is in a ratio of 4:3:3:3:2. The fine powder formula used in this study was provided by the Koda pharmaceutical company (Taichung, Taiwan). Aqueous extracts of WLS formula were prepared by putting 100g of formula into a bottle containing 500 ml d.d. water; the solution was then heated for 15 min in an autoclave at 121°C. The resulting jelly-like product was dissolved in d.d. water to a final volume of 750 ml and stored at 4°C for 7 days. The solution was then centrifuged at 1,500 rpm for 10 min. The concentration of the extract was measured by weighing 1 ml of the supernatant which had been dried in a 60°C oven for 1 day (82 mg/ml). Finally, the solution was then used to prepare five concentrations: 25, 3.125, 6.25, 12.5, and 50 mg per ml. The placebo, which had similar drug color, the same sodium content, equal osmolality and uniform bottle cover as WLS formula, was also provided by the Koda pharmaceutical company (Taichung, Taiwan).
Study Protocol
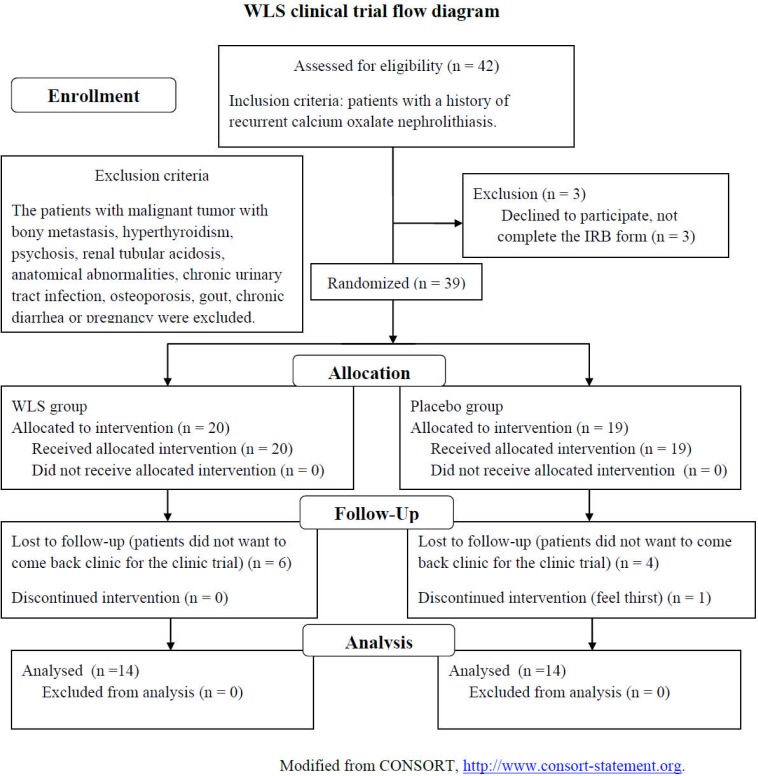
This experimental protocol was approved by the Institutional Review Board of Show Chwan Memorial Hospital (IRB, SCMH No: 960202). This was a prospective pilot study that evaluated the effect of WLS formula on 39 patients with recurrent calcium oxalate nephrolithiasis. The stones analyses for proving calcium oxalate nephrolithiasis were performed in all patients. Patients with malignant tumors with bony metastasis, hyperthyroidism, psychosis, renal tubular acidosis, anatomical abnormalities, chronic urinary tract infection, osteoporosis, gout, chronic diarrhea or pregnancy were excluded. The patients were randomized and divided into two groups (Figure 1). The medication group took 2 gm WLS formula three times daily for 1 month. The control group took 2 gm placebo formula three times daily for 1 month. The patients were asked to drink enough fluid to urinate at least 2 L daily during the study period. Before treatment, a 24-hour urine sample was collected from each patient, who had been kept on a random diet, for measurement of calcium, uric acid, pH and total urine volume. A venous serum sample was collected for calcium, phosphate, sodium, potassium, creatinine, GOT (AST), GPT (ALT) and uric acid. At the beginning and 1 month later, image follow-up with a single KUB (kidneys, ureters and bladder) x-ray film and renal ultrasonography was done in all patients. The severity of stone disease was determined by calculating the sum of the long-axis diameter of the largest stone (1 point, largest stone size < 1 cm; 2 points, largest stone size 1–2 cm; and 3 points, largest stone size > 2 cm) and the number of stones (1 point, one stone; 2 points, two stones; 3 points, more than two stones) from the KUB image (Liu et al., 2007). Statistical analysis was performed using the independent t-test, P<0.05 was considered statistically significant.
Figure 1.
Wu-Ling-San clinical trial flow diagram.
Results
A total of 39 patients were enrolled, of whom 31 (79.5%) were men and 8 (20.5%) were women. Six patients of the WLS group and 5 patients of the placebo group withdrew before the end of the study. Twenty-eight patients completed the study, of whom 23 (82.1%) were men and 5 (17.9%) were women. The major reason for withdrawal was that the patients did not want to come back to the clinic for the clinic trial. Only one patient in placebo group complained of thirst (Figure 1). The mean age of the patients was 46.8 ± 8.8years (range 30 to 63). The patients reported a lifetime total of 3 to 6 (average 4.2) stone episodes. The patients had undergone 2 to 3 (average 2.5) stone procedures. There were no differences in baseline data between the two groups, including age, male/female ratio, stone severity index, urolithiasis procedures and BMI (Table 1). The baseline urine output was 2485.7 ± 386.5ml/day (mean ± SD) in WLS group and 2732.1 ± 797.9 ml/day in placebo group. After treatment, the mean urine output level increased to 2796.4 ± 525.7 ml/day (percentage of change, 13.9 %) in WLS formula group and decreased slightly to 2521.4 ± 762.7ml/day (percentage of change, −5.7 %) in placebo group. The percentage of changes was significantly different between the two groups (independent t-test, P=0.02, Table 2).
Table 1.
Patient data at baseline.
| Wu-ling-san group | Placebo group | P value(t-test) | |
| Men(n) | 12 | 11 | 0.63 |
| Women(n) | 2 | 3 | |
| Mean age (years old) | 44.8 ± 8.7 | 48.8 ± 8.7 | 0.23 |
| Urolithiasis procedure | 2.6 ± 0.5 | 2.4 ± 0.5 | 0.690 |
| Stone severity index | 3.8 ± 1.1 | 3.4 ± 0.5 | 0.421 |
| BMI (kg/m2) | 26.3 ± 3.0 | 26.2 ± 3.0 | 1.000 |
Table 2.
Urine parameters before and after treatment were checked in Wu-ling-san formula or placebo group.
| Wu-ling-san group | Placebo group |
P value (% of change) |
|||||
| Pretreatment | Post-treatment | % of change | Pretreatment | Post-reatment | % of change | ||
| Urine volume(ml/day) | 2485.7 ± 386.5 | 2796.4 ± 525.7 | 13.9% | 2732.1 ± 797.9 | 2521.4 ± 762.7 | −5.7% | 0.02* |
| Urine Ca (mg/day) | 249.5 ± 132.2 | 302.1 ± 106.5 | 44.6% | 266.3 ± 101.6 | 415.1 ± 290.7 | 62.7% | 0.62 |
| Urine UA (mg/day) | 749.6 ± 336.6 | 893.5 ± 334.1 | 27.6% | 760.6 ± 246.2 | 849.3 ± 478.4 | 9.5% | 0.22 |
| Urine Cr (mg/dl) | 59.1 ± 21.7 | 71.1 ± 24.4 | 42.9% | 66.0 ± 33.5 | 80.4 ± 27.6 | 36.6% | 0.84 |
| Urine PH | 6.6 ± 0.6 | 6.5 ± 0.7 | −0.02% | 6.3 ± 0.8 | 6.3 ± 0.7 | 0.6% | 0.91 |
| 24hr Urine Ccr (ml/min) | 89.2 ± 26.4 | 107.5 ± 30.9 | 26.2% | 133.4 ± 37.1 | 114.1 ± 44.0 | 14.5% | 0.53 |
Data presented as mean ±SD
Percentage of change between groups P < 0.05 (independent t-test)
The baseline serum BUN was 14.7 ± 2.7mg/dl in WLS group and 15.0±4.6 mg/dl in placebo group. After treatment, the mean BUN level decreased to 13.8 ± 3.9 mg/dl (percentage of change, −6.9 %) in WLS group and increased slightly to 16.9 ± 5.3 mg/dl (percentage of change, 13.8 %) in placebo group. The percentage of change was significantly different between the two groups (independent t-test, P=0.006, Table 3). We believe that these results were related to the diuretic effect of WLS.
Table 3.
Renal and liver function in Wu-ling-san formula and placebo groups.
| Wu-ling-san group | Placebo group |
P value (% of change) |
|||||
| Pretreatment | Post-treatment | % of change | Pretreatment | Post-reatment | % of change | ||
| Serum BUN (mg/dl) | 14.7 ± 2.7 | 13.8 ± 3.9 | −6.9% | 15.1 ± 4.6 | 16.9 ± 5.3 | 13.8% | 0.006* |
| Serum Cr (mg/dl) | 1.1 ± 0.1 | 1.1 ± 0.1 | −0.4% | 1.2 ± 0.1 | 1.2 ± 0.2 | 2.8% | 0.44 |
| Serum GOT (IU/L) | 24.6 ± 12.1 | 23.7 ± 9.6 | −0.04% | 23.6 ± 6.6 | 22.2 ± 7.0 | −4.3% | 0.60 |
| Serum GPT (IU/L) | 36.0 ± 32.1 | 36.7 ± 27.9 | 7.7% | 32.8 ± 18.4 | 31.1 ± 20.1 | −4.9% | 0.25 |
Data presented as mean ±SD
Percentage of change between groups P < 0.05 (independent t-test)
Commonly used drugs for urolithiasis prophylaxis, such as potassium citrate, alkalinize urine. We checked the urine pH before and after treatment. The baseline urine pH was 6.6 ± 0.6 in WLS group and 6.3 ± 0.8 in placebo group. After treatment, the urine pH was 6.5 ± 0.7 (percentage of change, −0.02 %) in WLS formula group and 6.3 ± 0.7 (percentage of change, 0.6%) in placebo group. The percentage of change between the WLS and placebo group was not significantly different (independent t-test, P=0.91, Table 2). These data suggested that WLS had no direct effect on urine pH.
For the study of possible drug toxicity, we checked the liver and renal function of patients before and after treatment. After treatment, the mean GOT level was 23.7 ± 9.6IU/L (percentage of change, −0.04 %) in WLS formula group and 22.2 ± 7.0IU/L (percentage of change, −4.3 %) in placebo group. The percentage of change between the WLS and placebo group was not significantly different (independent t-test, P=0.6). Similarly, the mean GPT level was 36.7± 27.9 IU/L (percentage of change, 7.7 %) in WLS formula group and 31.1 ± 20.1 IU/L (percentage of change, −4.9 %) in placebo group post treatment. The percentage of change between the WLS and placebo group was not significantly different (independent t-test, P=0.25, Table 3). About renal function test, after treatment, the serum creatinine level was 1.1 ± 0.1 mg/dl (percentage of change, −0.4 %) in WLS formula group and 1.2 ± 0.2 mg/dl (percentage of change, 2.8%) in placebo group. The percentage of change between the WLS and placebo group was not significantly different (independent t-test, P=0.44, Table 3). Furthermore, the percentage of change of 24-hour Urine Ccr are slightly increased in WLS group than placebo group (26.2% versus 14.5%), but not significantly different (independent t-test, P=0.53, Table 2). These data suggested that WLS was safe and did not affect liver or renal function.
Urinary parameters, including 24-hour urine calcium and uric acid were checked. After treatment, the 24-hour urine calcium increased to 302.1 ± 106.5mg/day (percentage of change, 44.6 %) in WLS formula group and 415.1 ± 290.7mg/day (percentage of change, 62.7 %) in placebo group. The percentage of change in urinary calcium between the two groups was not significantly different (independent t-test, P=0.62). Similar results were noted on urine uric acid level. After treatment, the 24 hours urine uric acid increased to 893.5 ± 334.1mg/day (percentage of change, 27.6 %) in WLS formula group and 849.3 ± 478.4mg/day (percentage of change, 9.5%) in placebo group. The percentage of change between the two groups was not significantly different (independent t-test, P=0.22, Table 2).
Because some drugs used for urolithiasis prophylaxes such as diuretics have the side effect of serum electrolyte imbalance, we checked serum electrolytes including sodium and potassium before and after treatment. After treatment, the serum sodium level was 140.9 ± 1.2mmol/L (percentage of change, −0.5 %) in Wu-Ling-San formula group and 140.9 ± 1.7mmol/L (percentage of change, 0.2%) in placebo group. The percentage of change between the WLS and placebo group was not significantly different (independent t-test, P=0.12). Furthermore, the serum potassium level was 4.0 ± 0.3mmol/L (percentage of change, 0.7%) in WLS formula group and 4.0 ± 0.3mmol/L (percentage of change, −3.2%) in placebo group post treatment. The percentage of change between the two groups was not significantly different (independent t-test, P=0.16, Table 4). These data suggested that WLS did not induce serum electrolyte imbalance.
Table 4.
Serum electrolytes in Wu-ling-san formula and placebo groups.
| Wu-ling-san group (mean±S.D.) | Placebo group |
P value (% of change) |
|||||
| Pretreatment | Post-treatment | % of change | Pretreatment | Post-treatment | % of change | ||
| Serum Na (mmol/L) | 141.6 ± 1.3 | 140.9 ± 1.2 | −0.5% | 140.6 ± 1.3 | 140.9 ± 1.7 | 0.2% | 0.12 |
| Serum K (mmol/L) | 4.0 ± 0.3 | 4.0 ± 0.3 | 0.7% | 4.2 ± 0.5 | 4.0 ± 0.3 | −3.2% | 0.16 |
| Serum Ca (mg/dl) | 9.2 ± 0.3 | 9.1 ± 0.4 | −0.8% | 9.1 ± 0.3 | 9.2 ± 0.3 | 0.3% | 0.47 |
| Serum P (mg/dl) | 3.3 ± 0.6 | 3.3 ± 0.5 | 4.0% | 3.1 ± 0.6 | 3.2 ± 0.5 | 4.3% | 0.96 |
Data presented as mean ±SD
Percentage of change between groups P < 0.05 (independent t-test)
Serum calcium and phosphate concentrations are known risk factors of urolithiasis formation. We therefore checked the serum calcium and phosphate before and after treatment. After treatment, the serum calcium level was 9.1 ± 0.4 mg/dl (percentage of change, −0.8 %) in Wu-Ling-San formula group and 9.2 ± 0.3 mg/dl (percentage of change, 0.3%) in placebo group. The percentage of change between the WLS and placebo groups was not significantly different (independent t-test, P=0.47). The serum phosphate level was 3.3 ± 0.5 mg/dl (percentage of change, 4.0 %) in Wu-Ling-San formula group and 3.2 ± 0.5 mg/dl (percentage of change, 4.3%) in placebo group post treatment. The percentage of change on serum phosphate level between the WLS and placebo group was not significantly different (independent t-test, P=0.96, Table 4). These data suggested that WLS had no direct effect on serum calcium and phosphate.
During the study period, no patient complained of side effects, such as fatigue, dizziness, musculoskeletal symptoms, body weight loss or gastrointestinal disturbance. No statistical significant differences in urine calcium, urine uric acid, urine creatinine and urine pH were noted between the two groups (Table 2). The changes in liver function, renal function and serum electrolytes were not significantly different between the two groups (Table 3, 4).
Discussion
Many traditional Chinese medicines (TCM) are used to treat urolithiasis, and WLS is one of them (Freitas et al., 2002; Gohel et al., 2006). WLS is a TCM formula mainly used for treatment of uremia, dropsy, nephrosis and to promote urination. The formula for WLS was first recorded in the book “Shang Han Lun” (Treatise of Cold-induced Disorders) written by Zhong-Jing Zhang. The original indications for WLS were symptoms of headache, fever, voiding difficulty, irritability, strong thirst with vomiting immediately after drinking and a floating pulse (Tsai et al., 2008). Treating urinary stone disease with WLS was first recorded in the book written by Ken-Tang Wang entitled “Zheng Zhi Zhun Sheng” (standards of patterns and treatment) during the Ming Dynasty (late sixteenth century). Since that time, the WLS formula has been commonly used for treating urinary difficulties and febrile diseases because of its diuretic properties (Tsai et al., 2008).
To the best of our knowledge, the possible antilithic mechanism of WLS remains unclear. Some studies have suggested that macromolecules may be involved in the antilithic mechanism of WLS (Tsai et al., 2008). One of the components of WLS, Alisma orientalis, has been shown to inhibit the stone formation process (Cao et al., 2004; Yasui et al., 1999). Furthermore, several studies have shown that this herb significantly decreased the formation of calcium oxalate (CaOx) deposits, and downregulated the expression of inter-alpha-trypsin inhibitor and bikunin (Cao et al., 2004). Yashimura et al. noted that WLS significantly inhibited CaOx crystallization in human urine in vitro (Yoshimura et al., 1998). Liu et al. reported that in reducing CaOx crystals, WLS also suppressed the development of hydroxylapatite renal calcinosis in rats fed a high phosphorus diet (Liu et al., 2001). Our previous study already concluded that WLS effectively inhibits the process of CaOx nucleation, crystallization and aggregation in vitro and in vivo (Chen et al., 2007; Tsai et al., 2008). All of these reports suggest that WLS may be a useful drug for preventing renal stones. We conducted this prospective randomized controlled clinical trial to investigate the effect of WLS in recurrent calcium oxalate nephrolithiasis patients.
This pilot study results showed that urine output increased after treatment with WLS group, and these increase was significant when compared to the change in urine output in patients treated with placebo (Table 2). Patients treated with WLS also had decreased serum BUN. We believe that this change in serum BUN is a diuretic effect. The percentage of change of 24-hr Urine Ccr are slightly increased in WLS group than placebo group (26.2% versus 14.5%), but not significantly different (independent t-test, P=0.53, Table 2). The results of this study have confirmed that WLS formula is effective in causing diuresis in recurrent calcium oxalate stone formers (Table 3). Hydration, diuresis and increasing urine output are effective methods for stone prevention (Trinchieri et al., 2005).
It is well known that hydration and increasing urine output are important for stone prevention. Borghi et al. recruited 101 controls and 199 patients from the first calcium stone episode. Patients were randomly divided into two groups and followed prospectively for 5 years. In group 1, patients increased their water intake without any dietetic change, while in group 2 patients did not receive any treatment. The results showed that baseline urine volume was lower in male and female stone formers /compared to controls (P < 0.0001). During the follow-up period, stone recurrence was noted in 12 of 99 (12%) group 1 patients and 27 of 100 (27%) group 2 patients (P=0.008). The average interval for recurrence was 38.7 months in group 1 and 25.1months in group 2 (P= 0.016) patients. They concluded that urine volume is a real stone risk factor in stone formers and more water intake is the initial therapy for prevention of stone recurrences (Borghi et al., 1996). In a review by Manz et al., maintaining good hydration status has been shown to positively affect urolithiasis (Manz, 2007). In contrast, dehydration is a risk factor of urolithiasis. Atan et al. reported the incidence of urinary stones among male employees in the steel industry who were exposed to a high-temperature work environment. Their results showed that 181 of 10,326 workers (1.75%) presented with at least one episode of urinary stones. Of these, 103 (56.9%) were hot-area workers and 78 (43.1%) were room-temperature workers (P < 0.001). They suggested that workers exposed to high temperatures with dehydration status presented with a nine-fold risk of urolithiasis (Atan et al., 2005).
Some popular stone prevention drugs have side effects. Potassium citrate may lead to gastrointestinal disturbance, although this may be prevented by diluting it in a large glass of water (Koff et al., 2007; Whalley et al., 1996). Loop diuretics such as the thiazides, may induce hypokalemic, hypochloremic, metabolic alkalosis and this may be treated with potassium chloride replacement. Thiazide diuretics have also been linked to glucose intolerance, which may be an effect of hypokalemia rather than the diuretic itself. Thiazides may lead to hyponatremia which may cause permanent neurologic damage (Greenberg, 2000). In the contrast, WLS was not associated with any side effects such as gastrointestinal disturbance, electrolyte imbalance or malaise in our series (Table 4).
An interesting finding from our study was the overall good compliance with the instruction to urinate 2 L daily. All the patients in the WLS and placebo groups met the goal. This may be because we educated the patients on the possible antilithic mechanism and diuretic effect of WLS. One should drink enough fluid to urinate at least 2 L daily for WLS to reach its best effect. Furthermore, WLS is also cost effective. In Taiwan, the WLS formula costs only 10.2 USD/month, while potassium citrate costs 40.9 USD/month.
The limitation of this study is that we did not examine the oxalate and citrate levels in urine. The statistical significance of this study was also limited due to the small number of subjects and the short treatment period. Another limitation of our clinical trial was we used daily urine amount as a marker of drug efficacy. It is not very scientific, because there are so many factors that could influence the daily urine amount. For example, the amount of fluid intake, the environment temperature, the water content of meal and the amount of insensible water loss. The better way to elucidate the diuretic effect of WLS is to admit the patient to a special ward, consume a constant diet, stay in a constant environment (such as standardization of the room temperature, humidity, etc.) and under close observation while collecting urine. But that was difficulty to reach under our current health care system and limited foundation.
Conclusions
Traditional Chinese herb medicine WLS is a safe promising adjunct to surgical and medical management of kidney stones. It might be better accepted by patients because it has a good safety profile and a low incidence of side effects. Active therapy with WLS formula increased urine volume without causing electrolyte imbalance. Patients should be educated and hydration encouraged to meet the goals of increased urinary output, and treatment with WLS formula may help patients reach these goals.
Acknowledgments
This work was supported by RD96008 plan of Show Chwan Memorial Hospital, Chang-hua, Taiwan. The authors thank Dr. Marcelo Chen (Mackay Memorial Hospital, Taipei, Taiwan) and Dr. Anita Mannikarottu (Albany College of Pharmacy, Albany, New York) for assistance with correcting English manuscript.
References
- 1.Atan L, Andreoni C, Ortiz V, Silva E K, Pitta R, Atan F, Srougi M. High kidney stone risk in men working in steel industry at hot temperatures. Urology. 2005;65:858–861. doi: 10.1016/j.urology.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 2.Barcelo P, Wuhl O, Servitge E, Rousaud A, Pak C Y. Randomized double-blind study of potassium citrate in idiopathic hypocitraturic calcium nephrolithiasis. J Urol. 1993;150:1761–1764. doi: 10.1016/s0022-5347(17)35888-3. [DOI] [PubMed] [Google Scholar]
- 3.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol. 1996;155:839–843. [PubMed] [Google Scholar]
- 4.Cao Z G, Liu J H, Zhou S W, Wu W, Yin C P, Wu J Z. The effects of the active constituents of Alisma orientalis on renal stone formation and bikunin expression in rat urolithiasis model. Zhonghua Yi Xue Za Zhi. 2004;84:1276–1279. [PubMed] [Google Scholar]
- 5.Chen Y C, Ho C Y, Chen L D, Hsu S F, Chen W C. Wu-Ling-San formula inhibits the crystallization of calcium oxalate in vitro. Am J Chin Med. 2007;35:533–541. doi: 10.1142/S0192415X07005041. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger B, Pak C Y, Citron J T, Thomas C, Adams-Huet B, Vangessel A. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol. 1997;158:2069–2073. doi: 10.1016/s0022-5347(01)68155-2. [DOI] [PubMed] [Google Scholar]
- 7.Freitas A M, Schor N, Boim M A. The effect of Phyllanthus niruri on urinary inhibitors of calcium oxalate crystallization and other factors associated with renal stone formation. BJU Int. 2002;89:829–834. doi: 10.1046/j.1464-410x.2002.02794.x. [DOI] [PubMed] [Google Scholar]
- 8.Gohel M D, Wong S P. Chinese herbal medicines and their efficacy in treating renal stones. Urol Res. 2006;34:365–372. doi: 10.1007/s00240-006-0068-y. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg A. Diuretic complications. Am J Med Sci. 2000;319:10–24. [PubMed] [Google Scholar]
- 10.Hofbauer J, Hobarth K, Szabo N, Marberger M. Alkali citrate prophylaxis in idiopathic recurrent calcium oxalate urolithiasis--a prospective randomized study. Br J Urol. 1994;73:362–365. doi: 10.1111/j.1464-410x.1994.tb07597.x. [DOI] [PubMed] [Google Scholar]
- 11.Koff S G, Paquette E L, Cullen J, Gancarczyk K K, Tucciarone P R, Schenkman N S. Comparison between lemonade and potassium citrate and impact on urine pH and 24-hour urine parameters in patients with kidney stone formation. Urology. 2007;69:1013–1016. doi: 10.1016/j.urology.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y H, Huang W C, Tsai J Y, Lu C M, Chen W C, Lee M H, Hsu H S, Huang J K, Chang L S. Epidemiological studies on the prevalence of upper urinary calculi in Taiwan. Urol Int. 2002;68:172–177. doi: 10.1159/000048445. [DOI] [PubMed] [Google Scholar]
- 13.Lewandowski S, Rodgers A L. Idiopathic calcium oxalate urolithiasis: risk factors and conservative treatment. Clin Chim Acta. 2004;345:17–34. doi: 10.1016/j.cccn.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Liu C C, Huang C H, Wu W J, Huang S P, Chou Y H, Li C C, Chai C Y, Wu M T. Association of vitamin D receptor (Fok-I) polymorphism with the clinical presentation of calcium urolithiasis. BJU Int. 2007;99:1534–1538. doi: 10.1111/j.1464-410X.2007.06792.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q L, Sato S, Kishikawa T, Matsuzaki H, Yamanaka N. Effectiveness of a traditional Chinese medicine, Wulingsan, in suppressing the development of nephrocalcinosis induced by a high phosphorus diet in young rats. Med Electron Microsc. 2001;34:103–114. doi: 10.1007/s007950170004. [DOI] [PubMed] [Google Scholar]
- 16.Manz F. Hydration and disease. J Am Coll Nutr. 2007;26:535S–541S. doi: 10.1080/07315724.2007.10719655. [DOI] [PubMed] [Google Scholar]
- 17.Trinchieri A, Castelnuovo C, Lizzano R, Zanetti G. Calcium stone disease: a multiform reality. Urol Res. 2005;33:194–198. doi: 10.1007/s00240-004-0459-x. [DOI] [PubMed] [Google Scholar]
- 18.Tsai C H, Chen Y C, Chen L D, Pan T C, Ho C Y, Lai M T, Tsai F J, Chen W C. A traditional Chinese herbal antilithic formula, Wulingsan, effectively prevents the renal deposition of calcium oxalate crystal in ethylene glycol-fed rats. Urol Res. 2008;36:17–24. doi: 10.1007/s00240-007-0122-4. [DOI] [PubMed] [Google Scholar]
- 19.Uribarri J, Oh M S, Carroll H J. The first kidney stone. Ann Intern Med. 1989;111:1006–1009. doi: 10.7326/0003-4819-111-12-1006. [DOI] [PubMed] [Google Scholar]
- 20.Whalley N A, Meyers A M, Martins M, Margolius L P. Long-term effects of potassium citrate therapy on the formation of new stones in groups of recurrent stone formers with hypocitraturia. Br J Urol. 1996;78:10–14. doi: 10.1046/j.1464-410x.1996.09852.x. [DOI] [PubMed] [Google Scholar]
- 21.Yasui T, Fujita K, Sato M, Sugimoto M, Iguchi M, Nomura S, Kohri K. The effect of takusha, a kampo medicine, on renal stone formation and osteopontin expression in a rat urolithiasis model. Urol Res. 1999;27:194–199. doi: 10.1007/s002400050109. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura K, Miyake O, Okuyama A, Yoshioka T, Honda M, Yamaguchi S, Koide T. Effect of chorei-to and gorei-san on calcium oxalate crystallization in human urine. Hinyokika Kiyo. 1998;44:13–16. [PubMed] [Google Scholar]