Abstract
This study attempted to elucidate the neurotransmitter systems involved in the neurophysiological properties of ethanolic extract, fractions and pure isolates of Spondias mombin leaves in mice (n = 6) after intraperitoneal (i.p.) route of administration.The crude ethanolic extract of Spondian mombin leaves was fractionated using the partitioning method to obtain the ethylacetate, butanolic and aqueous fractions. Open column chromatographic fractionation of the ethylacetate fraction yielded seven sub-fractions, out of which the pure coumaroyl, quercetin and gallic acid derivatives were obtained after purification on Sephadex LH 20. The ethanolic extract, butanolic fraction, ethylacetate subfractions and pure isolates of the Spondian mombin leaves were tested on novelty-induced rearing and grooming behaviours in mice with standard pharmacological tools using the open field method. The extract and its fractions decreased novelty-induced rearing in a dose-dependent manner. While the Coumaroyl derivative had no effect on novelty-induced rearing, it significantly reversed the inhibitory effect of yohimbine, propranolol and haloperidol on novelty-induced rearing. Quercetin significantly potentiated the inhibitory effect of yohimbine on novelty-induced rearing. Naloxone significantly potentiated the quercetin-induced suppression of novelty-induced rearing. Gallic acid derivative significantly potentiated the inhibitory effect of yohimbine on novelty-induced rearing. Naloxone, atropine and haloperidol pretreatments significantly potentiated gallic acid derivative-induced suppression of novelty-induced rearing.The extract and its fractions had biphasic effect on novelty-induced grooming in mice. Coumaroyl derivative significantly increased novelty-induced grooming, while quercetin and gallic acid derivative decreased novelty-induced grooming significantly. The three pure isolates significantly reversed the effects of yohimbine and atropine on the novelty-induced grooming in mice. Propranolol-induced increase in novelty-induced grooming was significantly reversed by coumaroyl and gallic acid derivatives. Pre-treatment with naloxone significantly increased the gallic acid derivative-induced suppression of novelty-induced grooming. Pre-treatment with haloperidol reversed the effect of coumaroyl derivative and potentiated the inhibitory effect of quercetin derivative and gallic acid derivative significantly. This study suggested that adrenergic and dopaminergic neuro-transmissions are strongly involved in the neural mechanisms of the effect of the three pure isolates derivative, while opioid neuro-transmission is strongly linked with the neural mechanism of behavioural effect of coumaroyl derivative.
Keywords: Spondias mombin, Anacardiaceae, explorative behaviours, neurotransmitters
Introduction
Spondias mombin belongs to the family L. Anacardiacae. It grows in the rain forest and in the coastal areas. Other common names, according to Morton (1987), include the following: Bala (Costa Rica), Jobito (Panama), Jobo Blanco (Colombia), Jobo Corronchoso (Venezuela), Hoeboe (Surinam), Acaiba, Caja, Pau da tapera (Brazil), Ubo (Peru), Hobo (Mexico), Iyeye (Yoruba), Uvuru (Igbo). Previous phytochemical analysis of the Spondian mombin leaves revealed the following constituents: carotenoids, phytoene, phytofluene, alpha-trans-beta-carotene, alpha-cartene, beta-cryptoxanthin (cis and trans), zeinoxanthin and lutein Hamano and Marcadante (2001). Multiple medicinal values of different parts of the Spondian mombin have been reported. These include antibacterial (Ajao and Shonukan, 1985; Verpoorte and Dihal, 1987), anti-inflammatory (De Ferreyra, 1981), antispasmodic (Jaramillo and Ahunada-Barona, 1983), anxiolytic, anticonvulsant and sedative effects (Ayoka et al, 2005; Ayoka et al., 2006). Crude Spondias mombin (L. Anacardiaceae) leaves extracts have been reported to modulate multiple behaviours (novelty-induced, sedative, antipsychotic, antiepileptic) in mice and rats (Ayoka et al. 2005). Previous studies have reported extraction of different chemical compounds (phenolic acid, 6-alkenylsalicylic acid, anarcardic acid and phenolic derivatives) from Spondias mombin, which exhibited different biological properties including antibacterial, molluscicidal, antiviral and antioxidant effects (Corthout et al., 1994; Coates et al., 1994; Castner et al., 1998).
In this study, the butanolic, ethanolic, ethyl acetate extracts and pure compounds (Coumaroyl, Quercentin and Gallic acid) were obtained from Spondias mombin leaves. These extracts, their sub-fractions and pure compounds isolated from Spondias mombin leaves were examined for their possible neuro-behavioral effects in rodents. The possible neuropharmacological mechanisms responsible for these effects were also evaluated with focus on rearing and grooming behaviours in mice. Since Spondias mombin leaves extracts have been reported to be used in traditional medicine for the treatment of behavioral disorders in parts of South Western Nigeria (Ayoka et al., 2005), the elucidation of neurotransmitter systems involved in the behavioural effects of the crude extracts, fractions, sub-fractions and the isolated pure compounds of Spondias mombin leaves will suggest the neuro-pharmacological basis of some observed effects of the leaves in traditional medicine. This may encourage new drug development from Spondias mombin leave extracts for the management of neuropsychiatric disorders.
Materials and Methods
Preparation of extracts, fractions, sub-fractions and pure compounds from Spondias mombin leaves
The crude ethanolic extract of Spondian mombin leaves was fractionated into ethylacetate, butanolic and aqueous fractions. Open column chromatographic fractionation of the ethylacetate fraction yielded seven sub-fractions, out of which the pure coumaroyl, quercetin and gallic acid were obtained after purification on Sephadex LH 20.
Animals
Adult mice of both sexes (average weight of 20 g) (Vom strain, National Veterinary Research Institute, Jos, Nigeria) were used as test animals. They were maintained under natural daylight/night condition at the Faculty of Health Sciences Animal House Obafemi Awolowo University. All the animals were fed with standard feeds (Pfizer Feed Plc., Lagos, Nigeria) and had free access to water.
Toxicity tests
Toxicity test was carried out on the ethanolic and butanolic extracts and ethyl-acetate fraction of Spondias mombin. Nineteen groups of five mice each were used for each of the tests as follows:
Groups 1 – 18 received 50, 100, 200, 400, 800 and 1600 mg/kg i.p. of either the ethanolic or butanolic extract or ethylacetate fraction.
Group 19 received normal saline as control.
For each treatment, mortality rate was observed and recorded for 48 hours after the treatment. The seven sub-fractions obtained from the ethyl acetate fraction of Spondias mombin and the pure isolates (Coumaroyl, Quercetin and Gallic acid) were subjected to pilot survey study to determine the effective non-toxic dose to be used. Animals were observed daily for seven days for toxic effects of isolates at 1, 5, 10 and 20 mg/kg.
Assessment of Novelty-Induced Rearing and Grooming Behaviours in Mice
These behavioural assessments were done as described by Ajayi and Ukponmwan (1994). The observation cage was an opaque Plexiglas observation cage measuring 45 by 25 by 25 cm with only one side transparent for observation. The Plexiglas was bedded with sawdust. At the beginning of the test, the mouse was placed in the central square and observed thereafter for 10 minutes for rearing and grooming (Sanberg et al, 1985; Cessana et al, 1995). The laboratory was illuminated by normal fluorescent room light. All mice were observed and assessed singly in the Plexiglass cage after injection of saline or the test drugs (Koek et al, 1987; Ajayi and Ukponmwan, 1994). The observation cage was cleaned with absolute ethanol before introduction of another animal to avoid influence of odour from the preceding animal.
Novelty-Induced Rearing (NIR): NIR considered as a central excitatory behaviour locomotor behaviour (Labella et al., 1979) was counted on the number of times the mouse was standing on the hind limb with its forelimbs against the wall of the observation cage or in the free air (Ajayi and Ukponmwan, 1994). The number of rears was counted for 10 mins.
Novelty Induced Grooming (NIG): Grooming comprises the cleaning and dressing of the face and body with the tongue, fore limbs or with gentle bite (Bolles, 1960).
The effect of extracts (ethanolic or butanolic) and ethylacetate fraction {12.5, 25, 50,100 (mg/kg i.p.)} was determined in groups of six mice per treatment dose of each extract/fraction. Control group (n=6) received equal volume of saline while the reference group (n=6) received the reference drug. The effect of 10 mg/kg i.p. of ethylacetate sub-fractions (S1–S7), was determined in groups of five mice per treatment group. The effect of 10 mg/kg i.p. of the three pure compounds isolated (Coumaroyl, Quercetin and gallic acid derivatives) was determined in groups of five mice per treatment group. The animals were submitted to observation for NIR and NIG, after intraperitoneal (i.p.) administration of the doses of the test extracts/drugs.
In another set of experiments, mice were pre-treated 15 min prior with neurotransmitter blockers to evaluate the mode of actions of the extracts/ fractions/pure compounds on NIR and NIG behaviours in mice. The following receptor blockers were used: yohimbine (α2 adrenergic receptors antagonist, 1 mg/kg i.p), atropine (muscarinic receptor antagonist, 0.5 mg/kg i.p.), propranolol (β receptor adrenergic antagonist, 1 mg/kg i.p.), naloxone (µ opioid receptor antagonist, 1 mg/kg i.p), and haloperidol (D2 receptor antagonist, 0.25 mg/kg i.p.). The control groups received the antagonists singly. The animals were submitted to observation for NIR and NIG, after intraperitoneal (i.p.) administration of the doses of the test drugs.
Chemicals and Drugs
The following drugs were used: Atropine sulphate (BDH Chemicals Ltd England), Yohimbine, Naloxone, Propranolol, and Haloperidol (Sigma Chemical Co. St. Louis, USA), Diazepam (Roche, Basel Switzerland).
Statistical Analysis
The significance of differences between groups was determined using one-way analysis of variance (ANOVA), followed by post hoc analysis using the Student-Newman-Keuls test. Statistical significance was accepted at P values less than or equal to 0.05. In all these statistical determinations, a computer program - the Primer of Biostatics (Version 3.01) was used (Glantz, 1992).
Results
LD50
The LD50 for ethanolic extract was 510 mg/kg i.p., while it was 480 mg/kg for ethylacetate fraction and 720 mg/kg for the Butanolic fraction of the extract. The dose that produced the most robust effect for all behaviours studied for the seven fractions and the pure isolates (coumaroyl, quercetin and gallic acid) was 10 mg/kg i.p.
The Effect of Spondias mombin Extracts/Fractions on Novelty-Induced Rearing (NIR) Behaviour in Mice
The intraperitoneal administration of ethanolic extract and ethylacetate fraction decreased the NIR in a dose dependent manner from 12.5 – 100 mg/kg (P < 0.05), while the butanolic fraction decreased NIR in a dose dependent manner from 12.5 – 50 mg/kg. However, NIR at 100 mg/kg of the butanolic extract was not significantly different from the effect at 50 mg/kg (P > 0.05) {Figures 1, 2 and 3}. One-way ANOVA revealed that there is significant difference between various treatment groups treated with ethanolic extract; F(6, 35) = 34.03, P < 0.001; butanolic extract; F(6, 35) = 70.49, P < 0.001 and ethylacetate fraction; F(6, 35) = 44.85, P < 0.001.
Figure 1.
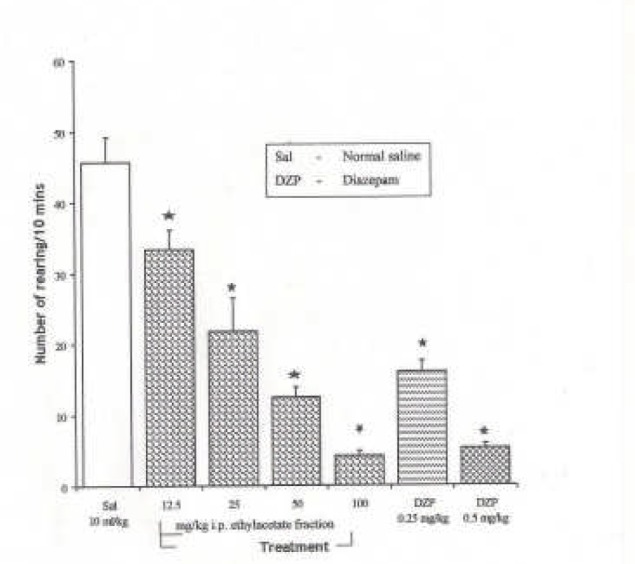
Effect of the Ethanolic extract of Spondias mombin on Novelty-Induced Rearing (NIR) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 44.03, P <0.001. Each bar is Mean ± S.E.M. (n = 6). Diazepam (DZP, 0.25 and 0.5 mg/kg i.p.) was used as the standard reference drug. * indicates significant difference from control. P < 0.05 (SNK test)
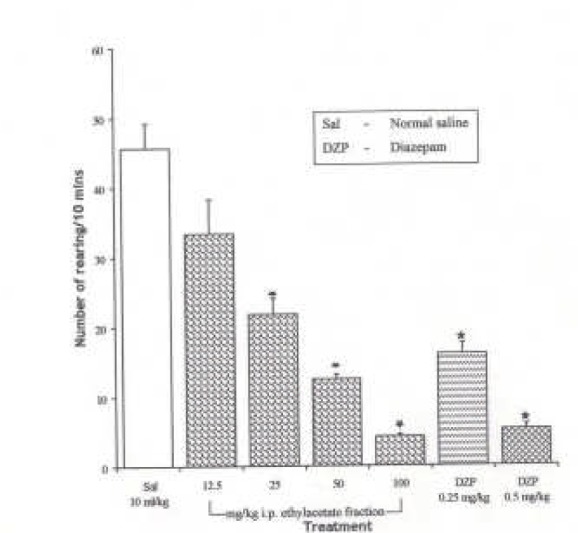
Figure 2.
Effect of the Ethylacetate Fraction of Spondias mombin on Novelty-Induced Rearing (NIR) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 44.85, P <0.001. Each bar is Mean ± S.E.M. (n = 6). Diazepam (DZP, 0.25 and 0.5 mg/kg i.p.) was used as the standard reference drug. * indicates significant difference from control. P < 0.05 (SNK test)
Figure 3.
Effect of Butanolic fraction of Spondian Mombin on Novelty-Induced Rearing (NIR) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 70.49, P < 0.001. Each bar is Mean ± S.E.M. (n = 6). Diazepam (DZP, 0.25 and 0.5 mg/kg i.p.) was used as the standard reference drug. * indicates significant difference from control. P < 0.05 (SNK test)
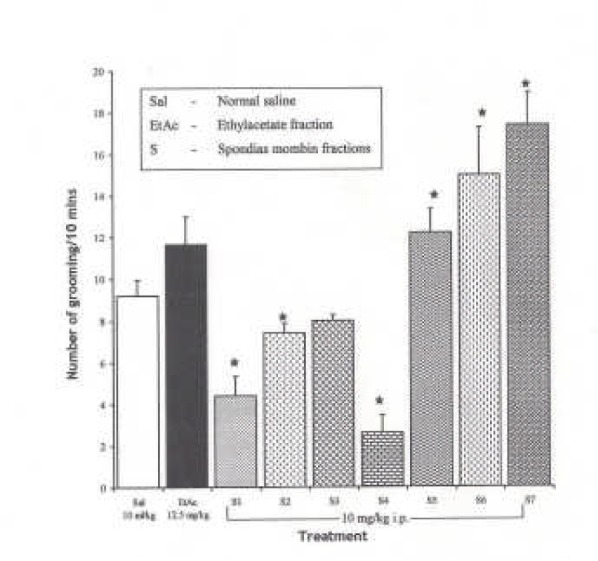
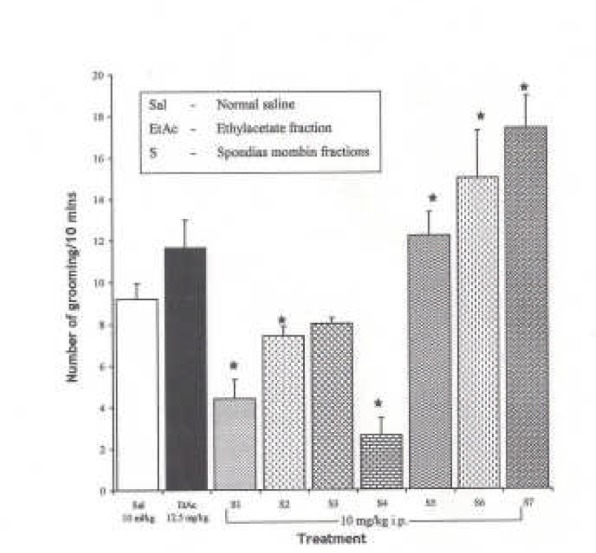
Effects of Spondias mombin Ethylacetate Sub-Fractions (S1 – S7) on Novelty-Induced Rearing Behaviour in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups treated with ethylacetate sub-fractions; F(8, 38) = 45.60, P < 0.001. The intraperitoneal administration of fractions, S1 – S7 decreased NIR relative to saline treated animals in mice except subfraction S2, which increased NIR in mice relative to saline (Figure 4).
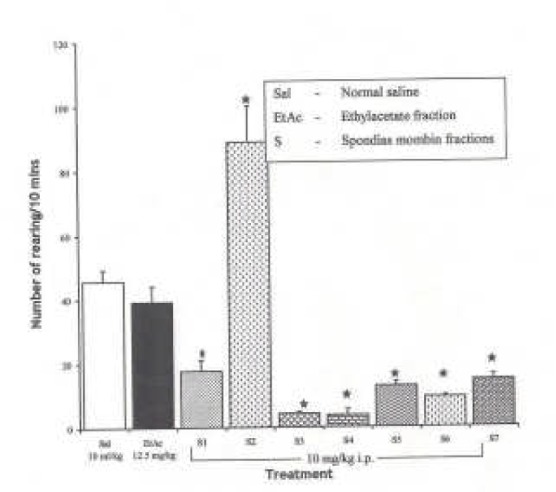
Fig.ure 4.
Effect of Spondias mombin Ethylacetate Sub Fractions (S1 – S7) on Novelty-Induced Rearing (NIR) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(8, 38) = 45.60, P < 0.001. Each bar is mean ± SEM. n = 6). S2 increased rearing while fractions S1, S2 – S7 decreased rearing behaviour. S1 — S7 were administered at 10 mg/kg i.p. * indicates significant difference from control. P < 0.05 (SNK test)
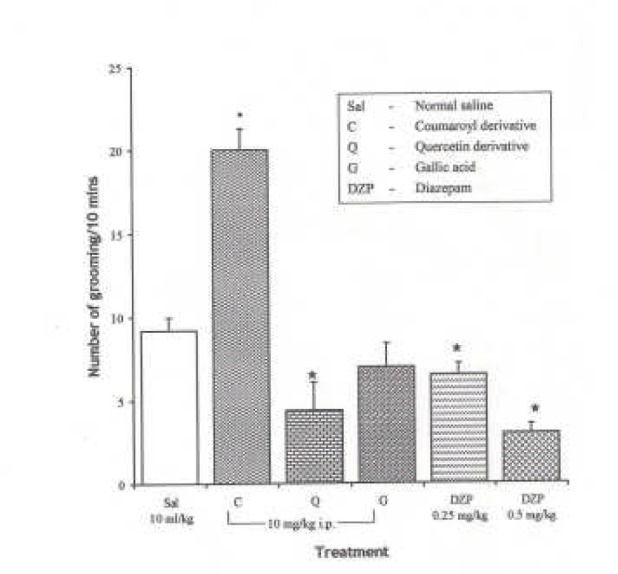
Effects of Pure Isolates from Spondias mombin leaves on Novelty-Induced Rearing (NIR) Behaviour in Mice
One-way ANOVA revealed that there is significant difference between various groups treated with pure isolates from spondian mombin leaves F(5, 27) = 19.48, P < 0.001. Intraperitoneal administration of Coumaroyl derivative had no significant effect on NIR relative to saline, whereas the Quercetin and Gallic acid decreased NIR in mice relative to saline control (Figure 5).
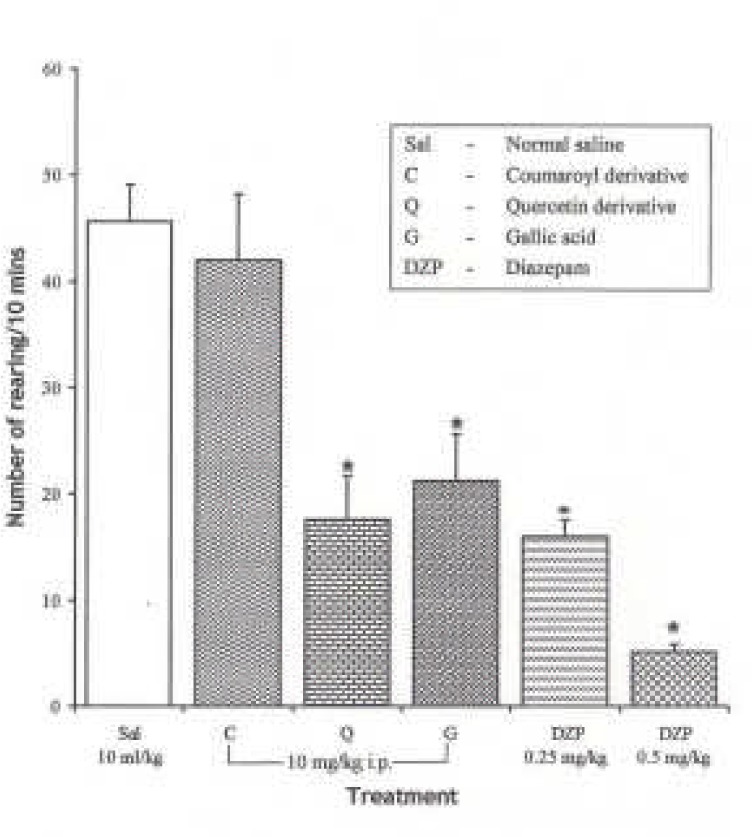
Figure 5.
Effect of the Pure Isolates on Novelty-Induced Rearing Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(5, 27) = 19.48, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Quercetin derivative and gallic acid decreased novelty-induced rearing behaviour in mice comparably with the reference drug. Diazepam (DZP 0.25 and 0.5 mg/kg I.P.) was used as the standard reference drug. * indicates significant difference from control. P < 0.05 (SNK test)
Effect of Yohimbine (1 mg/kg i.p.), α2 adrenergic receptor antagonist on the effect of the pure isolates from Spondias mombin on NIR in Mice
One-way ANOVA revealed that there is significant difference between the treatment groups; F(7, 33) = 13.74, P < 0.001. Yohimbine (1 mg/kg i.p.) decreased NIR in mice (P < 0.05). Quercetin and Gallic acid potentiate the yohimbine (1 mg/kg i.p.)-induced suppression of NIR in mice, while Coumaroyl reversed the inhibitory effect of yohimbine (1 mg/kg i.p.) on NIR in mice (Figure 6).
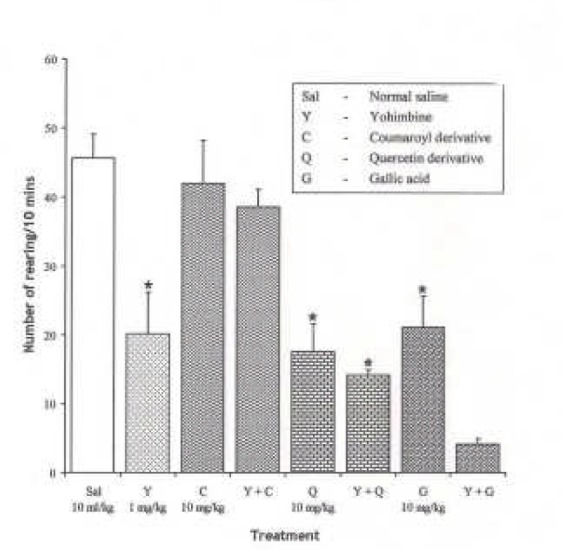
Figure 6.
Effect of Pre-treatment with Yohimbine (l mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Rearing (NIR) in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 13.74, P < 0.001. Each bar is mean ± S.E.M. (n = 6) Yohimbine (1 mg/kg i.p.) decreased rearing. Coumaroyl (10 mg/kg i.p.) that had no effect abolished the effect of yohimbine on NIR. Quercetin (10 mg/kg i.p.) decreased NIR and it is not affected by yohimbine. Gallic acid (10 mg/kg i.p.) decreased NIR which was further potentiated by yohimbine. * indicates significant difference from control. P < 0.05 (SNK test).
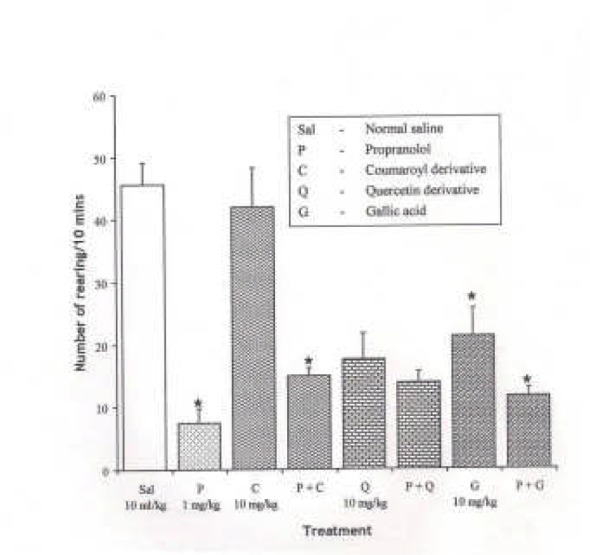
Effect of Propranolol (1 mg/kg i.p.), a β adrernergic receptor antagonist on the effect of the pure isolates from Spondias mombin on NIR in Mice
One-way ANOVA revealed that there is significant difference between the treatment groups; F(7, 33) = 16.83, P < 0.001. Propranolol (1 mg/kg i.p.) decreased NIR in mice (P < 0.05). Pre-treatment with propranolol (1 mg/kg i.p.) did not produce a significant change in the effects of Quercetin and Gallic acid on NIR, while Coumaroyl reversed the inhihibitory effect of proranolol (1 mg/kg i.p.) on NIR in mice (P < 0.05) {Figure 7}.
Fig.ure 7.
Effect of Pre-treatment with Propranolol (l mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Rearing (NIR) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 16.83, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Propranolol is 1 mg/kg i.p., while each of the pure isolates was given at 10 mg/kg i.p. Propranolol (l mg/kg i.p.) decreased NIR. Pre-treatment with propranolol (l mg/kg i.p.) had no effect on the effect of Quercetin and Gallic acid on NIR, while Coumaroyl reversed NIR. * indicates significant difference from control. P < 0.05 (SNK test).
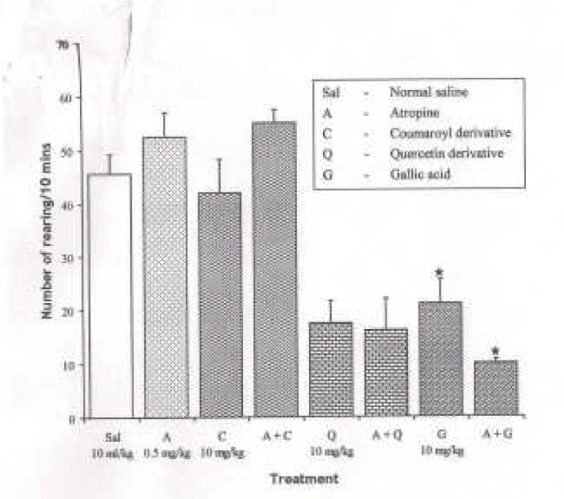
Effect of Atropine (0.5 mg/kg i.p.), a muscarinic antagonist on the effect of the pure isolates from Spondias mombin on NIR in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 17.33, P < 0.001. Atropine (0.5 mg/kg i.p.) did not alter the NIR in mice (P < 0.05). Coumaroyl had no effect either when given alone or when pre-treated with atropine (0.5 mg/kg i.p.). Pretreatment with atropine had no significant effect on Quercetininduced (10 mg/kg i.p.) decreased NIR. Gallic acid (10 mg/kg i.p.) decreased NIR alone and was further decreased when pretreated with atropine (Figure 8).
Figure 8.
Effect of Pre-treatment with Atropine (0.5 mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Rearing (NIR) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 17.33, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Atropine (0.5 mg/kg i.p.) did not alter NIR in mice. Coumaroyl had no effect either when given alone or when pre-treated with atropine (0.5 mg/kg i.p.). Quercetin (10 mg/kg i.p.) decreased NIR alone and reversed the effect of atropine. Gallic acid (10 mg/kg i.p.) decreased NIR alone and was further decreased when pre-treated with atropine. * indicates significant difference from control. P < 0.05 (SNK test).
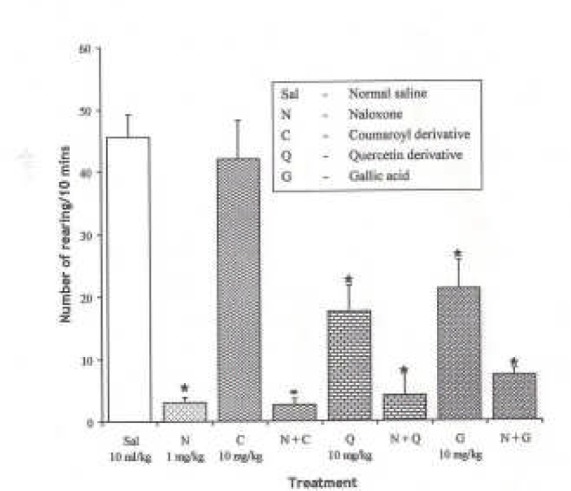
Effect of Naloxone (1 mg/kg i.p), µ opioid receptor antagonist on the effect of the pure isolates from Spondias mombin on NIR in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 23.83, P < 0.001. Naloxone (1 mg/kg i.p.) decreased NIR in mice (P < 0.05). Pre-treatment with Naloxone (1 mg/kg i.p.) decreased Coumaroyl NIR behaviour in mice. Quercetin and Gallic acid (P < 0.05) potentiated naloxone-induced decrease in NIR in mice {Figure 9}.
Figure 9.
Effect of Pre-treatment with Naloxone (l mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Rearing (NIR) Behaviour in Mice One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 23.83, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Naloxone (l mg/kg i.p.) decreased NIR in mice. Coumaroyl (10 mg/kg i.p.) had no effect on NIR and had no effect on the inhibitory effect of Naloxone. Quercetin (10 mg/kg i.p.) decreased NIR but had no effect on the inhibitory action of Naloxone. Gallic acid (10 mg/kg i.p.) decreased NIR but had no effect on the inhibitory action of Naloxone. That is, the effects of the isolates were reversed by Naloxone. * indicates significant difference from control. P < 0.05 (SNK test)
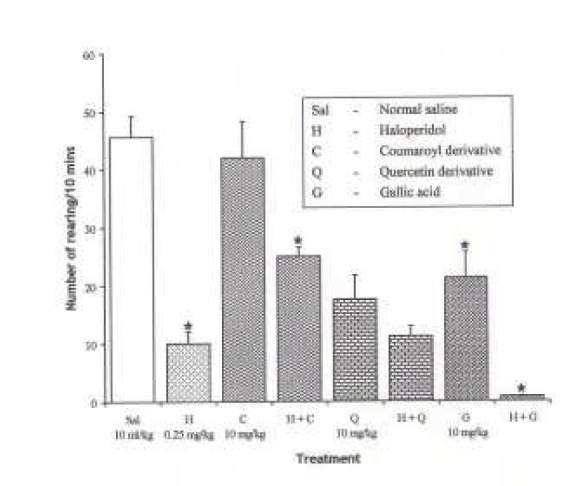
Effect of Haloperidol (0.25 mg/kg i.p.), D2 receptor antagonist on the effect of the pure isolates from Spondias mombin on NIR in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 20.25, P < 0.001. Haloperidol (0.25 mg/kg i.p.) decreased NIR in mice (P < 0.05). Pre-treatment with haloperidol (0.25 mg/kg i.p.) potentiated the inhibitory effect of Quercetin and Gallic acid on NIR in mice (P < 0.05) while the Coumaroyl derivative reduced the inhibitory effect of haloperidol on NIR {Figure 10}.
Figure 10.
Effect of Pre-treatment with Haloperidol (0.25 mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Rearing (NIR) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 20.25, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Haloperidol (0.25 mg/kg i.p) decreased NIR in mice. Coumaroyl (10 mg/kg i.p.) had no effect alone but reversed the effect of haloperidol. Quercetin decreased NIR and had no effect on haloperidol. Gallic acid decreased NIR and had additive effect on haloperidol inhibitory action on NIR. * indicates significant difference from control. P < 0.05 (SNK test)
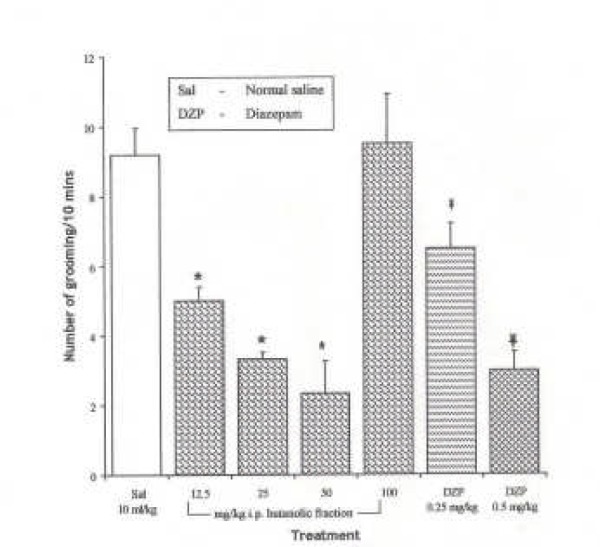
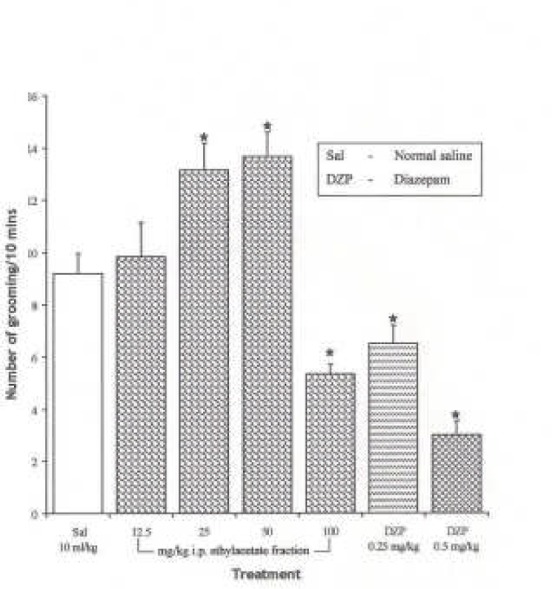
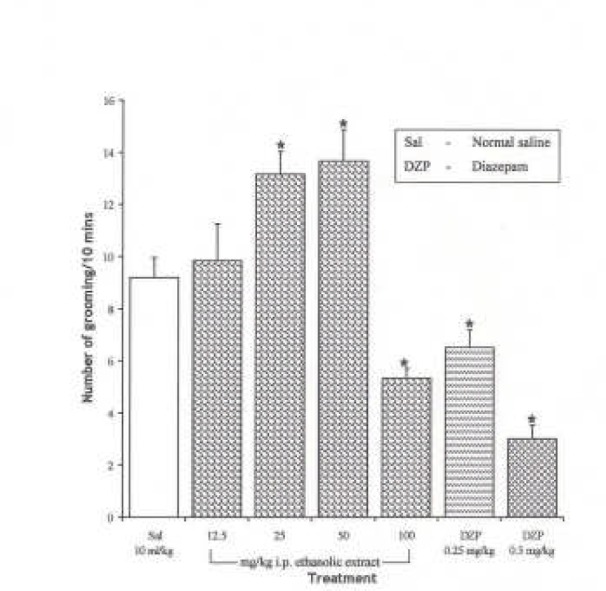
The Effect of Spondias mombin Extract/fraction on Novelty-Induced Grooming (NIG) Behaviour in Mice
Figure 11 shows the effect of ethanolic extract on grooming. There were initial increases at 25 and 50 mg/kg (P < 0.05) followed by a significant decrease at 100 mg/kg (P < 0.05). The ethylacetate fraction gave significant increases (P < 0.05). With butanolic fraction, there was dose dependent decrease between 12.5 and 50 mg/kg (P < 0.05) while at 100 mg/kg, there was increase in grooming that was not significant compared with control (P > 0.05) {Figures 11, 12 and 13}. For figure 11, one-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 19.54, P < 0.001. For figure 12, one-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 18.77, P < 0.001. For figure 13, one-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 13.58, P < 0.001.
Figure 11.
Effect of the Ethanolic extract of Spondias mombin on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 19.54, P < 0.001. Each bar is Mean ± S.E.M. (n = 6). Diazepam (DZP, 0.25 and 0.5 mg/kg i.p.) was used as the standard reference drug. There was increase in NIG behaviour from 12.5 to 50 mg/kg and a significant decrease at 100 mg/kg. * indicates significant difference from control. P < 0.05 (SNK test)
Figure 12.
Effect of the Ethylacetate Fraction of Spondias mombin on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 18.77, P < 0.001. Each bar is Mean ± S.E.M. (n = 6). Diazepam (DZP, 0.25 and 0.5 mg/kg i.p.) was used as the standard reference drug. There were increases in NIG behaviour at lower doses of 25 and 50 mg/kg and significant decreases at higher dose of 100 mg/kg. * indicates significant difference from control. P < 0.05 (SNK test)
Figure 13.
Effect of the Butanolic fraction of Spondias mombin on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(6, 35) = 13.58, P < 0.001. Each bar is Mean ± S.E.M. n = 6). Diazepam (DZP, 0.25 and 0.5 mg/kg i.p.) was used as the standard reference drug. There was significant decrease in NIG behaviour in a dose dependent manner from 12.5 to 50 mg/kg; while at 100 mg/kg there was no significant effect of the extract compared with the control. * indicates significant difference from control. P < 0.05 (SNK test)
Effects of the Spondias mombin Ethylacetate Sub Fractions (S1 – S7) on Novelty Induced Grooming (NIG) Behaviour in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(8, 38) = 19.84, P < 0.001. Intraperitoneal administration of S1 – S4 decreased NIG in mice while S5 – S7 increased NIG progressively. S4 gave the most potent inhibition while S7 gave the most potent increased (Figure 14).
Figure 14.
Effect of Spondias mombin Ethylacetate Fractions (S1 — S7) on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(8, 38) = 19.84, P < 0.001. Each bar is mean ± SEM. (n = 6) for control and the ethylacetate fractions and 5 for each of the fractions. Fractions S5, S6 and S7 significantly increased grooming while fractions S1 and S4 significantly decreased grooming. S1 — S7 were administered at 10 mg/kg i.p. * indicates significant difference from control. P < 0.05 (SNK test).
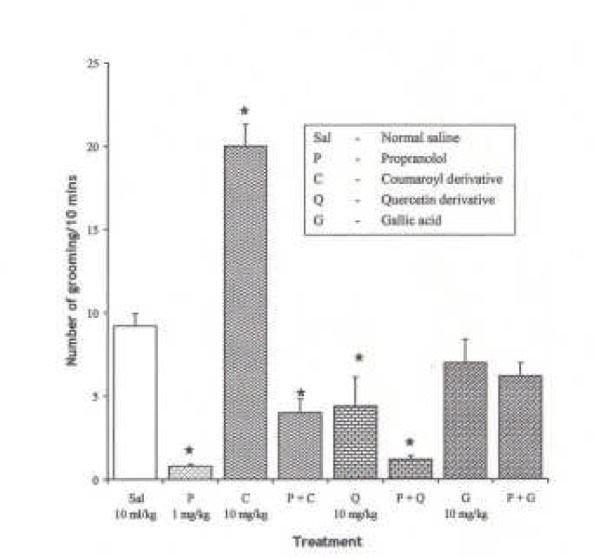
Effects of Pure Isolates from Spondias mombin Ethylacetate Sub Fractions on Novelty-Induced Grooming (NIG) Behaviour in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(5, 27) = 30.18, P < 0.001. Intraperitoneal administration of C (10 mg/kg i.p.) increased the NIG while intraperitoneal administration of Q (10 mg/kg i.p.) significantly decreased NIG in mice. Intraperitoneal administration of G at 10 mg/kg had no effect on NIG in mice (Figure 15).
Fig. 15.
Effect of the Pure Isolates on Novelty-Induced Grooming Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(5, 27) = 30.18, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Coumaroyl (10 mg/kg i.p.) significantly increased novelty-induced grooming behaviour in mice. Quercetin (10 mg/kg i.p.) significantly decreased novelty-induced grooming behaviour in mice. Gallic acid (10 mg/kg i.p.) is comparable to 0.25 mg/kg DPZ. Diazepam (DZP 0.25 and 0.5 mg/kg I.P.) was used as the standard reference drug. * indicates significant difference from control. P < 0.05 (SNK test)
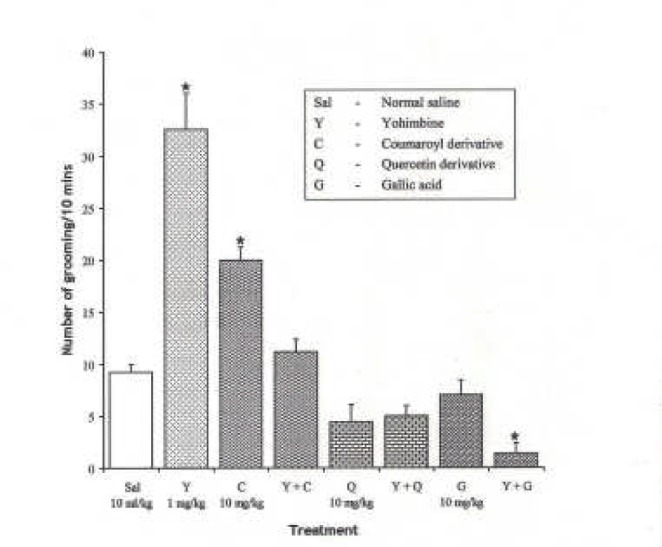
Effect of Pre-treatment with Yohimbine (1 mg/kg i.p.) on the Effect of the Pure Isolates from Spondias mombin on NIG in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 37.85, P < 0.001. Yohimbine (1 mg/kg i.p.) significantly increased NIG in mice (P < 0.05), while the isolates reversed the yohimbine (1 mg/kg i.p.)-induced increase in NIG in mice with Gallic acid being most potent.
Effect of Pre-treatment with Propranolol (1 mg/kg i.p.) on the Effect of the Pure Isolates from Spondias mombin on NIG in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 35.12, P < 0.001. Propranolol (1 mg/kg i.p.) decreased NIG in mice (P < 0.05). Pre-treatment with propranolol (1 mg/kg i.p.) reversed the effect of Coumaroyl and increased the inhibitory effect of Quercetin and Gallic acid on NIG in mice.
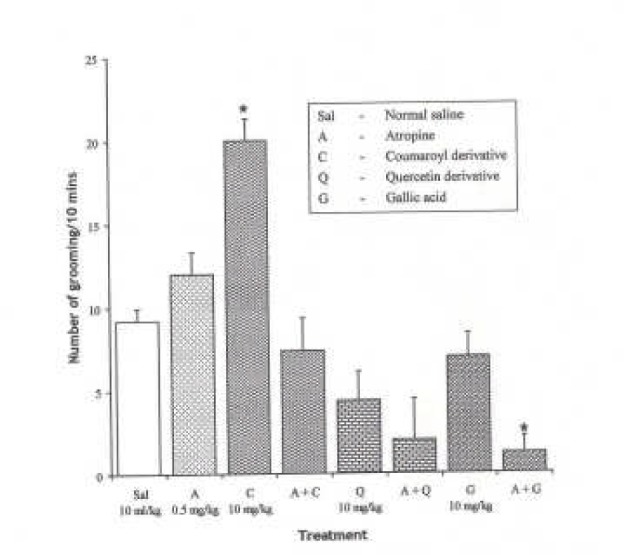
Effect of Pre-treatment with Atropine (0.5 mg/kg i.p.) on the Effect of the Pure Isolates from Spondias mombin on NIG in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 15.21, P < 0.001. Atropine (0.5 mg/kg i.p.) increased NIG in mice (P < 0.05). Pre-treatment with atropine (0.5 mg/kg i.p.) induced significant suppression of NIG behaviour by the Gallic acid isolate in mice, while the Quercetin-induced suppression of NIG was not reversed by atropine pretreatment. Pre-treatment with atropine (0.5 mg/kg i.p.) caused Coumaroyl to have no effect (P < 0.05).
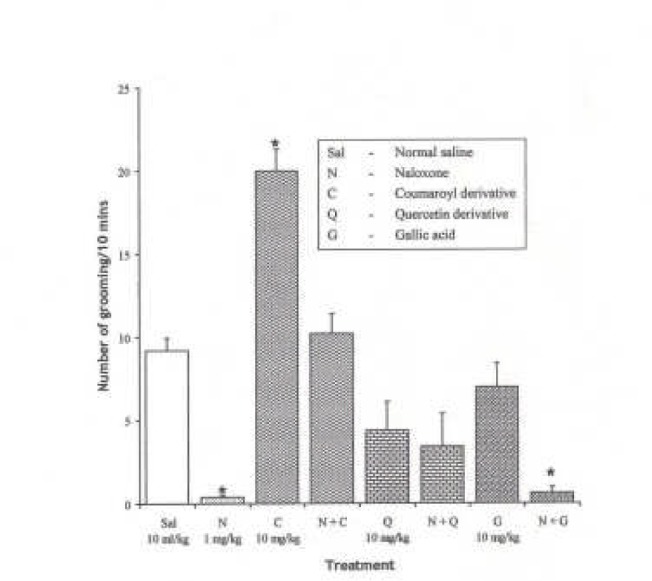
Effect of Pre-treatment with Naloxone (1 mg/kg i.p.) on the Effect of the Pure Isolates from Spondias mombin on NIG in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 26.15, P < 0.001. Naloxone (1 mg/kg i.p.) decreased NIG in mice (P < 0.05). Pre-treatment with Naloxone (1 mg/kg i.p.) did not affect the effect of the isolates (Coumaroyl, Quercetin and Gallic acid) on the NIG behaviour in mice (P > 0.05).
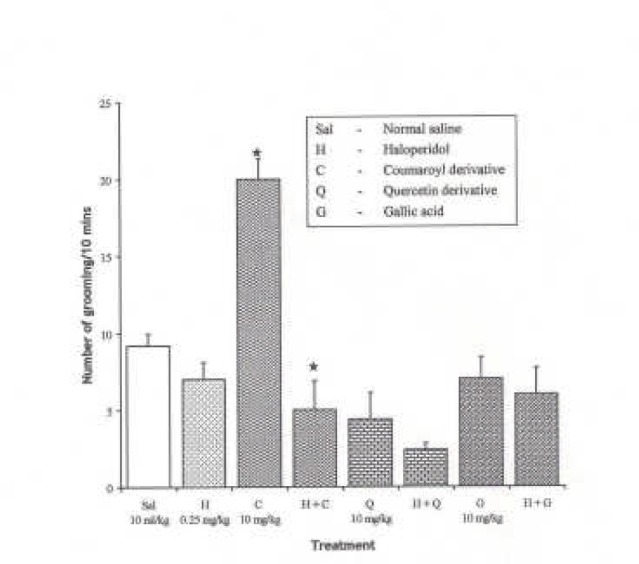
Effect of Pre-treatment with Haloperidol (0.25 mg/kg i.p.) on the Effect of the Pure Isolates from Spondias mombin on NIG in Mice
One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 15.64, P < 0.001. Haloperidol (0.25 mg/kg i.p.) had no effect on NIG in mice (P > 0.05). Pre-treatment with haloperidol (0.25 mg/kg i.p.) reversed the Coumaroyl-induced increase in NIG in mice, but had no significant effect on the Quercetin and Gallic acid-induced effect on the NIG behaviour in mice.
Discussion
It was reported in this study that novelty-induced rearing was decreased by the ethanolic extract of Spondias mombin. Among the fractions of the ethanolic extract, ethylacetate fraction produced the highest inhibitory effect. This result suggests that the active principle responsible for the suppression of NIR behaviour in mice is better extracted in ethanol and fractionated in ethylacetate. The decrease in rearing and grooming behaviours in mice by the ethanolic extract of the spondias mombin suggests a CNS depressant activity (Fujimori, 1965; Lu Ming-Chin, 1998).
Though the ethylacetate fraction of the Spondias mombin decreased novelty induced rearing NIR in mice, the ethylacetate sub-fractions produced different effects on NIR in mice. While the S2 subfraction had stimulatory effect on NIR, sub fractions S1, S3 – S7 were inhibitory to NIR behaviour in mice. Similarly, the three isolated compounds produced different effects on the NIR. While Coumaroyl derivative did not have any significant effect on NIR, the Quercetin and Gallic acid decreased NIR behaviour in mice. This finding suggests that Spondias mombin leaves contain different compound(s) with both stimulatory as in (S2) and suppressant as in (S1, S3–S7) on NIR in mice. However, the compounds with suppressant effect are present in a higher number of sub-fractions. This probably explains the gross suppressant effect of the ethanolic extract of Spondias mombin leaves on NIR.
Complex explorative behavioural paradigms like the novelty induced behaviours are regulated by multiple neurotransmitter systems, including adrenergic, cholinergic, opioid and dopaminergic pathways (Rodgers and Deacon, 1979; Bowman and Rand, 1980; Gispen et al, 1988; Watling, 1998; Rang et al, 2003). Hence, the possible involvement of these neurotransmitter systems in the neurobehavioral effects of Spondias mombin fractions and isolated compounds on novelty induced rearing and grooming was explored using pharmacological tools.
An α2-adrenoreceptor blocker that selectively facilitates noradrenaline release, yohimbine (Doxey et al, 1977) and propranolol, a β-adrenergic blocker (Orzack and Branconnier, 1973) singly significantly decreased NIR relative to saline control in mice. Although the Coumaroyl alone had no significant effect on NIR relative to saline control, it reversed the inhibitory effect of yohimbine and propranolol on NIR. This suggests that the Coumaroyl derivative pocess α2 and β-receptors agonist activity.
While Quercetin and Gallic acid singly significantly suppressed NIR relative to saline control, they potentiated the inhibitory effect of yohimbine. Pretreatment with propranolol had no significant effect on Quecertine and Gallic acid on NIR. This result suggests that the Quercetin and the Gallic acid facilitate the inhibition of α2 receptors. The α2 receptors may be involved in the mechanism of neurobehavioral effect of Quercetin and the Gallic acid, while the β-receptors appear to play no role in the inhibitory effect of Quercetin and Gallic acid derivatives on NIR.
A muscarinic cholinergic receptor antagonist, atropine (Pereiro, 1996), alone did not significantly alter NIR relative to saline control. Pretreatment with atropine produced no significant effect on Quercetin-induced suppression of NIR while it potentiated Gallic acid-induced suppression of NIR. This result suggests that blockade of muscarinic receptors potentiated Gallic acid induced suppression of NIR. This suggests that muscarinic receptors may be relevant in the mechanism of neurobehavioral effects of Gallic acid.
A specific opiate antagonist, naloxone (Czech et al, 1983) alone, significantly suppressed NIR relative to saline control. Coumaroyl had no significant effect on the effect of naloxone, while pretreatment with naloxone potentiated Quercetin and Gallic acid-induced suppression of NIR. This result suggests that blockade of µ-opioid receptors potentiated Quercertine and Gallic acid induced suppression of NIR. Quercetin and Gallic acid are suggested to facilitate blockade of µ-opioid receptor.
A D2 antagonist, haloperidol (Watling, 1998; Rang et al, 2003) alone, significantly suppressed NIR relative to saline control. Coumaroyl significantly reversed the haloperidol-induced suppression of NIR. Pretreatment with haloperidol significantly potentiated the Gallic acid-induced suppression of NIR. This suggests that Coumaroyl facilitates dopaminergic transmission while the Gallic acid suppresses dopaminergic transmission.
The crude extract of Spondias mombin, the ethylacetate and butanolic fractions exhibited a biphasic (increased and decreased) effect depending on the doses on NIG in mice. The lower doses of the ethanolic extract and the ethylacetate fraction produced an increase while the highest dose decreased NIG. This pattern is similar to the effect of diazepam on NIG (Komorowska and Pellis, 2004). The lower doses of butanolic fraction produced an inhibitory effect on NIG while the highest dose increased NIG.
The Coumaroyl increased NIG, while Quercetin decreased NIG relative to saline control. The Gallic acid had no effect on NIG relative to saline control. The possible involvement of different neurotransmitter systems in the behavioural effects of pure isolates of Spondian mombin on novelty-induced grooming was evaluated using pharmacological tools.
Yohimbine, a α2-adrenoreceptor blocker that selectively facilitates noradrenaline release (Doxey et al, 1977)-induced increase in NIG in mice was reversed by Coumaroyl, Quercetin and Gallic acid. This result suggests that Coumaroyl, Quercetin and Gallic acid facilitate α2 receptor function to regulate grooming behaviour.
A β-adrenergic blocking agent, propranolol (Orzack and Branconnier, 1973), inhibits NIG in rats relative to saline control. The propanolol-induced inhibition of NIG was reversed by Coumaroyl and Gallic acid, while Quercetin had no significant effect on propranolol-induced inhibition of NIG. This result suggests that β receptors are implicated in the neurobiological behavioural effects of the Coumaroyl and the Gallic acid.
A muscarinic cholinergic receptor antagonist, atropine (Pereiro, 1996), alone increased NIG in mice. Pretreatment with atropine reversed the Coumaroyl-induced increase in NIG, while Quercetin and Gallic acid reversed the atropine-induced increase in NIG. This result suggests that the Coumarol, Quercetin and Gallic acid facilitate cholinergic transmission.
A specific opiate antagonist, naloxone (Czech et al, 1983), decreased NIG relative to saline control. Pretreatment with naloxone reversed the Coumaroyl-induced increase in NIG, potentiated the Gallic acid derivative-induced suppression of NIG but had no effect on Quercetin induced suppression of NIG. This result suggests that blockade of µ-opioid receptor reversed the effect of Coumaroyl derivative but potentiated the effect of Gallic acid derivative. Hence, Coumaroyl derivative may be facilitating µ-opioid receptor while the Gallic acid derivate may be suppressing the µ-opioid receptor.
A D2 antagonist, haloperidol (Watling, 1998; Rang et al, 2003), had no effect on NIG in mice. Pretreatment with haloperidol reversed the effect of Coumaroyl but had no effect on Quercetin and Gallic acid suggesting that NIG behaviour effect of Coumaroyl may be mediated via dopaminergic transmission.
These results indicate that the isolated compounds displayed different effects on different receptors on the different paradigms explored. The Coumaroyl had fairly consistent facilitatory effects on the α2, β adrenergic and D2 receptors in both of the paradigms of explorative behaviours studied. It had an inconsistent facilitatory effect on the muscarinic receptors which is noticeable in the NIG, but absent in the NIR paradigm. The effect on the µ-opioid receptor across the two paradigms was also mixed, being facilitatory in NIG, but absent in the NIR. It is thus suggested that the adrenergic and the dopaminergic systems are strongly linked with the neural mechanism of psychotropic effect of the Coumaroyl.
The Quercetin had a fairly consistent inhibitory effect on the opioid transmission, while its effect on the α2-adrenoreceptor was not consistent, being stimulatory in the NIG but inhibitory in the NIR paradigm. It is thus suggested that among the neurotransmitter systems explored in this study, the opioid system is most likely to be involved in the neural mechanism of the behavioural effect of the Quercetin.
The effect of the Gallic acid on the neurotransmitter systems explored in this study is consistently inhibitory in the NIR behaviour, while it facilitated adrenergic and muscarinic transmission in the NIG behaviour. It is thus suggested that the Gallic acid be studied on more behavioural paradigms to ascertain the neurotransmitter system through which it exerts its behavioural effects.
Conclusion
In conclusion, this study implied that the effect of the ethylacetate fraction on gross exploratory behaviours in mice is more expressed than the butanolic fraction extract. This suggests that the polar nature of the Spondian mombin constituents is responsible for these biological activities.
Coumaroyl, Quercetin and Gallic acid displayed different effects on different receptors on the different paradigms explored. Facilitation of adrenergic and dopaminergic transmission is suggested to be strongly involved in the neural mechanisms of the effect of Coumaroyl, while inhibition of opioid transmission was strongly linked with the neural mechanism of behavioural effect of Quercentin.
Fig. 16.
Effect of Pre-treatment with Yohimbine (l mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 37.85, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Coumaroyl (10 mg/kg i.p.) increased NIG and abolished the effect of yohimbine (1 mg/kg i.p.) in NIG. Quercetin decreased NIG alone and had no effect when pre-treated with yohimbine. Gallic acid (10 mg/kg i.p.) had no effect alone but reversed the effect of yohimbine.* indicates significant difference from control. P < 0.05 (SNK test)
Figure 17.
Effect of Pre-treatment with Propranolol (l mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 35.12, P < 0.001. Each bar is mean ± S.E.M. (n = 6) Propranolol (l mg/kg i.p.) decreased NIG in mice. Coumaroyl (10 mg/kg i.p.) increased NIG which was reversed by propranolol. Quercetin (10 mg/kg i.p.) decreased NIG and had no effect on the inhibitory action of propranolol on NIG. Gallic acid (10 mg/kg i.p.) had no effect on propranolol. * indicates significant difference from control. P < 0.05 (SNK test)
Figure 18.
Effect of Pre-treatment with Atropine (0.5mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 15.21, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Atropine (0.5 mg/kg i.p.) increased grooming in mice. Coumaroyl (10 mg/kg i.p.) increased NIG that was abolished by atropine. Quercetin (10 mg/kg i.p.) decreased NIG and no effect on atropine. Gallic acid (10 mg/kg i.p.) had no effect alone and decreased NIG when pre-treated with atropine. * indicates significant difference from control. P < 0.05 (SNK test)
Figure 19.
Effect of Pre-treatment with Naloxone (l mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 26.15, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Naloxone (l mg/kg i.p.) decreased NIG in mice. Coumaroyl (10 mg/kg i.p.) increased NIG that was abolished by Naloxone. Quercetin (10 mg/kg i.p.) decreased NIG and had no effect on Naloxone. Gallic acid (10 mg/kg i.p.) had no effect on NIG and decreased NIG when pre-treated with Naloxone. * indicates significant difference from control. P < 0.05 (SNK test)
Figure 20.
Effect of Pre-treatment with Haloperidol (0.25 mg/kg i.p.) on the Pure Isolates from Spondias mombin Extract on Novelty-Induced Grooming (NIG) Behaviour in Mice. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 15.64, P < 0.001. One-way ANOVA revealed that there is significant difference between various treatment groups; F(7, 33) = 15.64, P < 0.001. Each bar is mean ± S.E.M. (n = 6). Haloperidol (0.25 mg/kg i.p) had no effect on NIG in mice. Coumaroyl (10 mg/kg i.p.) increased and had no effect on the effect of haloperidol. Quercetin decreased NIG and haloperidol had no effect on Quercetin derivative. Gallic acid (10 mg/kg i.p.) had no effect on NIG and no effect on haloperidol. * indicates significant difference from control. P < 0.05 (SNK test)
References
- 1.Ajayi A A, Ukponmwan O E. Evidence of angiotensin II and Endogenous opiod modulation of novelty induced rearing in the rat. African Journal of Medicine and Medical Sciences. 1994;23:287–290. [PubMed] [Google Scholar]
- 2.Ayoka A O, Akomolafe R O, Iwalewa E O, Ukponmwan O E. Studies on the anxiolytic effects of Spondias mombin L. (Anacardiaceae) extracts. African Journal of Traditional, Complementary and Alternative Medicine. 2005;2(2):153–165. [Google Scholar]
- 3.Ayoka A O, Akomolafe R O, Iwalewa E O, Akanmu M A, Ukponmwan O E. Sedative, antiepileptic and antipsychotic effects of Spondias mombin L. (Anacardiaceae) in mice and rats. Journal of Ethnopharmacology. 2006;103:166–175. doi: 10.1016/j.jep.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Bolles R C. Grooming behaviour in rat. Journal of Complimentary Physiology and Psychology. 1960;53:306–310. doi: 10.1037/h0045421. [DOI] [PubMed] [Google Scholar]
- 5.Bowman W C, Rand M J. Humoral mechanism in the central nervous system. In: Bowman W C, Rand M J, editors. textbook of Phamacology. 2nd Edition. Oxford, London: Blackwell Scientific Publication; 1980. pp. 14–22. [Google Scholar]
- 6.Castner J L, Timme S L, Duke J A. A Field Guide to Medicinal and Useful Plants of the Upper Amazon. Gainsville, FL: Feline Press; 1998. [Google Scholar]
- 7.Cessana R, Ciprandi C, Borsini F. The effect of BIMT 17, a new potential antidepressant in the forced swimming test in mice. Behavioural Pharmacology. 1995;6:688–694. [PubMed] [Google Scholar]
- 8.Coates N J, Gilpin M L, Gwynn M N, Lewis D E, Milner P H, Spear S R, Tyler J W. SB202742 a novel beta-lactamase inhibitor isolated from Spondias mombin. Journal of Natural Products. 1994;57:654–657. doi: 10.1021/np50107a016. [DOI] [PubMed] [Google Scholar]
- 9.Corthout J, Pieters L A, Claeys M, Vanden Berghe D A, Viletinck A J. Antibacterial and molluscicidal phenolic acid from Spondia mombin. Planta Medica. 1994;60:460–463. doi: 10.1055/s-2006-959532. [DOI] [PubMed] [Google Scholar]
- 10.Czech D A, Stein E A, Blake M J. Naloxone-induced hypodipsia: a CNS mapping study. Life Science. 1983;33(8):797–803. doi: 10.1016/0024-3205(83)90786-5. [DOI] [PubMed] [Google Scholar]
- 11.Doxey J C, Smith C F C, Walker J M. Selectivity of blocking agents for pre and postsynaptic α-adrenoceptors. British Journal of Pharmacology. 1977;60:91–96. doi: 10.1111/j.1476-5381.1977.tb16752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimori H. Potentiation of barbital hypnosis as an evaluation method for central nervous system depressant. Psychopharmacology. 1965;7:374–377. doi: 10.1007/BF00403761. [DOI] [PubMed] [Google Scholar]
- 13.Gispen W H, Colbern D L, Spruijt B M. Molecular transduction mechanisms in ACTH-induced grooming. Psychopharmacology Series. 1988;4:215–231. doi: 10.1007/978-3-642-73223-2_16. [DOI] [PubMed] [Google Scholar]
- 14.Glantz A S. Primer of Biostatistics (Version 301) McGraw-Hill Inc; 1992. [Google Scholar]
- 15.Koek W, Wood J H, Ornstein P. A simple and rapid method for screening similarities among directly observable behavioural effects of drugs. Psychopharmacology. 1987;91:297–304. doi: 10.1007/BF00518181. [DOI] [PubMed] [Google Scholar]
- 16.Komorowska J, Pellis S M. Regulatory mechanisms underlying novelty-induced grooming in the laboratory rat. Behavioural Processes. 2004;67(2):287–293. doi: 10.1016/j.beproc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Labella F S, Punsky C, Havlicek V. Morphine derivative with diminished opiate receptor potency show enhanced control excitatory activity. Brain Research. 1979;174:263–271. doi: 10.1016/0006-8993(79)90849-7. [DOI] [PubMed] [Google Scholar]
- 18.Lu Ming-Chin. Studies on the sedative effect of cistanche deserticola. Journal of Ethnopharmacology. 1998;59:1661–1665. doi: 10.1016/s0378-8741(97)00108-6. [DOI] [PubMed] [Google Scholar]
- 19.Orzack M H, Branconnier R. CNS Effects of Propranolol in Man. Psychopharmacologia (Berl) 1973;29:299–306. doi: 10.1007/BF00429277. [DOI] [PubMed] [Google Scholar]
- 20.Pereiro L M P. Atropine. Practical Procedures. 1996;6(5):1. [Google Scholar]
- 21.Rang H P, Dale M M, Rilter J M, Moore P K. Pharmacology. 5th Edition. Churchill: Livingstone; 2003. [Google Scholar]
- 22.Rodgers R J, Deacon R M. Effect of Naloxone on the behaviour of rats exposed to a novel environment. Psychopharmacology (Berl) 1979;65(1):103–105. doi: 10.1007/BF00491988. [DOI] [PubMed] [Google Scholar]
- 23.Sanberg P R, Hangenmeyer S H, Henault M A. Automated measurement of multivariate locomotor behaviour in rodents. Neurobehaviour, Toxicology and Teratology. 1985;7:87–94. [PubMed] [Google Scholar]
- 24.Watling K J. Overview of central nervous system receptors. In: Watling Keith J, editor. The RBI Handbook of Receptor Clarification and signal Transduction. 3rd ed. Natick, MA: RBI; 1998. pp. 2–45. [Google Scholar]


