Abstract
Medicinal plants have been used in healthcare since time immemorial. Studies have been carried out globally to verify their efficacy and some of the findings have led to the production of plant-based medicines. The global market value of medicinal plant products exceeds $100 billion per annum. This paper discusses the role, contributions and usefulness of medicinal plants in tackling the diseases of public health importance, with particular emphasis on the current strategic approaches to disease prevention. A comparison is drawn between the ‘whole population’ and ‘high-risk’ strategies. The usefulness of the common-factor approach as a method of engaging other health promoters in propagating the ideals of medicinal plants is highlighted. The place of medicinal plants in preventing common diseases is further examined under the five core principles of the Primary Health Care (PHC) approach. Medicinal plants play vital roles in disease prevention and their promotion and use fit into all existing prevention strategies. However, conscious efforts need to be made to properly identify, recognise and position medicinal plants in the design and implementation of these strategies. These approaches present interesting and emerging perspectives in the field of medicinal plants. Recommendations are proposed for strategising the future role and place for medicinal plants in disease prevention.
Keywords: Medicinal Plants, Prevention, Strategy, Primary Health Care
Introduction
The emphasis on the use of medicinal plants had hitherto been placed on the treatment rather than prevention of diseases. However, there exists in the literature considerable report in recent times on research work on the use of medicinal plants and their constituents in disease prevention. A World Health Organisation (WHO) Expert Group defined Traditional Medicine as the sum total of all knowledge and practices, whether explicable or not, used in diagnosis, prevention and elimination of physical, mental, or social imbalance and relying exclusively on practical experience and observation handed down from generation to generation, whether verbally or in writing (WHO, 1976). For Africa, this may be extended further by including an expression, such as ‘while bearing in mind the original concept of nature which includes the material world, the sociological environment whether living or dead and the metaphysical forces of the universe’.
Over 90% of traditional medicine recipes/remedies contain medicinal plants but this paper will address, specifically, the medicinal plants that have been implicated with preventive measures in disease control strategies. However, it must be noted that only a very thin divide exists between treatment and prevention in some cases. A quick example is the fact that by treating mild elevation of blood pressure renal disease can be prevented.
What is a medicinal plant?
A medicinal plant is any plant which, in one or more of its organs, contains substances that can be used for therapeutic purposes or which are precursors for the synthesis of useful drugs. This description makes it possible to distinguish between medicinal plants whose therapeutic properties and constituents have been established scientifically, and plants that are regarded as medicinal but which have not yet been subjected to a thorough scientific study.
A number of plants have been used in traditional medicine for many years. Some do seem to work although there may not be sufficient scientific data (double-blind trials, for example) to confirm their efficacy. Such plants should qualify as medicinal plants. The term ‘crude drugs of natural or biological origin’ is used by pharmacists and pharmacologists to describe whole plants or parts of plants which have medicinal properties. A definition of medicinal plants for the purpose of this presentation should include the following (Sofowora 2008; Evans, 2008):
plants or plant parts used medicinally in galenical preparations (e.g. decoctions, infusions, etc.) e.g. Cascara bark;
plants used for extraction of pure substances either for direct medicinal use or for the hemi-synthesis of medicinal compounds (e.g. hemi-synthesis of sex hormones from diosgenin obtained from Dioscorea yams);
food, spice, and perfumery plants used medicinally, e.g. ginger;
microscopic plants, e.g. fungi, actinomycetes, used for isolation of drugs, especially antibiotics. Examples are ergot (Claviceps purpurea growing on rye) or Streptomyces griseus; and
fibre plants, e.g. cotton, flax, jute, used for the preparation of surgical dressings.
The growing importance of medicinal plants can be appreciated from the economic stand point when the following facts are considered:
Global trade in herbs is over USD 100 Billion per annum
India and China's medicinal plant trade is about two to five billion US dollars annually
In Germany, it is over one billion US dollars annually
Rose Periwinkle which is endemic to Madagascar fetches US$100 million per annum
China trades in 7,000 species and 700,000 tons of medicinal plants per annum
India trades in 7,000 species of medicinal plants
Morocco exports 58.7 tons of medicinal plants annually
In the last 5 years, sales of medicinal plants doubled in China, tripled in India and grew by 25% in Europe.
A Presidential Initiative Committee on the Development, Promotion, and Commercialisation of Nigerian Herbal Medicinal Products was inaugurated on 30th May 2006 and was given a target of US$1billion sales of medicinal plants and its products within 10 years for Nigeria. Based on current research and financial investments, medicinal plants will, seemingly, continue to play an important role as a health aid (Hoareau and DaSilva, 1999). The use of traditional medicine and medicinal plants in most developing countries, as a normative basis for the maintenance of good health, has been widely observed (UNESCO, 1996). Furthermore, an increasing reliance on the use of medicinal plants in the industrialised societies has been traced to the extraction and development of several drugs and chemotherapeutics from these plants as well as from traditionally used rural herbal remedies (UNESCO, 1998). Moreover, in these societies, herbal remedies have become more popular in the treatment of minor ailments, and also on account of the increasing costs of personal health maintenance. Indeed, the market and public demand has been so great that there is a great risk that many medicinal plants today face either extinction or loss of genetic diversity.
Preventive strategies
Health promotion, disease prevention and chronic disease management are proactive approaches to health care that stresses prevention at different points along the health care continuum. Health promotion and disease prevention strategies focus on keeping people well and preventing diseases from occurring. These strategies are referred to as primary prevention activities. Prevention is categorised into three levels (Commission on Chronic Illness, 1957):
- Primary Prevention, which seeks to decrease the number of new cases of a disorder or illness. At this level of prevention we have:
- Health promotion/education, and
- Specific protective measures (such as immunisation)
Secondary Prevention, which seeks to lower the rate of established cases of a disorder or illness in the population (prevalence). This level essentially involves measures that ensure early diagnosis (such as screening) and prompt management
- Tertiary Prevention, which seeks to decrease the amount of disability associated with an existing disorder. This level involves:
- Disability limitation and
- Rehabilitation
The secondary and tertiary prevention activities focus on maintaining the health of individuals with chronic conditions, delaying progression of their conditions, and preventing complications.
Disease prevention should focus on strategies that reduce the risk of disease, identify risk factors, or detect disease in its early, most treatable stages. Examples of disease prevention activities include well-baby visits, immunisations, calcium and Vitamin D supplements to reduce the risk of osteoporosis, blood pressure and cholesterol assessments during annual health exams, and screening for illnesses such as breast, cervical, colorectal and prostate cancer (Family Health Teams, 2006).
Public health, diet, food production and the environment are deeply interrelated, and understanding these relationships is crucial in pursuing a liveable future. Sometimes therefore, there is only a thin line between treatment and prevention of certain diseases. For example, treatment of mild hypertension will prevent many chronic renal diseases. This is also true for obesity, cancers, coronary heart diseases (CHDs) as well as diabetes and its sequelae, though these are non-communicable diseases.
The burden of healthcare and its human and financial resources requirement
In developing countries all over the world, large numbers of people die daily of preventable or curable diseases because of the lack of even simple health care. Diseases in these countries are often associated with malnutrition. As a result, those that do survive often never recover completely from the effects. The developing world is not a homogenous entity, but is made up of a variety of widely differing countries and areas which are at different stages of development. Nevertheless, these developing countries have certain features in common, including extremely limited resources, poor communications, vast distances, low levels of education, and individual and community poverty. These factors act together to keep these countries in a perpetual state of poverty. Yet, their populations continue to rise, especially in the rural regions which usually account for about 80 per cent of the total population.
Another special characteristic of the developing world is the nomadic lifestyle of some of its people. Some 50 to 100 million nomads have been estimated to be present in the world, and 90 per cent of these live in Africa or Asia. Nomads have their own needs and problems peculiar to their lifestyle. Because of their constant movement and dispersion it is difficult for conventional health services to reach these people. The striking difference between the developed and the developing world in terms of health care is reflected in the differing life expectancy of their populations. For example, according to WHO (2012), in 2009 the life expectancy at birth was estimated at 51 years in Angola and Burkina Faso, while for Malawi it was 47 years (African countries) compared with 80 years in the United Kingdom, USA and Austria. Although the situation is improving in some African countries, this gap is still wide. Twenty-six African countries are ranked among the ‘Low Income Group’, 17 in the ‘Lower Middle Income Group’ and 9 in the ‘Upper Middle Income Group’, while only Equatorial Guinea is ranked in the ‘High Income Group’ (WHO, 2012). Several of the people in Africa earn less than US$2 per day.
Any strategy for disease prevention in the developing countries, especially in Africa, must take these socioeconomic factors into account. With the abundant biodiversity of plants in the African Region and the relative lower cost of using plant derived medicines instead of processed synthetic drugs, medicinal plants should have a role to play in disease prevention strategies in Africa.
Strategies planned for traditional medicine development (First decade and second decade for Traditional Medicine in Africa)
For WHO, the main principles of the strategy for developing traditional medicine (TM) globally were thus:
Policy: Integrate TM/CAM with national health care systems, as appropriate, by developing and implementing national TM/CAM policies and programmes
Safety, efficacy and quality: Promote the safety, efficacy and quality of TM/CAM by expanding the knowledge-base on TM/CAM, and by providing guidance on regulatory and quality assurance standards
Access: Increase the availability and affordability of TM/CAM, with an emphasis on access for poor populations
Rational use: Promote therapeutically sound use of appropriate TM/CAM by providers and consumers
Strategies for development of TM in the African Region (WHO-AFRO, Brazzaville)
For WHO-AFRO, the priority interventions for the development of TM during first and second decades (i.e. 2001–2010 and 2011–2020) for African TM are as follows:
Policy formulation;
Capacity building;
Research promotion;
Support for the local production of Traditional Medicines including cultivation of medicinal plants;
Protection of intellectual property rights and traditional medical knowledge.
Global Disease Burden
Diseases have been grouped as communicable or non-communicable based on the involvement or otherwise of a transmissible biologic disease causing agent. Until recently, communicable diseases (CDs) were the major causes of ill-health and deaths in the developing (low and middle resource) countries while non-communicable diseases were prevalent in the developed (high resource) countries, where improvement in living conditions and widespread deployment of technology had brought the CDs under control. However, the optimism that communicable diseases would be less of a health problem in the developed countries appears to have been misplaced with the appearance of new infectious diseases and re-emergence of older disease agents. Similarly, non-communicable diseases are already a major cause of morbidity and mortality as a consequence of globalisation and changing lifestyle in developing countries. Globally therefore, NCDs and CDs account for about equal quantities of morbidity and mortality, thus making all countries to currently face the double disease burden. The overall picture is, however, graver for low and middle income countries in terms of the health and socio-economic impacts.
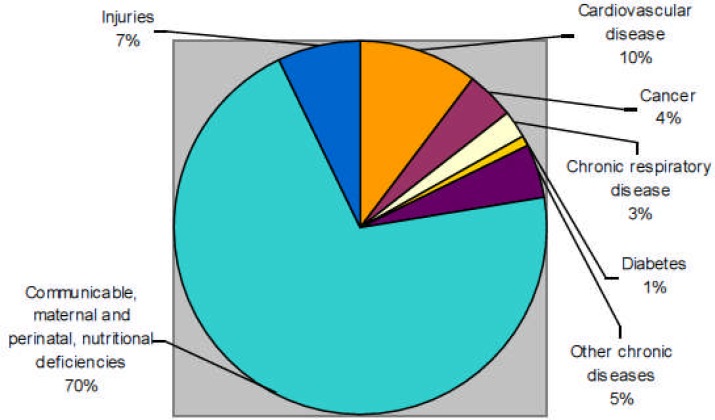
For example, estimates of projected causes of all deaths for the African Region indicate that injuries and chronic communicable diseases accounted for 30% of all deaths for 2005. (Figure 1)
Figure 1.
Projected deaths by cause, all ages, WHO African Region, 2005
Source: Moeti (2008). Data estimated by WHO using standard methods to maximize cross-border comparability. They are not necessarily the official statistics of Member States.
Medicinal plants and disease prevention
Strategies for the Prevention of Communicable diseases
Three core approaches - surveillance, outbreak investigations and immunisation - are fundamental to the prevention of communicable diseases. While medicinal plants may appear to have limited role in these approaches, several medicinal plants and traditional medicines derived from them have been used to enhance immune response to several disease agents (Di Pierro et al., 2012; Ramakrishna et al., 2011).
Strategies for Prevention of Non-Communicable Diseases
The WHO 2008 to 2013 Action Plan for the Global Strategy for the Prevention and Control of NCDs articulated an intersectoral, multi-level plan to curb the rising global prevalence of NCDs with particular focus on the low and middle income countries. The overall foci of the plan were to
map the emerging NCD epidemic and ascertain their social, economic, behavioural and political determinants;
reduce the level of exposure of individuals and population to the common modifiable risk factors - tobacco use, unhealthy diet, physical inactivity, etc.; and
strengthen health care for people with non-communicable diseases through the development of evidence-based norms, standards and guidelines for cost effective interventions.
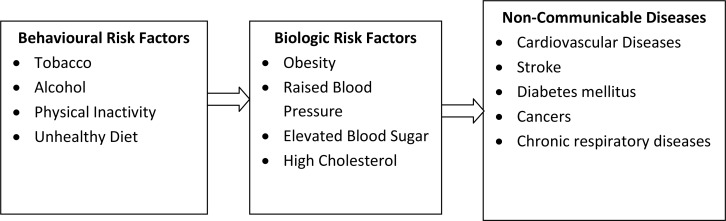
Related to the third focus above, an examination of the causal chain of risk factors for NCDs (Figure 2) is helpful in illuminating the potential role of medicinal plants in the prevention of NCDs. Medicinal plants have specific roles in strengthening health care opportunities for people with NCDs as well as in the management of the biologic risk factors for NCDs, especially in the early stage (Jung et al., 2012; Tan et al., 2010).
Figure 2.
Causal chain of risk factors for NCDs
The ‘whole population’ and ‘high-risk’ strategies
Two main types of approaches have been advocated in tackling major public health problems. The whole-population strategy targets the community as a whole to control the occurrence of new diseases in the population. The high-risk strategy on the other hand aims to identify individuals most at risk for a disease or outcome and then target preventive efforts at that group. These were first defined by Geoffrey Rose (Rose, 1985). In promoting the use of medicinal plants in disease prevention, the whole-population strategy will have the global community as the target, whereas the high-risk strategy may focus on rural communities, especially in the developing countries. It may also on the other hand wish to focus on refining medicinal plants for use in specific disease conditions that presently defy conventional treatment, such as cancer, HIV infection and AIDS.
Utilising the Common Factor approach
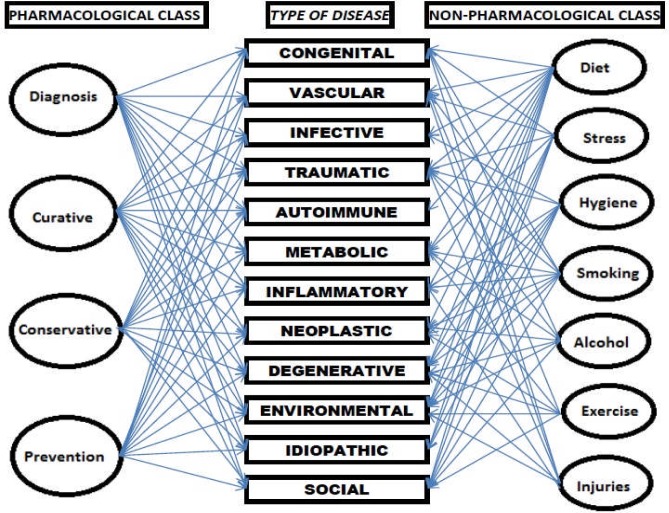
The common risk factor approach aims at bringing together several health promoters working on eliminating common-risk factors as a way of preventing diseases. (Sheiham and Walt, 2000) Poor Diet for example can lead to obesity, diabetes, cancers, and dental caries. Hence, nutritionists, diabetologists, oncologists, dental practitioners can work together with diet as common theme. A modified form of this approach (Figure 3) can be a useful tool in engaging other health promoters, in tackling the different forms of disease, and in propagating the ideals of medicinal plants. Working with various groups, for example, appropriate medicinal plants can be incorporated into the diets to alleviate disease and suffering. This approach will enable those working to promote the use of medicinal plants to collaborate with other health promoters in areas such as malaria, diabetes, cancers, cardiovascular diseases, tuberculosis, HIV/AIDS, oral diseases, dermatological problems, etc.
Figure 3.
The Common Factor Approach (Adapted from Sheiham and Walt, 2000)
The Primary Health Care (PHC) Approach
Primary Health Care was defined in Alma Ata (WHO, 1978) as essential care based on practical, scientifically sound and socially acceptable method and technology made universally accessible to individuals and families in the community through their full participation and at a cost they and the country can afford to maintain in the spirit of self-reliance and self-determination. The PHC philosophy recognises that each discipline contributes to health and health services delivery within a PHC model, both in a unique sense and through collaborative interdisciplinary practice.
The five core principles of the PHC approach include the following:
Equitable distribution
Community Participation (as issues that local people identify rather than predetermined services introduced by professionals, working within existing community organisations and local government structures, etc.)
Focus on Prevention
Appropriate Technology
Multisectoral Approach - Emphasis should be made that the reason for the failure of many programmes is due to the fact that they operate in isolation, separate from the general health care structure and without the support of other relevant sectors. The need for programme cooperation and collaboration cannot be over-emphasised.
The elements (or components) of PHC include (but not limited to) Immunisation, Maternal and Child Health (MCH) Care, Essential Drugs, Food and Nutrition, Education, Common Illnesses and injury, Water and Sanitation, Endemic Infectious Diseases, Mental health and Oral health.
All African countries have adopted PHC as the over-arching strategy to achieve health for their citizens. Strategies for the promotion of medicinal plants for the prevention of diseases in Africa must therefore take into cognisance the PHC approach. It must essentially follow the 5-key principles outlined above and be integrated into the elements. Medicinal plants will be useful for Maternal and Child health care, as essential drugs, in food and nutrition, for common illnesses and injury, for endemic infectious diseases, mental health and oral health.
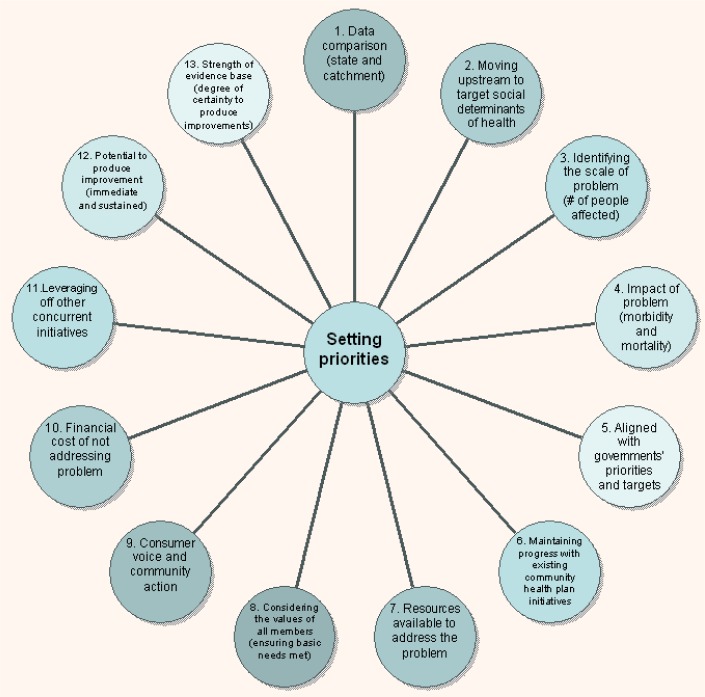
Medicinal plants also fit perfectly into the modelling for priorities in Primary Health Care as proposed by McDonald and Ollerenshaw (2011). (Figure 5)
Figure 5.
Model for Priority setting in Primary Health Care (McDonald & Ollerenshaw, 2011)
In summary, from the above considerations of available strategies, medicinal plants can play vital roles in disease prevention and their promotion and use fit into all existing prevention strategies. However, conscious efforts need to be made to properly identify, recognise and position medicinal plants in the design and implementation of these strategies. These approaches present interesting and emerging perspectives in the field of medicinal plants.
Ethnobotanical Studies on Medicinal Plants Used in Disease Prevention
In order that a comprehensive compilation of medicinal plants that can be used in disease prevention is obtained, collation of original data from the traditional custodians of such knowledge is essential (Tan et al., 2010). This is especially so in the case of African Traditional Medicine (ATM) where information is passed on from generation to generation orally about the plants used. Unlike in Chinese Traditional Medicine (CTM) and the Indian systems of medicine (Ayurveda, Unani and Sithda) where the information is available in books (and now online), a lot of the information on African traditional medicine is yet to be documented. Efforts are, however, being made by WHO-AFRO to augment the various isolated databases on medicinal plants through the provision of guidelines for documentation of herbal recipes (WHO/AFRO 2012). Specific ethnobotanic surveys at village level using some of the methods described by Sofowora (2008) can be used. Such a survey by Biswas et al. (2011) on medicinal plants used for preventive medicinal purposes in Muktipara village, Chuadanga District of Bangladesh yielded 11 authentic plants including Azadirachta indica and Moringa oleifera which are quite common in Africa.
A similar survey conducted by Rahmatulla et al. (2011) among the Chakma residents of Hatimara (south) village of Rangamati district, also in Bangladesh, indicated that the mode of consumption of the plants differed to some extent. Some plants, like Spilanthes calva or Commelina paludosa, the leaves were boiled, mixed with crushed peppers and taken. The authors found that the addition of peppers did not serve any therapeutic purposes. Rather, peppers, particularly hot peppers, were added to make the dish more palatable and to impart flavour to the dish (Rahmatullah et al., 2009; Sofowora 2008; Abel and Busia, 2005). The juice of young leaves of Centella asiatica or juice of leaves of Solena amplexicaulis was taken in the raw state. The fruits of Gymnopetalum cochinchinense were used for prevention of ulcer, and Solanum torvum as a preventive measure against leucorrhoea, typhoid and tonsillitis. The barks and seeds of Saraca were mashed and taken in the raw state as prevention for irregular menstruation and menorrhagia.
International ethnobotanical surveys sponsored by ACCT (Agence de Cooperation Culturelle et Technique) into 17 Francophone African countries and 5 into Anglophone African countries sponsored by the African Union (AU/STRC) and most of which were led by Professor Edouard Adjanohoun (Benin now France) and Professor Laurent Ake Assi have been published. These need to be searched for plants used in disease prevention as they were general ethnobotanic surveys which probably emphasised more enquiries on the use of plants to treat disease. All the data from such surveys especially the new ones need to be stored in a database where it is not yet done and protected for only authorised access.
Medicinal plants used to prevent cancer
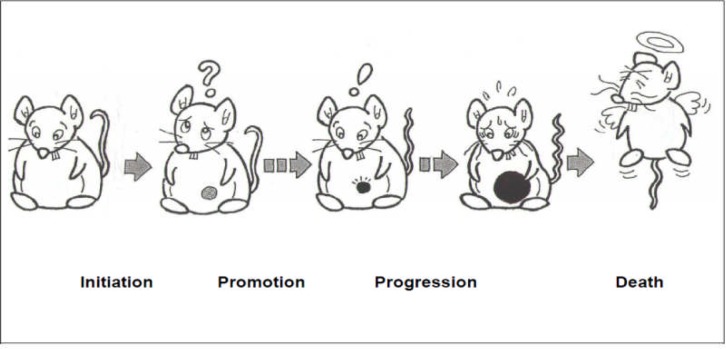
Yasukawa (2012) has reviewed the chemopreventive activity of natural sources, foods, supplements, crude drugs and Kampo medicines (traditional Japanese herbal prescriptions). In that review, he observed that cancer chemoprevention is currently one of the most urgent projects in public health. Cancer chemoprevention is defined as the use of specific natural and synthetic chemical agents to reverse or suppress carcinogenesis and prevent the development of invasive cancers. Recently, dietary non-nutrient compounds have demonstrated important effects as chemo-preventive agents, and considerable work on the cancer chemopreventive effects of such compounds in animal models has been undertaken. Epidemiological surveys have shown that the majority of human cancers are related to two factors, namely, diet and smoking (Banning, 2005; Hirayama, 1984). However, in the general population, daily consumption of certain foods has also been shown to have anticancer effects. This highlights the importance of environmental factors such as diet in cancer chemoprevention (Banning, 2005). An understanding of the mechanisms of carcinogenesis is essential for cancer chemoprevention. Most cancer prevention research is based on the concept of multistage carcinogenesis: initiation → promotion → progression (Pitot and Dragan (1991)]; Morse and Stoner, 1993). (Figure 6)
Figure 6.
Stages in Cancer Prevention Research
In contrast to both the initiation and progression stages, animal studies have indicated that the promotion stage occurs over a long time period and may be reversible, at least early on. For this reason, it is expected that inhibition of tumour promotion should be an efficient approach to cancer control (Murakami, et al., 1996). Yasukawa and his team have found in the search for potential anti-tumour promoters (cancer chemopreventive agents) from edible plants and fungi, and from crude drugs, that various triterpene alcohols and sterols and their oxygenated derivatives showed inhibitory effects on mouse ear inflammation induced by 12-Otetradecanoylphorbol-13-acetate (TPA). Primary prevention of cancer aims to avoid the development of cancer. Therefore, initiation and/or promotion of carcinogenesis should be inhibited. However, the adult population bears tumour cells that cannot revert to normal cells, and thus effective strategies to prevent cancer include avoiding continuous contact between these cells and promoters and/or aggressively inhibiting the tumour promoter effects. Therefore, to prevent cancer, it is essential to find plants that contain effective compounds (anti-tumour promoters) that delay, inhibit or block tumour promotion, which is a reversible and long-term process (Yasukawa, 2012). A few examples of such plants of interest are shown below:
(Pygeum) Prunus spp (Family Rosaceae) e.g. African Prune or African Plum Tree
Prostate cancer is a very good example for chemoprevention because prostate cancer is typically slow growing and is usually diagnosed in elderly males. The extract of the bark of Pygeum africanum (Prunus Africana) has been used in Europe as a prevention and treatment of prostate disorders including benign prostatic hypertrophy (BPH). In tissue culture, ethanolic extracts (30%) of the bark inhibited the growth of PC-3 and LNCaP cells; induced apoptosis and altered cell kinetics; down-regulated ERalpha and PKC-alpha protein, and demonstrated good binding ability to both mouse uterine oestrogen receptors and LNCaP human androgen receptors. TRAMP mice fed with P. africanum showed a significant reduction (P = 0.034) in prostate cancer incidence (35%) compared to casein fed mice (62.5%). P. africanum therefore has a significant role in regulation of prostate cancer both in vitro and in vivo and therefore may be a useful supplement for people at high risk for developing prostate cancer (Shenouda et al, 2007). Katz (2002) had observed that the consumption of isoflavones found in legumes and other plants is related to lower rates of BPH and prostate cancer among Asian men (Katz, 2002). The methanol extract of Prunus jamasakura Sieb. ex Koidz. inhibited two-stage carcinogenesis by DMBA/TPA in mouse skin (Yasukawa, et al., 1998). Octacosyl ferulate, from the active fraction of the plant, inhibited tumour promotion by DMBA/TPA in mouse skin (Yasukawa, et al., 1998) (Figure 7). The compound also inhibited the phosphorylation of histone by protein kinase C (PKC) in a concentration-dependent manner.
Figure 7.
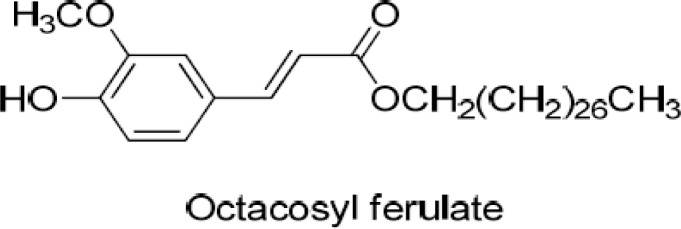
Octacosyl ferulate from Pruni Cortex
Azadirachta indica (Family Meliaceae) Neem
Over 60 different types of biochemicals including terpenoids and steroids have been purified from this plant. The anticancer properties of the plant have been studied largely in terms of its preventive, protective, tumour-suppressive, immunomodulatory and apoptotic effects against various types of cancer and their molecular mechanisms (Paul et al, 2011). Triple-negative breast cancer (TNBC) accounts for 15–20% of all breast tumours and these breast tumours are usually aggressive and highly metastatic. Unfortunately, treatment options for TNBCs are limited. A novel compound, 2′-3′-dehydrosalannol (DHS) isolated from A. indica uncrushed leaves, inhibited growth and induced apoptosis in TNBC cell lines. Molecular analysis suggested that DHS inhibited cathepsin-mediated pro-survival signalling [pAKT: phosphorylated protein kinase B; BCL-2: B-cell lymphoma 2 and cyclin D1] and induced pro-apoptotic markers such as BAX [BCL-2-associated X protein] and cleaved caspase-3 (Boopalan et al, 2012, Malathi et al, 2002). Also, Neem leaves were found to inhibit tumour promotion by DMBA/TPA in mouse skin (Arora et al., 2011). Inhibition of carcinogenesis in response to neem treatment was accompanied by an over expression of signal transducer and activator of transcription 1 (STAT1) and activator protein 1 (AP-1) and decrease in nuclear factor-kappa B (NF-κB) expression (Arora et al., 2011). In a recent study, Bharati et al, (2012) evaluated the anticarcinogenic potential of aqueous A. indica leaf extract against N-nitrosodiethylamine (NDEA)-induced hepatocarcinogenesis. They reported a significant reduction in tumour incidence (33%), tumour multiplicity (42%), and increase in survival (34%) upon administration of the aqueous extract to NDEA-abused mice. Transmission and scanning electron microscopic investigations showed severe alterations in organelle organisation, cellular arrangement, degree of differentiation, cellular metabolism, and morphology of the hepatocytes. They concluded that these changes appeared to be distinctly delayed upon supplementation with the leaf extract of the plant. The results suggest that A. indica may have anticancer potential against NDEA-induced hepatic cancer.
Rosmarinus officinalis L (Family Labiatae) Rosemary
Colorectal cancer is the second leading cause of cancer death in Australia. Ngo et al. (2011) reviewed scientific evidence from all studies published from 1996 to March 2010 and which examined the protective effects of rosemary on colorectal cancer and other types of cancer. They concluded that evidence from animal and cell culture studies demonstrates the anticancer potential of rosemary extract as well as only the following constituents of it: carnosol, carnosic acid, ursolic acid, and rosmarinic acid. The reported anticancer properties were found to arise through the molecular changes in the multiple-stage process of cancer development, which are dose related and not tissue or species specific. López-Jiménez (2013) demonstrated that the anti-angiogenic activity of carnosol and carnosic acid could contribute to the chemopreventive, antitumoral and antimetastatic activities of rosemary extracts and suggested their potential in the treatment of other angiogenesis-related malignancies. Ursolic acid and carnosol were isolated from the methanol extract of Rosemary, and inhibited DMBA/TPA-promoted two-stage carcinogenesis in mouse skin (Huang et al., 1994) (Figure 8).
Figure 8.
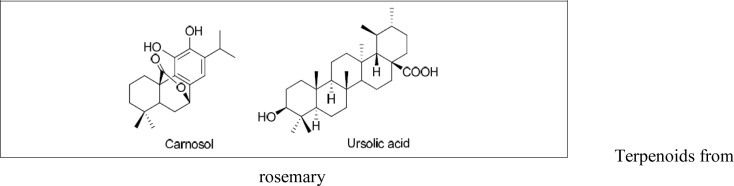
Terpenoids from rosemary
Vitis vinifera L. (Family Vitaceae) Grape
Dietary intake of foods rich in antioxidant properties is suggested to be cancer protective. Foods rich in antioxidant properties include grape (Vitis vinifera). Grape skin and seed extracts exert strong free radical scavenging and chelating activities and inhibit lipid oxidation in various food and cell models in vitro. The use of grape antioxidants are promising against a broad range of cancer cells by targeting epidermal growth factor receptor (EGFR) and its downstream pathways, inhibiting over-expression of COX-2 and prostaglandin E2 receptors, or modifying oestrogen receptor pathways, resulting in cell cycle arrest and apoptosis (Zhou and Raffoul, 2012).
Filip et al. (2011) in their studies on Photoprotective effects of two natural products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin reported that their results suggest that Calluna vulgaris and Vitis vinifera extracts might be chemopreventive candidates for reducing UV-induced risk for skin cancer.
Currants and Sultanas (Vitis vinifera L.) are dried vine products produced in Greece. Kaliora et al. (2008) investigated the gastric cancer preventive activity of methanol extracts obtained from currants from three different origins in Greece (Vostizza, Nemea, and Messinia), as well as methanol extracts obtained from Sultanas cultivated on the island of Crete as to inhibition of cell proliferation, induction of apoptosis, and inhibition of inflammation. All extracts from 500 micrograms of dried raisins studied suppressed cell proliferation, significantly those obtained from Sultanas from Crete and currants from Nemea. The French eat higher levels of animal fat, but their incidence of heart disease remains surprisingly low. This ‘French Paradox’ is thought to be due to the benefits they derive from consuming red wine. The ethanol extract of grapes inhibited tumour promotion by DMBA/TPA in mouse skin (Alam et al., 2002). Resveratrol, in a dose-dependent manner, reduced the incidence, total number and multiplicity of visible hepatocyte nodules (Bishayee and Dhir, 2009) (Figure 9).
Figure 9.
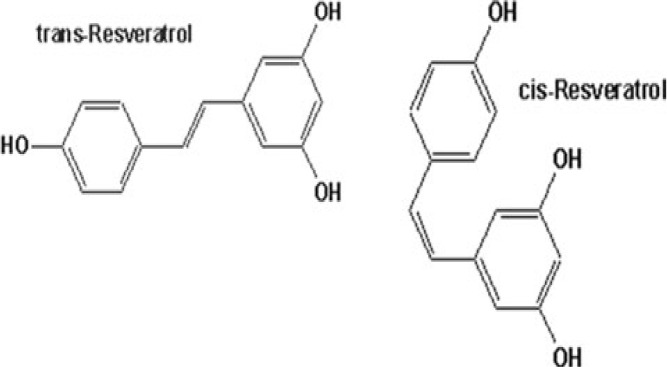
Resveratrol from grape
On the other hand, grape seeds are a rich source of monomeric, dimeric and oligomeric proanthocyanidins. The polyphenolic fraction of grape seeds suppressed tumour promotion by DMBA/TPA in mouse skin (Bomser, et al., 1999; Zhao, et al., 1999).
Glycine max or G. soya (Family Leguminosae) Soya milk
Genistein, the most abundant phytoestrogen in soybeans, may bind to oestrogen receptors and perform anticancer activities. Choriocarcinoma is a malignant, trophoblastic and aggressive cancer of the placenta. Liu et al (2011) investigated the effect of genistein on the invasive potential of the choriocarcinoma cell line JAR and its underlying mechanism and found that genistein inhibited JAR cell invasion in a dose-dependent manner by a matrigel invasion assay. Their findings have significant implications for the prevention and therapy of choriocarcinoma. However, Khan et al (2012) tested the hypothesis that Soy isoflavone consumption may protect against breast cancer development. They found a lack of efficacy for breast cancer prevention and a possible adverse effect in premenopausal women. Soy milk inhibited 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanoneinduced mammary carcinogenesis in rats (Ohta et al., 2000). Soy beans contain high amounts of isoflavonoids and saponins; isoflavonoids have been shown to have phytoestrogenic activity (Moliteni et al., 1995; Katz, 2002).
Zingiber officinale Roscoe (Family Zingiberaceae) Ginger
Dehydrozingerone a pungent constituent of ginger is a vanillyl ketone. Structurally, it is representative of half the chemical structure of curcumin which is a promising phytochemical for the inhibition of malignant tumours, including colon cancer. Yogosawa et al. (2012) evaluated the antiproliferative effects of dehydrozingerone against HT-29 human colon cancer cells, and it was found that it dose-dependently inhibited growth at the G2/M phase with up-regulation of p21. Dehydrozingerone additionally led to the accumulation of intracellular ROS, although most radical scavengers could not clearly repress the cell-cycle arrest at the G2/M phase. Their results suggest that analogues of dehydrozingerone may be potential chemotherapeutic agents for colon cancer (Škrovánková, 2012). Kurapati et al. (2012) investigated the combinatorial cytotoxic effects of Curcuma longa and Zingiber officinale on the PC-3M prostate cancer cell line. The two extracts separately showed significant inhibitory effects on colony-forming ability. However, when both the agents were tested together at the same concentrations, the combined effects were much more significant than their individual ones, suggesting the role of multiple components and their synergistic mode of actions to elicit stronger beneficial effects.
Hexahydrocurcumin, extracted from Zingiber officinale, was shown to be cytotoxic to colorectal cancer cells by Chen et al. (2011). Treatment of SW480 cells with hexahydrocurcumin (100 microM) resulted in a massive accumulation of the cells in the G1/G0 phase of the cell cycle. This compound could prove useful in cancer prevention.
The protective and/or preventive activity of various spices against various cancers and gastric ulcer has been reviewed by Sumbul et al. (2011) and by Sung et al. (2012).
Topical application of an ethanol extract of ginger inhibited TPA-induced tumour promotion during two-stage carcinogenesis in mouse skin (Katiyar et al., 1996). Pre-application of an ethanol extract of ginger onto the skin of SENCAR mice resulted in significant inhibition of TPA-induced epidermal ODC, COX and lipoxygenase activities as well as ODC mRNA expression in a dose-dependent manner. Topical application of [6]-gingerol inhibited tumour promotion by DMBA/TPA in mouse skin, and also suppressed TPA-induced epidermal ODC activity and inflammation (Park et al., 1998) (Figure 10).
Figure 10.
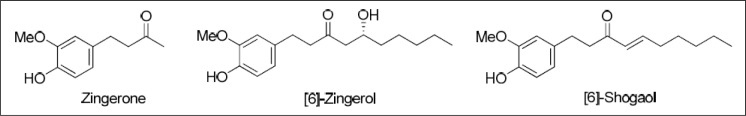
Some phenolics from Zingiber officinale
Food Supplements
Allium cepa and A. sativum (Family Liliaceae) Onion and Garlic
Antony and Singh (2011) have reviewed the mechanisms and targets of cancer chemoprevention by diallyl trisulfide (DATS) extracted from Allium species, while Zhou et al (2011) in a meta-analysis, in which they pooled analysis of all studies, concluded that the consumption of large amounts of Allium vegetables (in a comparison of the highest and lowest consumption groups) reduced the risk for gastric cancer (odds ratio, 0.54; 95% confidence interval, 0.43–0.65). Allium sativum (garlic) and its chemical constituents, especially Allicin, Dially Disulfide, Diallyl Trisulfide, have also been shown in recent studies to be chemopreventive agents for lung cancer and breast cancer (Chu et al., 2012; Nkrumah-Elie et al., 2012; Li et al., 2012). Allicin was also reported by Wang et al. (2012) to induce apoptosis in EL-4 cells in a time-and concentration-dependent manner, in which the mitochondrial pathway might play a central role.
Onion oil inhibited tumour promotion by DMBA/TPA (Belman, 1983; Perchellt et al., 1990) and by BP/croton oil (Sadhana et al., 1988) in mouse skin, while garlic oil inhibited tumour promotion by DMBA/TPA in mouse skin (Belman, 1983; Perchellt et al., 1990).
Panax ginseng C.A. Mayer (Family Araliaceae). Ginseng
Li et al. (2012) characterised a homogeneous polysaccharide (PGPW1) from the root of Panax ginseng with molecular weight as 3.5×10(5) Da. PGPW1 contained Glcucose, Galactose, Mannose and Arabinose in the molar ratio of 3.3:1.2:0.5:1.1. It dose-dependently displayed potent anti-proliferation and anti-metastatic activities. They also found that the attenuated expression of M3 muscarinic receptor on the surface of T24 cells by PGPW1 would contribute to its antitumour functions. All the data indicated the potential of its clinical application for the prevention and treatment of bladder cancer metastasis. Dong et al. (2011) have evaluated the cytotoxic potency of ginsenosides and their synthetic derivatives against a variety of cancer cells with a view to determining the structure activity relationship. The results clearly indicated that the compound with less polar chemical structures possesses higher cytotoxic activity towards cancer cells. In their own investigation on ginsenoside Rp1, Kang et al. (2011) found that it inhibited breast cancer cell proliferation and inhibited both anchorage-dependent and -independent breast cancer cell colony formation. In addition, Rp1 induced cycle arrest and apoptosis-mediated cell growth suppression. Rp1 also decreased the stability of the IGF-1R protein in breast cancer cells. They therefore suggested that Rp1 has potential as an anticancer drug and that IGF-1R is an important target for treatment and prevention of breast cancer. In their own studies, Cui et al. (2010) found that when American ginseng was tested using the azoxymethane (AOM)/dextran sulphate sodium (DSS) mouse model of ulcerative colitis, they demonstrated that ginseng can suppress colon cancer associated with colitis.
Oral administration of white and red ginseng (Panax ginseng C.A. Mayer) suppressed colon carcinogenesis by 1,2-dimethylhydrazine (DMH) in rat (Fukushima, et al., 2001). The ginsenosides Rg3, Rg5 and Rh2 are active components in ginseng, and act either singularly or synergistically in cancer prevention (Yun, et al., 2001). The methanol extract of san-chi ginseng (P. notoginseng (Burk.) F.H. Chen) suppressed skin carcinogenesis by DMBA/TPA, liver carcinogenesis by DEN/Phenobarbital, lung carcinogenesis by 4NQO/glycerol in mice (Konoshima et al., 1996). Moreover, the methanol extract of san-chi inhibited skin carcinogenesis by NOR-1/TPA, as well as DMBA/fumonisin B1 in mice (Konoshima et al., 1999). The ginsenoside Rg1 slightly suppressed tumour promotion by DMBA/TPA in mouse skin (Konoshima et al., 1996) (Figure 11).
Figure 11.

Ginsenosides from Ginseng
Prevention and treatment of male osteoporosis due to androgen deficiency by using the medicinal plant: Eurycoma longifolia
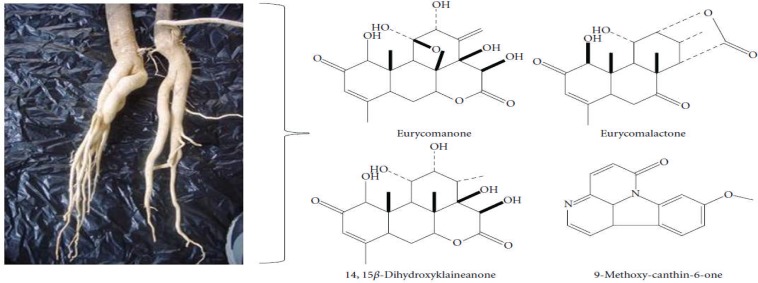
Osteoporosis in elderly men is now becoming an alarming health issue due to its relation with a higher mortality rate compared to osteoporosis in women. Androgen deficiency (hypogonadism) is one of the major factors of male osteoporosis and it can be treated with testosterone replacement therapy (TRT). However, one medicinal plant, Eurycoma longifolia Jack (EL), can be used as an alternative treatment to prevent and treat male osteoporosis without causing the side effects associated with TRT. EL exerts proandrogenic effects that enhance testosterone level, as well as stimulate osteoblast proliferation and osteoclast apoptosis. This will maintain bone remodelling activity and reduce bone loss. Phytochemical components of EL may also prevent osteoporosis via its antioxidative property (Figure 12). Hence, EL has the potential as a complementary treatment for male osteoporosis (Effendy et al., 2012).
Figure 12.
Some of the chemical constituents isolated from the root of Eurycoma longifolia Jack (Source: Effendy et al., 2012)
Plants used for the prevention of Coronary Heart Disease (CHD)
Coronary Heart Disease (CHD) is the primary contributor to morbidity and mortality worldwide. A great deal of research is now focused on identifying new therapeutic alternatives to prevent and treat CHD. The most consistent recommendations from a Public Health perspective involve multiple changes in diet and exercise. Medicinal plants are also a viable option for its prevention and treatment. Clinical and preclinical data on some medicinal plants used as dietary supplements show that they may be useful in the strategies to reduce the prevalence and mortality of CHD either in the general population or in the subsets of individuals at high risk. Such plants include, for example, artichoke, garlic, gingko, guggul, hawthorn and tea.
Artichoke Cynara scolymus L. (Family Asteraceae)
Dried leaves and lower part of the flower of this plant contain 6% phenolic acids, 5% sequiterpene latones. Three sesquiterpenes, cynaropicrin, aguerine B, and grosheimin were isolated as the active components of the artichoke extract which reduced serum triglyceride levels in olive oil-loaded mice. Aqueous and ethanolic extracts of artichoke also reduced intracellular oxidative stress stimulated by inflammatory mediators such as tumour necrosis factor alpha (TNFa) and lipopolysacchaide (LPS), as well as ox-low density lipoprotein (ox-LDL) in endothelial cells and monocytes.
Garlic (Alium sativum, (Family Liliaceae)
Over 35 randomised clinical trials on garlic were carried out to investigate the effect of garlic on cardiovascular end points. Overall, there is evidence from randomised controlled trials (RCT) in adults that the use of garlic preparations can lead to a small but statistically significant reduction in total cholesterol levels compared with controls.
Guggul Commiphora mukul.(Jacq) Engler, (Family Burseraceae)
The Mukul Myrrh tree has been used as far back as 600 BC. According to the recent WHO Monograph for guggul, the plant is useful for the treatment of hyperlipideamia and hypercholesterolemia. The plant sterols, E- and Z-guggulesterone, are believed to be the bioactive compounds.
Strategies for combating micronutrient deficiencies: Food-based approaches
Multiple micronutrient deficiencies abound in developing countries (Ramakrishnan, 2002). They are caused by inadequate intakes but genetic, parasitic and infectious diseases may also play a role (Fishman, et al., 2000; Stoltzfus, 2001). Micro nutrient deficiencies can have major adverse health consequences, contributing to impairment in growth, immune competence, mental and physical development, and poor reproductive outcomes (Viteri and Gonzales, 2002) that cannot always be reversed by nutrition interventions. Inadequate intakes of certain micronutrients such as iodine, selenium and zinc can also be exacerbated by environmental factors, as their content in plant based foods is dependent on soil trace elements (Gibson and Hotz, 2001).
There is therefore a strong need for strategies to reduce micronutrient deficiencies in developing countries. Strategies commonly used are supplementation and food-based approaches, preferably in conjunction with public health interventions such as promotion and support of breast feeding and control of infectious and parasitic diseases. Fruits and vegetables are a good source of vitamins and minerals. Faber and Laurie (2011) described a home garden strategy that integrates gardening activities with nutrition education, using community based growth monitoring as an entry point in South Africa. A positive effect was observed that Provitamin A rich vegetables and fruits contributed significantly towards achieving the recommended dietary intake of Vitamin A and other micronutrients. They concluded that home gardening is a long term strategy that contributes to combating Vitamin A and other nutritional deficiencies. This is only one of the several individual investigations reported by Thompson and Amoroso (2011) to pinpoint the fact that food based strategies are viable, sustainable and long term solutions to overcoming micronutrient malnutrition.
The strategy of using antioxidant activity of medicinal plants in prevention of diseases
Oxidative stress, caused by reactive oxygen species, plays an important role in many chronic and degenerative diseases, such as atherosclerosis, ischemic heart disease, cancer, diabetes mellitus, neurodegenerative diseases and ageing (Azizova, 2002). The body's non-enzymatic antioxidant defence system includes some antioxidants, such as vitamin C, vitamin E, vitamin K and glutathione. Some synthetic antioxidants, widely used in food industry to protect food from oxidation and spoiling, are harmful because of their potential toxicity and carcinogenicity (Botterweck et al., 2000). However, natural antioxidants in fruits and vegetables are inversely related with the risk of the chronic diseases mentioned above (Leifert and Abeywardena, 2008). Natural antioxidants, therefore, provide alternative strategy to prevention as well as treatment of these diseases. Phenolic compounds because of their oxidative activity are potential agents for preventing and treating many oxidative stress-related diseases. The antioxidant activity of polyphenols is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers, metal chelators and reductants of ferryl hemoglobin (Kratchanova et al., 2010). Some medicinal plants possess more potent antioxidant activity than common dietary plants (Cai et al., 2004). Therefore, their extract, if not toxic, can serve as food additive and can be used for disease prevention (Liu, 2003; Liu et al., 2008).
Oral Diseases prevention with medicinal plants
Oral diseases are major health problems with dental caries and periodontal diseases among the most prevalent, preventable global infectious diseases. Oral health influences the general quality of life and poor oral health is linked to chronic conditions and systemic diseases. The association between oral diseases and the oral microbiota is well established. Although there are more than 750 species of bacteria that inhabit the oral cavity, most are normal commensals and only a few are implicated in oral diseases. The development of dental caries involves acidogenic and aciduric Gram-positive bacteria (mutans streptococci, lactobacilli and actinomycetes). Periodontal diseases have been linked to anaerobic Gram-negative bacteria (Porphyromonas gingivalis, Actinobacillus, Prevotella and Fusobacterium). Given the incidence of oral disease, increased resistance by bacteria to antibiotics, adverse effects of some antibacterial agents currently used in dentistry and financial considerations in developing countries, there is a need for alternative prevention and treatment options that are safe, effective and economical. While several agents are commercially available, these chemicals can alter oral microbiota and have undesirable side-effects, including tooth staining. Hence, the search for alternative products continues and natural phytochemicals isolated from plants used as traditional medicines are considered as good alternatives. Palombo (2011) concluded that there is considerable evidence that plant extracts, essential oils and purified phytochemicals have the potential to be developed into agents that can be used as preventive or treatment therapies for oral diseases. While it is encouraging to see a number of clinical trials of such products, further studies of the safety and efficacy of these agents will be important to establish whether they offer therapeutic benefits, either alone or in combination with conventional therapies, that can help to reduce the overall burden of oral diseases worldwide. In particular, studies that address issues such as adequate statistical power, blinding, standardisation of extracts or purified compounds, and quality control would be of great value (Palombo, 2011)
Some miscellaneous practices for the prevention of diseases with medicinal plants in Africa
According to Koumare (2008) repeated sore throat especially in children is often prevented in Mali and other African countries by using gargles made with the following plants: Spilanthes sp, Guiera senegalensis and Waltheria indica. Traore (1975) had also compiled some cultural practices of the Malian people in the prevention of dental problems with medicinal plants (Troure, 1975). The efficacy of chewing sticks used for preventive dental care had been reported by Sofowora and others (Sofowora, 2008). It is common knowledge among women in Africa, especially in Burkina Faso, to rub the skin of new borne babies with various medicinal plants (e.g. Pterocarpus osun) soaked in oil to prevent them from bacterial infection as they are carried by various people. Also, because the mothers do not know whether the visitors carrying the newborn have evil intentions, the preparation with which the baby is rubbed often contains plants with occult powers to ward off any spiritual attack on the baby by visitors. Various antenatal practices using medicinal plants exist in Africa. The pregnant woman is made to bathe in some decoctions of herbs and required to drink a little of it periodically throughout the period of pregnancy to sustain her. There are also herbs to be taken just before delivery to hasten or to ease delivery. However, the use of herbs in late pregnancy could be for their oxytocic properties. Some preparations for preventing road accidents exist in Africa just as there are similar preparations utilising the occult power of herbs to eject a person from an accident vehicle before the accident (Egbe in Yoruba). These latter preventive uses of medicinal plants involving the occult power of herbs may be beyond present capabilities of scientific experimentation to prove their efficacy.
Conclusions
Efforts must be geared towards measures that will enhance the effectiveness, efficacy and rational use of medicinal plants, especially through the integration into national, regional and local health policies and programmes. Most African countries, for example, hinge their health care system on the Primary Health Care (PHC) strategy and it is necessary to incorporate the use of medicinal plants into all the components of PHC in these countries. The following are also recommended: a) Collation of data from books, research articles, conducting of ethnobotanical surveys (because Africans have only recently started documentation of medicinal plants and their uses; oral tradition had been the mainstay) specifically to look for plants used in preventing diseases in our communities as was done in Muktipara village of Sri Lanka. Database searches on medicinal plants will also yield useful results (Table 1); b) collaborative research with Institutes for preventive medicine as well as departments of preventive dentistry in teaching hospitals; and c) coordination of the research to avoid duplication of efforts.
Table 1.
plants with potentials in preventive medicine practice
| S/No | Plant | Family | Common Names |
Local Names | Parts Used | Chemical Constituents |
Action | References |
| 1 |
Hygrophila auriculata |
Acanthaceae | Indian paint brush |
Hausa: zazargiwa, kayarukumi |
Whole plant | Steroids, Alkaloids | Antibiotic | Odugbemi (2006) |
| 2 |
Peristrophe bicalyculata |
Acanthaceae | Tabanin dawaki, hargowandoki |
Whole plant | Alkaloids, Essential oils, Fatty acids |
Antimicrobial, it prevent Tuberculosis |
Okujagu (2008) | |
| 3 |
Amaranthus spinosus L. |
Amaranthaceae | Thorny pig weed |
Hausa :zaaki banza mai kaya,Igbo:inine ogwu,yoruba:tete kekere |
Whole plant | Tannins,saponins,amino acids,methyl derivative,cholesterol, oleanolic acid,stigmasterol,camp esterol |
Antipyretic | Okujagu (2008) |
| 4 |
Celosia isertii C.C. Townsend |
Amaranthaceae | Hausa: Nannaho Igbo: Eli-emi Onu Yoruba: Ajifowo |
Whole plant | Amaranthin celosianin Betanin |
Antihelmintic | Okujagu (2008) | |
| 5 | Mangifera indica | Anacardiaceae | Mango | Hausa: mangwaro Igbo: mangoro |
Stem bark, leaves, fruit |
Mangiferine | Anti-emetic, Anti-inflammatory | Okujagu (2008) |
| 6 |
Spondias mombin L. |
Anacardiaceae | Akika (Yoruba), Ijikere (Igbo) |
Leaves | Tannins, phenolic esters, Alkaloids, saponin |
Antiviral Antibacterial |
Okwu and Okwu (2004) | |
| 7 |
Xylopia aethiopica (Dunal) A. Rich. |
Annonaceae | Ethiopian pepper seed |
Sesedo (Yoruba), Uda (Igbo) |
Fruits | Volatile oil | Antimicrobial, | |
| 8 |
Catharanthus roseus L. G. Don |
Apocynaceae | Rose periwinkle |
Apabida (Yoruba) | Leaves | Vincrstine, vinblastine | Anti-leukaemic, anti-cancer, antihypertensive |
Elujoba et al, (2005) |
| 9 |
Rauwolfia vomitoriaAfz Or R. serpentine (L.) Benth ex. Kurz |
Apocynaceae | Serpent wood | Asofeyeje (Yoruba), Akata (Igbo) |
Leaves, Seeds |
Reserpine, deserpidine, rescinamine, yohimbine |
Antihypertensive |
Kokwaro, (1993); Sofowora (2008) |
| 10 |
Crytolopsis sanguinolenta (lindl( |
Apocynaceae | Igbo: oba Yoruba: igisoba |
Fruit | hydrocyanic acids | Anti-tumor, anti-inflammatory | ||
| 11 |
Aristolochia albida Duchartre |
Aristolochiaceae | Dutchman pipe |
Hausa :duman dutse,Igbo:isi okuko,Yoruba:para n funfun |
root | Aristolochic acids,aristolachine,aris tinic acids,aristidinic acids |
Anthelmintic,anti-inflammatory | Okujagu (2008) |
| 12 |
Ageratum conyzoides L. |
Asteraceae | Goat weed | Imiesu (Yoruba) | Whole plant | Phenols, Alkaloids | Antimicrobial, anti-inflammatory, anti-allergic properties. |
Kokwaro (1993) |
| 13 |
Kigelia Africana (Lam.) Benth |
Bignoniaceae | Sausage tree | Pandoro (Yoruba) Ishi (Igbo), Rahaina, nonon giwa (Hausa) |
Bark, Seed. | Naphthoquinones, Iridoids, |
Anti-malaria | |
| 14 |
Adansonia digitata L. |
Bombacaceae | Baobab, Monkey bread |
Kuka (Hausa), Osa (Yoruba) Osi (Edo), Oyili-akpu (Igbo), Kuwa (kanuri) |
Root, bark, seed, leaves, flower. |
Hydroquinone, proanthocyanidins |
Anti-inflammation | |
| 15 |
Afzelia africana Smith |
Caesalpiniaceae | Hausa: kawo Igbo: Akpalata Yoruba: Api Edo: Ariyan Efik: Enyin mbukpo |
Bark | Alkaloids, Tanins | Antihelmintic | Okujagu (2008) | |
| 16 | Cassia alataL. | Caesalpinaceae | Cassia | Asunran (Yoruba) | Leaves | Alkaloids, Saponins, Tannins, anthracionones and carbohydrates. |
Antiseptic, laxative and it also inhibits fungal growth |
Gill (1992); Sule et al (2010) |
| 17 | Cassia tora | Caesalpinaceae | Hausa: Talasa Yoruba Aborere |
Whole plant | Tannis, Alkaloids, Anthraquinones, Glycosides, Oil, Hydrocyanic acid, fatty acid |
Antimicrobial Activity | Sofowora (2008) | |
| 18 |
Daniella oliveri (Rolfe) |
Caesalpiniaceae | Balsam tree | Hausa: maje Igbo: Agba Yoruba: Ojia, iya |
Gum of bark | Alkaloid, Essential oil, tannin and oleoresin |
Anti-parasite | Onwukaeme (1993) |
| 19 | Cleome viscose L. | Capparidaceae | Spider plant | Hausa :tafarnuwan,Igbo;k eerem kerem,Yoruba a:ekuya |
Seeds, root | Coumarinolignans, cleomiscosin, marcrocyclic diterpenes |
Antiscorbutic,anthelmintic | Okujagu (2008) |
| 20 | Carica papaya L. | Caricaseae | Paw paw | Ibepe (Yoruba) | Fruits, latex, leaves |
Alkaloids, Flavonoids, Triterpenes |
anthelmintic, antifungal |
Gill (1992). |
| 21 |
Bryophyllum pinnatum Lam Oken |
Crassulaceae | Ressurection plant, Life plant |
Eru-odundun, Abomoda (Yoruba), Odaa opue (Igbo) |
Leaves, Roots, Leaf sap |
alkaloids, flavonoids and essential oils |
Antifungal, Antimicrobial, Anticancer. antihypertensive activity, prevent and treat Leishmaniasis, protect against chemically induced anaphylactic reactions |
Ghasi et al (2011) |
| 22 |
Mormodica charantia |
Curcubitaceae | Bitter lemon | Hausa Garapunu | Fruit | Steroids | Antidiabetic, Antioxidant | Abe (1974) |
| 23. |
Telferia occidentalis |
Curcubitaceae | Fluted pumpkin | Igbo: ugu | Seeds | Steriods, tannic acid | Prevention of adnopause, prevention of anaemia |
Tan (2002) |
| 24 |
Acalypha godseffiana pax |
Euphorbiaceae | Golden acalypha |
Yoruba: Jinwinni | Leaf Poultice |
antibiotic, bacteristatic, fungistatic |
Antibacterial | Odugbemi (2006) |
| 25 |
Acalypha wilkesiana Müll.-Arg. |
Euphorbiaceae | Copper leaf, beef steak plant |
Yoruba: Jinwinni, (Ijebu) aworoso |
Leaf | antibiotic, bacteristatic, fungistatic |
antimicrobials | Odugbemi (2006) |
| 26 |
Acalypha wilkesiana |
Euphobiaceae | Acalypha | Hausa: Jinwinni, Igbo: Jiwene, Yoruba aworoso |
leaf | Ethanol | Antmicrobial | Odugbemi (2006) |
| 27 |
Alchornea cordifolia_(Schum. & Thonn.) |
Euphorbiaceae | Christmas bush, Oliver |
Yoruba: èpa, esin, Hausa: bambami, Igbo: ubebe, |
Leaf | Alkaloids glycosides, saponins, steroids tannins, astringents |
Antiaborifacients | Burkill (1985): |
| 28 | Euphorbia hirta l | Euphobiaceae | Hausa: udani Igbo: |
Whole plant | Alkaloid, glycoside, tarderpenoids, sterols |
Antibacterial, anti-diarrhoea, Anti-asthmatic, | Onwukaeme (1993) | |
| 29 | Jatrophacurcas L. | Euphorbiaceae | Red fig nut flower |
Lapalapa (Yoruba) | Leaves | Volatile oil, Curcin, Saponins, Tannins |
Antimicrobial, antiseptic | Thomas (1989) |
| 30 |
Mallotus oppositifolius (Geisel) mull. Arg. |
Euphorbiaceae | Ukpo (Igbo), Kafar mutuwaa (Hausa), Eja (Yoruba) |
Leaves | Alkaliods, phenols, anthocyanins, butacyanin, flavonoids, steroids and tannins |
Anti-malarial and inhibits fungal growth. |
Farombi et al., 2001 | |
| 31 | Phyllanthus amarus | Euphorbiaceae | Stone breaker | Ite kwonwa nazu (Igbo), Geeron tsutsaayee (Hausa), Eyin olubi (Yoruba), Ebebenizo (Benin). |
Whole plant | Insulin, Saponin, Tannins. |
||
| 32 |
Ocimum gratissimum L |
Lamiaceae | Clove basil, Sweet basil, tea bush, Scent leaf or fever plant |
Efinrin (Yoruba), Nchu-anwu, Ahuji (Igbo), Daidoya (Hausa) |
Leaves, Essential oils |
Curcumin, flavonoids, isoflavone, flavones, anthocyanin, catechin and isocatechin; Alkaloids, Saponins, Cardiac glycosides and Anthraquinones |
It prevents breast cancer and fat accumulation. It inhibits growth of disease causative microorganisms (antimicrobial). It has antioxidant activity and also preventing the formation of oxidized Low-density Lipoprotein (LDL), which is considered to induce cardiovascular disease |
Obho (2006)
Amic et al., (2003); Nwinyi et. al. (2009); |
| 33 | Allium cepa Linn | Liliaceae | Onions | Alubosa (Yoruba) | Bulbs | Alicin, quercetin, allyl propyl disulfide |
Prevents irregular heart beat and abnormal blood pressure. It also prevents cancers, coronary heart disease, arteriosclerosis (hardening of the arteries), bronchitis, dry or stubborn cough and blood clots. |
Ozougwu and Eyo (2011) |
| 34 | Allium sativum L. | Liliaceae | Garlic | Aayu (Yoruba) | Bulbs | peptides, steroids, terpenoids, flavonoids, and phenols |
Anti-bacteria and also prevents high blood pressure |
Gill, 1992 |
| 35 |
Erythrophleum suaveolens Guill. & Perr. |
Leguminosae | sassy, sasswood, red water tree and ordeal tree |
Obo Erunobo, olu-obo, Ajeku, Obo (Yoruba), Gwaska (Hausa), inyi,akpa. (Igbo) |
Stem bark | Saponins | Anti-oxidants, antiviral, Antihelminthic, antimicrobial, anticancer |
Lawal et al, 2010; Akinpelu et al, 2012 |
| 36 |
Abelmoschus esculentus_(Linn.) |
Malvaceae | Okro, lady's fingers. |
Yoruba: ila | Fruit | alkaloids. uricant. aromatic substances |
antibiotic, bacteristatic, fungistatic | Odugbemi (2006) |
| 37 |
Hibiscus sabdariffa L. |
Malvaceae | Red Sorrel | Yakuwa (Hausa), Karasu (Kanuri), Tabwa (babur) |
Whole plant | Protocatechuic acid | Anti-malaria | |
| 38 | Sida acuta Burm. F | Malvaceae | Horn bean leaf |
Esoketu, Iseketu (Yoruba) |
Leaves, Roots |
Alkaloids, Phenols, Fatty acids, Coumarins, Sterols, Triterpenoids |
Antipyretic and has inhibitory activity against disease causing bacteria |
Iroha et al 2009 |
| 39 |
Azadirachtaindica (A.Juss.) |
Meliaceae | Neem | Dongoyaro | leaves | Quinine | Anti-malaria. | Lawal et al, 2010 |
| 40 |
Azadirachta indica A.Juss. |
Meliaceae | Neem | Dongoyaro | Leaves | Quinine | Anti malaria, insecticide | Lawal et al, 2010 |
| 41 |
Acacia polyacantha Wild. |
Mimosaceae | Edi (Yoruba) | Bark | Alkaloid, Tannins, Saponins, Resins |
Anti-malaria | ||
| 42 |
Pentacletha macrophylla Benth |
Mimosoideae | Oil bean tree | Igbo: ugba, ukpaka, akpaka Ijaw: ukpakara Yoruba Apara |
Leaves | Saponin, tannins, paucine |
Anticarcinogenic | Onwukaeme (1993) |
| 43 |
Artocarpus heterophyllus Lam |
Moraceae | Jack fruit | Fruits | dihydromorin, steppogenin, norartocarpetin, artocarpanone, and artocarpesin |
Anti-ulcer | Lawal et al, 2010 | |
| 44 |
Moringa oleifera Lam. |
Moringaceae | Drumstick tree |
Moringa | Leaves, Seeds |
Saponins, Riboflavin, Sterols |
Antioxidants, antimicrobial | Odugbemi (2006) |
| 45 |
Passiflora foetida L |
Passifloraceae | Stinking passion flower |
Nine-nine | Roots, Leaves, Fruits |
chrysoeriol, apigenin, luteolin, kaempferol, isoschaftoside, 2′-xylosylvitexin, isovitexin and vitexin |
Antispasmodic, antibacterial, antihypertension, antiproliferative activity on human breast adenocarcinoma |
Moongkarndi et al., 2004 |
| 46 |
Cymbopogon citrates |
Poaceae | Lemon grass | Kooko oba (Yoruba) Isauri (Hausa), Achara ehi (Igbo Ikon (Efik). |
Leaves | Essential oils (e.g Citral), Limonine, Camphene, citronella, Geraniol, Alkaloids. |
Anti-malaria. | Odugbemi (2006) |
| 47 |
Piper guineense Schum |
Piperaceae | African black pepper |
Iyere, Ata-iyere (Yoruba) |
Fruits, Leaves, Seeds |
alkaloidal amides (Piperanine, dihydrowasanine, isobutyl-(E,E)-2, 4-decadienamide) |
Antipyretic, Antiemetic, antiparasitic, antimicrobial and antifungal activities |
Ngane et al., (2003); Ekanem et al., (2004) |
| 48 | Piper nigrum L. | Piperaceae | Black pepper | Uziza (Igbo), Iyere (Yoruba), Masoro (Hausa) |
Fruits | eugenol, kaempferol, myrcene, piperine, quercetin, chavicin and rutin black pepper. |
It inhibits pro-inflammatory cytokines that are produced by tumour cells (anti-tumourigenic). It is also immunostimmulatory, stomachic, carminative, anticholestrolemic and anti-oxidant. It also inhibits the growth of malaria parasite. |
Shaba et al. (2012) |
| 49 |
Peperomia Pellucida L. |
Piperaceae | pepper elder | Bark | alkaloids, flavonoids, tannin, saponins and Tepernoid |
Prevents gout, anti-arthritic, Anti-cancer, Antioxidant, antimicrobial, antfungal |
Odugbemi (2006) | |
| 50 | Citrus aurantifolia | Rutaceae | Orange (Lime) |
Olome (Igbo), Osan-wewe (Yoruba) Lemun tsami (Hausa), Lemun tsamtsam (Kanuri) |
Fruit, Leaves | Ascorbic acid, asparagine, acetic acid, essential oils. |
Anti-malaria | Odugbemi (2006) |
| 51 | Capsicum annum | Solanaceae | Ball pepper | Hausa: kananan barkomo Yoruba: Ata wewe Igbo: ose ogologo |
Fruits, and leaves |
Capsaicin, capsaicinoids |
Antimicrobial, prevention of heart attack and stroke |
Odugbemi (2006) |
| 52 |
Cola millenii K. Schum |
Sterculiaceae | monkey cola | Obi-edun (Yoruba) | Fruits | phytosterols, polyphenols (tannins, flavonoids), saponins, alkaloids, saponin glycosides, steroids and triterpenoids, glycosides, hydrolysable tannins, phenols and volatile oils |
Antiviral, reduces the low density lipoprotein, i.e. the cholesterol involved in depositing fat in the Arteries prevent blood clotting which can reduce risk of heart attack or stroke. |
Lawal et al, 2010 |
| 53 | Cucurma longa L. | Zingiberaceae | Tumeric | Tumeric | Roots | Curcumin, Tumeric oils, Terpenoids |
It prevents cancerous growth. It also aids digestion of fats. It helpful in preventing the blockage of arteries that can gradually cause a heart attack or stroke |
Odugbemi (2006) |
| 54 |
Zingiber officinale Roscoe |
Zingiberaceae | Ginger | Ginger | Rhizome | Gingerols, shogaols, zingerone and paradol |
It prevents chilblains and circulatory problems such as Raynaud's disease. It is highly antiseptic, activating immunity and dispelling a whole variety of bacterial and viral infections. It also inhibits blood clotting. |
Ozougwu and Eyo (2011) |
Figure 4.
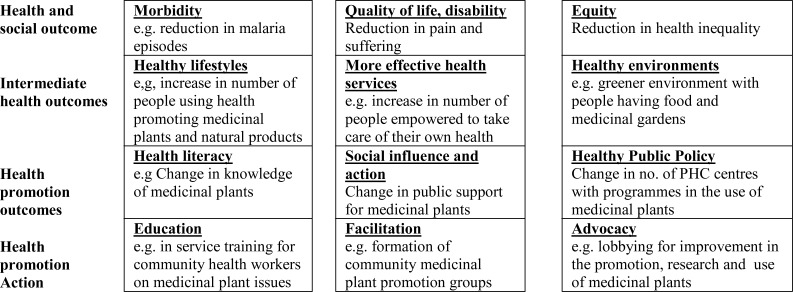
A Health Promotion Model for Medicinal Plants (Adapted from Nutbeam, 1998)
Acknowledgements
We would like to thank the organisers of the third CONGRESS INTERNATIONAL DE PHYTOTHERAPIE DE OUAGADOUGOU (CIPO) for inviting Professor Sofowora as a plenary lecturer to present this paper in Ouagadougou on 9th October, 2012. The assistance of the Nigeria Natural Medicine Development Agency (NNMDA) in the generation of the table of plants used in disease prevention through their virtual library as well as the provision of some original research articles by Professor (Mrs.)' Lanre Omobuwajo is hereby acknowledged.
References
- 1.Abe H. Catalase. In method of enzymatic analysis. New York: Academic Press; 1974. pp. 673–684. [Google Scholar]
- 2.Akinpelu et al. deleteandlistallnames, author. Effect of stem - bark of Erythrophleum suaveolens (Guill. & Perri.) saponin on fresh water snail (Lanistes lybicus) tissues. African Journal of Environmental Science and Technology. 2012;6(11):446–451. [Google Scholar]
- 3.Alam A, Khan N, Sharma S, Saleem M, Sultana S. Chemopreventive effect of Vitis vinifera extract on 12-O-tetradecanoyl-13-phorbol acetate-induced cutaneous oxidative stress and tumor promotion in murine skin. Pharmacological Research. 2002;46(6):557–564. doi: 10.1016/s1043661802002268. [DOI] [PubMed] [Google Scholar]
- 4.Alma Ata. International Conference on Primary Health Care. 11. Vol. 32. WHO Chron; 1978. Nov, Declaration of Alma-Ata; pp. 428–430. [PubMed] [Google Scholar]
- 5.Amic D, Davidovic-Amic D, Beslo D, Trinajstic N. Structure-Radical scavenging activity relationship of flavonoids. Croatia Chem Acta. 2003;76:75–61. [Google Scholar]
- 6.Antony ML, Singh SV. Molecular mechanisms and targets of cancer chemoprevention by garlic-derived bioactive compound diallyl trisulfide. Indian J Exp Biol. 2011;49(11):805–816. [PMC free article] [PubMed] [Google Scholar]
- 7.Arora N, Bansal MP, Koul A. Azadirachta indica exerts chemopreventive action against murine skin cancer: studies on histopathological, ultrastructural changes and modulation of NF-kappaB, AP-1, and STAT1. Oncology Research. 2011;19(5):179–191. doi: 10.3727/096504011x12970940207724. [DOI] [PubMed] [Google Scholar]
- 8.Azizova OA. Role of free radical processes in the development of atherosclerosis. Biol Memb. 2002;9:451–471. [Google Scholar]
- 9.Bharati S, Rishi P, Koul A. Azadirachta indica exhibits chemopreventive action against hepatic cancer: Studies on associated histopathological and ultrastructural changes. Microsc Res Tech. 2012;75(5):586–595. doi: 10.1002/jemt.21095. [DOI] [PubMed] [Google Scholar]
- 10.Belman S. Onion and garlic oils inhibit tumor promotion. Carcinogenesis. 1983;4(8):1063–1065. doi: 10.1093/carcin/4.8.1063. 1983. [DOI] [PubMed] [Google Scholar]
- 11.Banning M. The carcinogenic and protective effects of food. British Journal of Nursing. 2005;14(20):1070–1074. doi: 10.12968/bjon.2005.14.20.20049. [DOI] [PubMed] [Google Scholar]
- 12.Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocercinogenesis: inhibition of cell proliferation and induction of apoptosis. Chemico-Biological Interactions. 2009;179(2–3):131–144. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Biswas KR, Ishika T, Rahman M, Khan T, Swarna A, Monalisa MN, Sanam S, Malek I, Rahmatulla M. American-Eurasian Journal of Sustainable Agriculture. 2011;5(2):247–251. [Google Scholar]
- 14.Bomser JA, Singletary KW, Wallig MA, Smith MAL. Inhibition of TPA induced tumor promotion in CD-1 mouse epidermis by a polyphenolic fraction from grape seeds. Cancer Letters. 1999;135(2):151–157. doi: 10.1016/s0304-3835(98)00289-4. [DOI] [PubMed] [Google Scholar]
- 15.Boopalan T, Arumugam A, Damodaran C, Rajkumar L. The anticancer effect of 2′-3′-dehydrosalannol on triple-negative breast cancer cells. Anticancer Res. 2012;32(7):2801–2806. 2012. [PubMed] [Google Scholar]
- 16.Botterweck AAM, Verhagen H, Goldbohm RA, Kleinjans J, Van den Brandt PA. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: results from analyses in the Netherlands cohort study. Food Chem Toxicol. 2000;38:599–605. doi: 10.1016/s0278-6915(00)00042-9. [DOI] [PubMed] [Google Scholar]
- 17.Burkill HM. The useful plants of West Africa. Kew: Royal Botanical Gardens; 1985. [Google Scholar]
- 18.Abel C, Busia K. An exploratory ethnobotanical study of the practice of herbal medicine by the Akan Peoples of Ghana. Alternative Medicine Review. 2005;10(2):111–122. [PubMed] [Google Scholar]
- 19.Cai YZ, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CY, Yang WL, Kuo SY. Cytotoxic activity and cell cycle analysis of hexahydrocurcumin on SW 480 human colorectal cancer cells. 2011;6(11):1671–1672. [PubMed] [Google Scholar]
- 21.Chu YL, Ho CT, Chung JG, Rajasekaran R, Sheen LY. Allicin Induces p53-Mediated Autophagy in Hep G2 Human Liver Cancer Cells'. J Agric Food Chem. 2012 doi: 10.1021/jf301298y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Cui X, Jin Y, Poudyal D, Chumanevich AA, Davis T, Winds A, Hofseth A, Wu W, Habiger J, Pena E, Wood P, Nagarkatti M, Nagarkatti PS, Hofseth L. Mechanistic insight into the ability of American ginseng to suppress colon cancer associated with colitis. Carcinogenesis. 2010;31(10):1734–1741. doi: 10.1093/carcin/bgq163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Pierro F, Rapacioli G, Ferrara T, Togni S. Use of a standardized extract from Echinacea angustifolia (Polinaceae) for the prevention of respiratory tract infections'. Altern Med Rev. 2012;17(1):36–41. [PubMed] [Google Scholar]
- 24.Dong H, Bai LP, Wong VK, Zhou H, Wang JR, Liu Y, Jiang ZH, Liu L. The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules. 2011;16(12):10619–10630. doi: 10.3390/molecules161210619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Effendy NM, Mohamed N, Muhammad N, Mohamad IN, Shuid AN. Eurycoma longifolia: Medicinal Plant in the Prevention and Treatment of Male Osteoporosis due to Androgen Deficiency. Evidence-Based Complementary and Alternative Medicine 1-9. 2012 doi: 10.1155/2012/125761. Article ID 125761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekanem AP, Wang M, Simon JE, Obiekezie AI, Morah F. In vivo and in vitro activities of seed extract of Piper guineense Shum and Thonn. Against skin and gill Monogenean parasites of Gold fish (Carassius auratus auratus) Phytother Res. 2004;3:97. doi: 10.1002/ptr.1550. [DOI] [PubMed] [Google Scholar]
- 27.Elujoba AA, Odeleye OM, Ogunyemi CM. Traditional Medical Development for Medical and Dental Primary Healthcare Delivery System in Africa. Afr J Traditional, Complementary and Alternate Medicine. 2005;2(1):46–61. [Google Scholar]
- 28.Evans WC. Trease and Evans' Pharmacognosy. 16th Edition. London: WB Saunders Company Ltd; 2008. [Google Scholar]
- 29.Faber M, Laurie S. A home gardening Approach Developed in South Africa to address Vitamin A Deficiency. In: Thompson B, Amoroso L, editors. “Combating Micronutrient deficiencies: Food-based Approaches”. Rome: Pub.: CABI and FAO; 2011. Chapter 9. [Google Scholar]
- 30.Family Health Teams, author. Guide to Health Promotion and Disease Prevention. Ontario: Canada: 2006. [Google Scholar]
- 31.Farombi A. Antioxidant and Antinflammatory activities of Mallotus oppositifolius (Geisel) mull. Arg In Model Systems Afr J Med Sci. 2001;30:213–215. [PubMed] [Google Scholar]
- 32.Filip A, Daicoviciu D, Clichici S, Mocan T, Muresan A, Postescu ID. Chemopreventive effects of Calluna vulgaris and Vitis vinifera extracts on UVB-induced skin damage in SKH-1 hairless mice. J Med Food. 2011;14(7–8):761–766. [PubMed] [Google Scholar]
- 33.Fishman SM, Christian P, West KP. The role of vitamins in the prevention and control of anaemia. Public Health Nutr. 2000 Jun;3(2):125–150. doi: 10.1017/s1368980000000173. [DOI] [PubMed] [Google Scholar]
- 34.Ghasi S, Egwuibe C, Achukwu PU, Onyeanusi JC. Assessment of the medical benefit in the folkloric use of Bryophyllum Pinnatum leaf among the Igbos of Nigeria for the treatment of hypertension. African Journal of Pharmacy and Pharmacology. 2011;5(1):83–92. [Google Scholar]
- 35.Gibson RS, Hotz C. Dietary diversification/modification strategies to enhance micronutrient content and bioavailability of diets in developing countries. Br J Nutr. 2001 May;85(Suppl 2S):159–166. doi: 10.1079/bjn2001309. [DOI] [PubMed] [Google Scholar]
- 36.Gill LS. Ethnomedical uses of plants in Nigeria. Nigeria: University of Benin press; 1992. [Google Scholar]
- 37.Hirayama T. Epidemiology of stomach cancer in Japan. With special reference to the strategy for the primary prevention. Japanese Journal of Clinical Oncology. 1984;14(2):159–168. [PubMed] [Google Scholar]
- 38.Hoareau H, DaSilva EJ. Medicinal plants: a re-emerging health aid’. Electronic Journal of Biotechnology. 1999;2(2) Issue of August 15, Available on line at http://www.ejb.org/content/vol2/issue2/full/2/ [Google Scholar]
- 39.Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Research. 1994;54(3):701–708. [PubMed] [Google Scholar]
- 40.Iroha IR, Amadi ES, Nwuzo AC, Afiukwa FN. Evaluation of the Antibacterial Activity of Extracts of Sida acuta Against Clinical Isolates of Staphylococcus aureus Isolated from Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome Patients. Research Journal of Pharmacology. 2009;3(2):22–25. [Google Scholar]
- 41.Jung CH, Ahn J, Jeon TI, Kim TW, Ha TY. Syzigium aromaticum ethanol extract reduces high-fat diet-induced obesity in mice through down regulation of adipogenic and lipogenic gene expression’. Exp Ther Med. 2012;4(3):409–414. doi: 10.3892/etm.2012.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katiyar SK, Agarwal R, Mukhtar H. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale rhizome. Cancer Research. 1996;56(5):1023–1030. [PubMed] [Google Scholar]
- 43.Katz AE. Flavonoid and botanical approaches to prostate health. J Altern Complement Med. 2002;8(6):813–821. doi: 10.1089/10755530260511829. [DOI] [PubMed] [Google Scholar]
- 44.Kaliora AC, Kountouri AM, Karathanos VT, Koumbi L, Papadopoulos NG, Andrikopoulos NK. Effect of Greek raisins (Vitis vinifera L.) from different origins on gastric cancer cell growth. Nutr Cancer. 2008;60(6):792–799. doi: 10.1080/01635580802295776. [DOI] [PubMed] [Google Scholar]
- 45.Kang JH, Song KH, Woo JK, Park MH, Rhee MH, Choi C, Oh SH. Ginsenoside Rp1 from Panax ginseng exhibits anti-cancer activity by down-regulation of the IGF-1R/Akt pathway in breast cancer cells. Plant Foods Hum Nutr. 2011;66(3):298–305. doi: 10.1007/s11130-011-0242-4. [DOI] [PubMed] [Google Scholar]
- 46.Khan SA, Chatterton RT, Michel N, Bryk M, Lee O, Ivancic D, Heinz R, Zalles CM, Helenowski IB, Jovanovic BD, Franke AA, Bosland MC, Wang J, Hansen NM, Bethke KP, Dew A, Coomes M, Bergan RC. Soy isoflavone supplementation for breast cancer risk reduction: a randomized phase II trial. Cancer Prev Res (Phila) 2012;5(2):309–319. doi: 10.1158/1940-6207.CAPR-11-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kokwaro JO. Medicinal Plants of East Africa. 2nd Edition. Nairobi: East African Literature Bureau; 1993. [Google Scholar]
- 48.Koumare AK, Sissoko F, Diop AK, Ongoiba N, Maiga I, Bougoudogo F, Soumare S, Sangare D, Ouattara K, Diallo A, Doumbia D, Dembele S. Factors affecting nosocomial infection in the surgery setting at the Hospital of Point “G”] Mali Med. 2008;23(3):44–46. [PubMed] [Google Scholar]
- 49.Kurapati KR, Samikkannu T, Kadiyala DB, Zainulabedin SM, Gandhi N, Sathaye SS, Indap MA, Boukli N, Rodriguez JW, Nair MP. Combinatorial cytotoxic effects of Curcuma longa and Zingiber officinale on the PC-3M prostate cancer cell line. J Basic Clin Physiol Pharmacol. 2012:1–8. doi: 10.1515/jbcpp-2012-0021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konoshima T, Takasaki M, Tokuda H. Anti-tumor-promoting activity of the roots of Panax notoginseng (1) Natural Medicines. 1996;50(2):158–162. [Google Scholar]
- 51.Konoshima T, Takasaki M, Tokuda H. Anti-carcinogenesis activity of the roots of Panax notoginseng. II, Biological & Pharmaceutical Bulletin. 1999;22(10):1150–1152. doi: 10.1248/bpb.22.1150. [DOI] [PubMed] [Google Scholar]
- 52.Kratchanova M, Denev P, Ciz M, Lojek A, Mihailov A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochimica Polonica. 2010;57(2):229–234. [PubMed] [Google Scholar]
- 53.Lawal IO, Uzokwe NE, Igboanugo ABI, Adio AF, Awosan EA, Nwogwugwu JO, Faloye B, Olatunji BP, Adesoga AA. Ethno medicinal information on collation and identification of some medicinal plants in Research Institutes of South-west Nigeria. African Journal of Pharmacy and Pharmacology. 2010;4(1):001–007. [Google Scholar]
- 54.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008;28:729–737. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Li W, Tian H, Li L, Li S, Yue W, Chen Z, Qi L, Hu W, Zhu Y, Hao B, Gao C, Si L, Gao F. Diallyl trisulfide induces apoptosis and inhibits proliferation of A549 cells in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai) 2012;44(7):577–583. doi: 10.1093/abbs/gms033. [DOI] [PubMed] [Google Scholar]
- 56.Li C, Cai J, Geng J, Li Y, Wang Z, Li R. Purification, characterization and anticancer activity of a polysaccharide from Panax ginseng. Int J Biol Macromol. 2012a;51(5):968–973. doi: 10.1016/j.ijbiomac.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Li X, Yin L, Ding J, Jin H, Feng Y. Genistein inhibits placental choriocarcinoma cell line JAR invasion through ERβ/MTA3/Snail/E-cadherin pathway. Oncol Lett. 2011;2(5):891–897. doi: 10.3892/ol.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Amer J Clin Nutr. 2003;78:517–520. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 59.Liu HY, Qiu NX, Ding HH, Yao RQ. Polyphenols content and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res Intern. 2008;41:363–370. [Google Scholar]
- 60.López-Jiménez A, García-Caballero M, Medina MÁ, Quesada AR. Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur J Nutr. 2013;52(1):85–95. doi: 10.1007/s00394-011-0289-x. [DOI] [PubMed] [Google Scholar]
- 61.Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J Ethnopharmacol. 2004;90(1):161–166. doi: 10.1016/j.jep.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 62.Ngono Ngane A, Biyiti L, Bouchet P, Nkengfack A, Amvam Zollo PH. Antifungal activities of Piper guineense of Cameroun. Fitoter. 2003;4(5):464–468. doi: 10.1016/s0367-326x(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 63.Nwinyi OC, Chinedu NS, Ajani OO, Ikpo CO, Ogunniran KO. Antibacterial effect of extracts of Ocimum gratissimum and Piper guineense on Escherichia coli and Staphylococcus aureus. African Journal of Food Scienc. 2009;e3(3):77–81. [Google Scholar]
- 64.Malathi R, Rajan SS, Gopalakrishnan G, Suresh G. 2′,3′-Dehydrosalannol. Acta Crystallogr C. 2002;58(Pt 11):o681–o682. doi: 10.1107/s010827010201747x. Epub 2002 Oct 31. [DOI] [PubMed] [Google Scholar]
- 65.McDonald J, Ollerenshaw A. Priority setting in primary health care: a framework for local catchments. Rural Remote Health. 2011;11(2):1714. Epub 2011 Jun 6. [PubMed] [Google Scholar]
- 66.Moeti M. Noncommunicable diseases: An overview of Africa's new silent killers. African Health Monitor. 2008;8(1):2–5. [Google Scholar]
- 67.Morse MA, Stoner GD. Cancer chemoprevention: principles and prospects. Carcinogenesis. 1993;14(9):1737–1746. doi: 10.1093/carcin/14.9.1737. [DOI] [PubMed] [Google Scholar]
- 68.Murakami A, Ohigashi H, Koshimizu K. Anti-tumor promotion with food phytochemicals: a strategy for cancer chemoprevention. Bioscience, Biotechonology and Biochemistry. 1996;60(1):1–8. doi: 10.1271/bbb.60.1. [DOI] [PubMed] [Google Scholar]
- 69.Ngo SN, Williams DB, Head RJ. Rosemary and cancer prevention: preclinical perspectives. Crit Rev Food Sci Nutr. 2011;51(10):946–954. doi: 10.1080/10408398.2010.490883. [DOI] [PubMed] [Google Scholar]
- 70.Nkrumah-Elie YM, Reuben JS, Hudson AM, Taka E, Badisa R, Ardley T, Israel B, Sadrud-Din SY, Oriaku ET, Darling-Reed SF. The attenuation of early benzo(a)pyrene-induced carcinogenic insults by diallyl disulfide (DADS) in MCF-10A cells. Nutr Cancer. 2012;64(7):1112–1121. doi: 10.1080/01635581.2012.712738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nutbeam D. Evaluating health promotion-progress, problems and solutions. Health Promotion International. 1998;13(1):27–44. [Google Scholar]
- 72.Obho G. Antioxidant and Antimicrobial Properties of Ethanolic Extract of Ocimum gratissimum Linn. Leaves. 2006;1(1):47–53. [Google Scholar]
- 73.Odugbemi T. Outlines and pictures of medicinal plants from Nigeria. Lagos: University of Lagos Press; 2006. 283pp. [Google Scholar]
- 74.Ohta T, Nakatsugi S, Watanabe K, Kawamori T, Ishikawa F, Morotomi M, Sugie S, Toda T, Sugimura T, Wakabayashi K. Inhibitory effects of Bifidobacterium-fermented soy milk on 2-amino-1-methyl-6-phenylimidazol[4,5-b]-pyridine-induced rat mammary carcinogenesis, with a partial contribution of its component isoflavones, Carcinogenesis. 21. 2000;5:937–941. doi: 10.1093/carcin/21.5.937. [DOI] [PubMed] [Google Scholar]
- 75.Okujagu TF, Etatuvie SO, Eze I, Jimoh B, Nwokereke C, Mbaoji C, Mohammed Z. Medicinal plant of Nigeria, north-west Nigeria. Vol 1 Nigerian Journal of Natural Product & Medicine (177) 2008;1:32–34. [Google Scholar]
- 76.Okwu DE, Okwu ME. Chemical Composition of Spondias mombin Linn Plant Parts J. Sustain Agric Environ. 2004;6(2):140–147. [Google Scholar]
- 77.Onwukaeme ND. Medicinal plant of Nigeria Natural Medicine Development Agency Federal Ministry of Science and Technology. Phytotherapy research. 1995;9:306–308. [Google Scholar]
- 78.Ozougwu, Eyo Evaluation Of The Activity Of Zingiber Officinale (Ginger) Aqueous Extracts On Alloxan-Induced Diabetic Rats. Pharmacologyonline. 2011;1:258–269. [Google Scholar]
- 79.Palombo EA. Evidence-Based Complementary and Alternative Medicine. 2011:1–15. doi: 10.1155/2016/4142104. 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Letters. 1998;129(2):139–144. doi: 10.1016/s0304-3835(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 81.Paul R, Prasad M, Sah NK. Anticancer biology of Azadirachta indica L (neem): a mini review. Cancer Biol Ther. 2011;12(6):467–476. doi: 10.4161/cbt.12.6.16850. [DOI] [PubMed] [Google Scholar]
- 82.Perchellet JP, Perchellet EM, Belman S. Inhibition of DMBA-induced mouse skin tumorigenesis by garlic oil and inhibition of two tumor-promotion stage by garlic and onion oils. Nutrition and Cancer. 1990;14(3–4):183–193. doi: 10.1080/01635589009514093. [DOI] [PubMed] [Google Scholar]
- 83.Pitot HC, Dragan YP. Facts and theories concerning the mechanisms of carcinogenesis. The FASEB Journal. 1991;5(9):2280–2286. [PubMed] [Google Scholar]
- 84.Ramakrishnan U. Prevalence of micronutrient malnutrition worldwide. Nutr Rev. 2002;60(5 Pt 2):S46–S52. doi: 10.1301/00296640260130731. [DOI] [PubMed] [Google Scholar]
- 85.Ramakrishna Y, Goda H, Baliga MS, Munsh AK. Decreasing cariogenic bacteria with a natural alternative prevention therapy using phytochemistry (plant extracts) J Clin Paediatr Dent. 2011;36(1):55–63. doi: 10.17796/jcpd.36.1.f485870h90174311. [DOI] [PubMed] [Google Scholar]
- 86.Chowdhury MH, Haque WM. A survey of medicinal plants used by Kavirajes of Chalna area, Khulna district, Bangladesh. Afr J Tradit Complement Altern Med. 2009 Dec 30;7(2):91–97. doi: 10.4314/ajtcam.v7i2.50859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rose G. Sick individuals and sick populations. International Journal of Epidemiology. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 88.Sadhana AS, Rao AR, Kucheria K, Bijani V. Inhibitory action of garlic oil on the initiation of benz[a]pyrene-induced skin carcinogenesis in mice. Cancer Letters. 1988;40(2):193–197. doi: 10.1016/0304-3835(88)90010-9. [DOI] [PubMed] [Google Scholar]
- 89.Shaba P, Pandey NN, Sharma OP, Rao JR, Singh RK. Anti-trypanosomal Activity of Piper Nigrum L (Black pepper) Against Trypanosoma Evansi. J Vet Adv. 2012;(4):161–167. [Google Scholar]
- 90.Sheiham A, Watt RG. The Common Risk Factor Approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000;28:399–406. doi: 10.1034/j.1600-0528.2000.028006399.x. [DOI] [PubMed] [Google Scholar]
- 91.Shenouda NS, Sakla MS, Newton LG, Besch-Williford C, Greenberg NM, MacDonald RS, Lubahn DB. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine. 2007 Feb;31(1):72–81. doi: 10.1007/s12020-007-0014-y. [DOI] [PubMed] [Google Scholar]
- 92.Škrovánková S, Mišurcová L, Machů L. Antioxidant activity and protecting health effects of common medicinal plants. Adv Food Nutr Res. 2012;67:75–139. doi: 10.1016/B978-0-12-394598-3.00003-4. [DOI] [PubMed] [Google Scholar]
- 93.Stoltzfus RJ. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. J Nutr Feb. 2001;131(2S-2):697–701. doi: 10.1093/jn/131.2.697S. [DOI] [PubMed] [Google Scholar]
- 94.Sule WF, Okonko IO, Joseph TA, Ojezele MO, Nwanze JC, Alli JA, Adewale OG, Ojezele OJ. In vitro-antifungal activity of Senna alata linn crude leaf extract. Adv Appl Sc Res. 2010;1(2):14. [Google Scholar]
- 95.Sumbul S, Ahmad MA, Mohd A, Mohd A. Role of phenolic compounds in peptic ulcer: An overview. J Pharm Bioallied Sci. 2011;3(3):361–367. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sung B, Prasad S, Yadav VR, Aggarwal BB. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr Cancer. 2012;64(2):173–197. doi: 10.1080/01635581.2012.630551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sofowora A. Medicinal Plants and Traditional Medicine in Africa'. 3rd edn. Ibadan: Spectrum Books; 2008. [Google Scholar]
- 98.Tan AC, Konczak I, Sze DM, Ramzan I. Towards the discovery of novel phytochemicals for disease prevention from native Australian plants: an ethnobotanical approach. Asian Pac J Clin Nutr. 2010;19(3):330–334. [PubMed] [Google Scholar]
- 99.Tan RS. Androgen: introducing the concept of relative hypogonadism in aging males. Int j Important res. 2002;14:319. doi: 10.1038/sj.ijir.3900861. [DOI] [PubMed] [Google Scholar]
- 100.Thompson B, Amoroso L. Combating Micronutrient deficiencies: Food-based Approaches. Rome: CABI and FAO; 2011. [Google Scholar]
- 101.Thomas OO. Re-examination of the antimicrobial activities of Xylopia aethiopica, Carica papaya, Ocimium gratissimum and Jatropha curcas. Fitoterapia. 1989;60:147–161. [Google Scholar]
- 102.Traoré M. PhD Thesis in Dental Surgery. Thesis No 23. Senegal: University of Dakar; 1975. Title: “Coutumes et thérapeutiques traditionnelles odonto-stomatologiques au Mali. 13 Décembre. [Google Scholar]
- 103.UNESCO, author. Culture and Health, Orientation Texts-World Decade for Cultural Development 1988 — 1997. Paris, France: 1996. Document CLT/DEC/PRO - 1996. pgs. 129. [Google Scholar]
- 104.UNESCO, author. FIT/504-RAF-48 Terminal Report: Promotion of Ethnobotany and the Sustainable Use of Plant Resources in Africa. Paris: 1998. p. 60. [Google Scholar]
- 105.Viteri FE, Gonzalez H. Adverse outcomes of poor micronutrient status in childhood and adolescence. Nutr Rev,ay. 2002;60(5 Pt 2):S77–S83. doi: 10.1301/00296640260130795. [DOI] [PubMed] [Google Scholar]
- 106.Wang Z, Liu Z, Cao Z, Li L. Allicin induces apoptosis in EL-4 cells in vitro by activation of expression of caspase-3 and -12 and up-regulation of the ratio of Bax/Bcl-2.'. Nat Prod Res. 2012;26(11):1033–1037. doi: 10.1080/14786419.2010.550894. [DOI] [PubMed] [Google Scholar]
- 107.WHO/AFRO, author. ‘African Traditional Medicine’. Brazzaville: 1976. Technical report series, No. 1; pp. 3–4. Report of the Regional Expert Committee. [Google Scholar]
- 108.WHO, author. The Promotion and Development of Traditional Medicine. Report of a WHO meeting. Geneva: World Health Organization; 1978. Technical report series 622. [PubMed] [Google Scholar]
- 109.WHO, author. Health Statistics. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 110.Yasukawa K, Demitrijevic SM, Evans FJ, Kawabata S, Takido M. Inhibitory effect of Prunus Cortex extract and its component, octacosyl ferulate, on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Phytotherapy Research. 1998;12(4):261–265. [Google Scholar]
- 111.Yasukawa K. In: Medicinal and Edible Plants as Cancer Preventive Agents, Drug Discovery Research in: Pharmacognosy. Prof Omboon Vallisuta., editor. InTech; 2012. [March 14, 2013]. ISBN: 978-953-51-0213-7, Available from: http://www.intechopen.com/books/drug-discovery-research-in-pharmacognosy/medicinal-and-edible-plants-as-cancer-preventive-agent. [Google Scholar]
- 112.Yogosawa S, Yamada Y, Yasuda S, Sun Q, Takizawa K, Sakai T. Dehydrozingerone, a Structural Analogue of Curcumin, Induces Cell-Cycle Arrest at the G2/M Phase and Accumulates Intracellular ROS in HT-29 Human Colon Cancer Cells. J Nat Prod. 2012;75(12):2088–2093. doi: 10.1021/np300465f. 2012. [DOI] [PubMed] [Google Scholar]
- 113.Yun TK, Lee YS, Lee YH, Kim SI, Yun HY. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. Journal of Korean Medical Science. 2001;(16 Suppl):S6–S18. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao J, Wang J, Chen Y, Agarwl R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotin protocol and identification of procyanidin B5-3'-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20(9):1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 115.Zhou K, Raffoul JJ. Potential anticancer properties of grape antioxidants. J Oncol. 2012;2012:803294. doi: 10.1155/2012/803294. 2012. Epub Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. 2011;141(1):80–89. doi: 10.1053/j.gastro.2011.03.057. [DOI] [PubMed] [Google Scholar]