Abstract
Alendronate belongs to a class of drugs called bisphosphonates. Bisphosphonates (BP) therapy is a vital option to reduce the risk of bone fracture in people who suffer from osteoporosis. Yet, bisphosphonate have displayed several side effects. Lepidium sativum (LS) seeds have been used in traditional folk medicine to heal fractured bones. However, there is a dearth of information on the impact of LS on bone metabolism especially in cases of glucocorticoids induced osteoporosis. Therefore, the aim of the study was to compare the biochemical bone markers and histological responses of LS alone (6 g of LS seeds in diet daily, n=8), ALD (alendronate, 70 mg/kg s.c.; n=8) alone, or LS and ALD combined in a rat model of glucocorticoid-induced osteoporosis (GIO) by injecting rats with methylprednisolone 3.5 mg/kg per day for 4 weeks. Serum calcium (Ca), albumin, phosphorus (P), bone-specific alkaline phosphatase (b-ALP), and tartrate-resistant acid phosphatase (TRAP) were measured 4 weeks after induction of GIO. GIO-group showed significantly increased serum TRAP and decreased b-ALP. GIO-group also showed significantly decreased serum P and unaltered Ca concentrations. Histological examination of GIO-group tibia bones indicated an osteoporotic change and a concomitant decrease in percentage of trabecular area or bone marrow area (PTB) in the proximal femoral epiphysis. Treatment with either LS and/or ALD ameliorated the above mentioned changes with variable degrees, with a net results of enhanced serum calcium, bone architecture, PTB, b-ALP and decreased TRAP in LS and LS+ALD groups compared to that of animals treated with alendronate alone. In conclusion, our findings present evidence supporting the potential benefits of LS in reducing the burden of GCs on bone health.
Keywords: Osteoporosis, Bisphosphonates, Lepidium sativum, bone turnover markers
Introduction
Osteoporosis is a skeletal disorder in which bone strength is compromised, resulting in an increased risk of fracture (Giangregorio et al., 2009). Sustaining a hip fracture is one of the most serious consequences of osteoporosis. Nearly one third of those who sustain osteoporotic hip fractures enter nursing homes within the year following the fracture, and one person in five dies within one year of experiencing an osteoporotic hip fracture (Rackoff and Rosen 1998).
Glucocorticoids are important in the management of chronic, noninfectious, inflammatory and rheumatic diseases (Goulding, 2004, Meduri et al., 2002, Ono et al., 2006). Bone loss is one of the most devastating side effects of glucocorticoids because they inhibit calcium transport, cause secondary hyperparathyroidism, hypogonadism, and impairment of osteoblast function (Patschan et al., 2001, Rackoff and Rosen 1998). In addition, pre-menopausal women requiring glucocorticoids are at a significant risk of developing glucocortiocoid-induced osteoporosis (Ledwich and Clarke 2009, Silverman and Lane 2009).
Bone loss can be minimised through proper nutrition, weight-bearing exercise, calcium and vitamin D supplementation, and, where indicated, bisphosphonate treatment (Garnero 2008, Siris et al., 2009). Bisphosphonate (alendronate) therapy is a vital option in the prevention and treatment of GIO (de Nijs et al., 2006). However, these agents have had disappointing results in clinical trials (Hoes et al., 2010, Jacobs et al., 2007). In addition, animal studies with bisphosphonates have displayed maternal toxicity, foetal underdevelopment, embryo-lethality, hypocalcaemia and skeletal retardation during pregnancy (Hassen-Zrour et al., 2009, Minsker et al., 1993). Bisphosphonates are therefore contra-indicated in pregnancy and have an FDA category C pregnancy risk (McNicholl and Heaney 2009). Their use in premenopausal women prior to conception may also pose a teratogenic risk because bisphosphonates remain in mineralised bone for several years. Consequently, a clinical dilemma exists in treating and preventing GIO in pre-menopausal women.
Herbal medication has been and remains commonly used instead of chemical drugs because of its minor side effects. Lepidium sativum L. (LS), or what is called locally “hab arachad”, is a native shrub belonging to Brassicaceae family, wildly grown in the Middle East where LS is largely recommended by traditional herbal healers for hypertension, diabetes control, renal disease and phytotherapy. The LS seeds are well known in Saudi Arabia and some other Arab countries as a good alternative medication for fracture healing (Juma, 2007). This property has attracted our interest to study its ability to treat GIO in rats.
Analysis of Lepidium sativum leaves showed that it consists of water, protein, fat, carbohydrate, mineral matter, calcium and phosphorus, trace elements: iron nickel cobalt and iodine; vitamins; vitamin A, thiamine, riboflavin, niacin and ascorbic acid. Cooked leaves contain vitamin A, thiamine, riboflavin, niacin, and ascorbic acid, moisture, protein, fat, ash, and sulphur. The seeds contain an alkaloid, glucotropaeloin, sinapin, sinapic acid, mucilaginous matter and uric acid. The oil contents of saturated and unsaturated acids are palmitic, stearic, arachidic behenic lignoceric, loeic, and linolenic. The usaponifiable matter contains β-sitosterol and α-tocopherol (Gokavi et al., 2004).
Many studies have assessed the influence of L sativum seeds (Juma, 2007) and roots (Zhang et al., 2206; Gonzales, 2012) on bone health. However, there is a dearth of information on the impact of Lepidium sativum on bone metabolism, especially in cases of glucocorticoids induced osteoporosis. In addition there is an urgent need to find out new modalities other than bisphosphonates therapy for osteoporosis due to its unsafe side effects. Therefore, we aimed to compare the biochemical effects of Lepidium sativum and alendronate on glucocorticoid-induced osteoporosis in adult female rats.
Materials and Methods
Experimental Animals
Forty female Wistar rats (40 female rats, weight 100–125 g) were housed in temperature and humidity controlled conditions and were fed standard rat chow containing 53% vegetable starch, 4.5% fat, 22% protein, 0.36% sodium, and 1.08% potassium prior to experiments. Animals were divided randomly into 5 groups (8 rats/group), and all procedures were conducted according to the guidelines of the Animal Scientific Procedures Act (United Kingdom, 1986). The animals were housed in stainless steel cages at room temperature of 27±2°C. All the test and control animals had free access to food and tap water till the end of the experiment. Control rats were fed with a normal diet, but the test animals had, in addition, 6 g of L. sativum seeds in their food daily. These seeds were obtained from the local market of the type grown in the Al-Qaseem area in Saudi Arabia (Juma, 2007). Rats were weighed weekly to detect any change in body weight.
Osteoporosis was induced in rats as previously reported (Hulley et al., 2002). Briefly, all rats except controls were injected subcutaneously (s.c.) with methylprednisolone 3.5 mg/kg per day for 4 weeks and were divided into the following groups: (GC) group included rats that were left without treatment to serve as osteoporosis control, (GC+ALD) group included osteoporotic rats injected subcutaneously with 70 mg/kg body weight of Alendronate (2 injections/week) (Sass et al., 1997), (GC+LS) group included osteoporotic rats fed 6 g LS seeds in diet daily, GC+ (LS+ALD) group were osteoporotic rats treated with alendronate and were fed 6g LS seeds in diet daily. Control rats were given a daily subcutaneous injection of saline (HC). Blood samples were taken from the retro-orbital sinus under light ether anaesthesia after an overnight fast in heparinzed capillary tubes and blood serum were separated and stored at −20°C until further analysis.
Biochemical Measurements
Blood serum samples were analysed for calcium (Ca), phosphorus (P), albumin (ALB) concentrations using an automated analyser (Cobas Diagnostic System, Hoffman La Roche Inc., Indianapolis, IN). Serum bone-specific alkaline phosphatase (b-ALP) concentrations were measured by immunoassay using the Access Ostase Assay (Beckman Access, Beckman Coulter Inc., Fullerton, CA, USA). Serum tartrate-resistant acid phosphatase (TRAP) concentrations were measured coloremetrically using commercially available test kit according to manufacturer guidelines (Cayman Chemical, Ann Arbor, MI, USA). The kit included L-tartrate, an inhibitor of non-tartrate resistant APs.
Measurement of Bone Ash and Ca Content
Each left femur was desiccated in different baths of alcohol and dried overnight at 100°C. The dry weight was then determined. Next, the vertebra was incinerated for 12 hours at 1000°C, and the ash weight was determined. The measurements obtained were expressed as the percentages of the ash amounts relative to the dry weight of the vertebra. The ash was then solubilized in 6 N HCl and analysed for Ca content as described previously (Shahnazari et al., 2009). Values were expressed as milligram of Ca per cubic centimetre of bone volume. Bone volume was measured in the right femur using Archimedes' principle (Kasra and Grynpas 1995).
Histopathological Examination
The right tibia was dissected from each animal, weighed, fixed in 70% (v/v) ethanol and embedded in paraffin wax for cross sectioning. Five micron sections were stained with Haematoxylin and Eosin (H&E) and examined under light microscopy. The trabecular bone tissue of the proximal epiphysis of the femur was photographed at 20X magnification. The cross-sectional total, bone and marrow areas were measured using ImageJ software (http://rsb.info.nih.gov/ij/), and the percentage of trabecular area/bone marrow area (PTB) was than calculated.
Statistical Analyses
Results were expressed as mean ± SD. All experimental data were analysed using one-way analysis of variance with Dunnett's test. Values of P < .05 were considered statistically significant.
Results
The initial weights and final weights are shown in Table 1. At the beginning and end of the experiment, there was no difference in weight between controls and GIO rats (p > 0.05). All animals increased their weight during the experiment. A significant increase in the body weight gain of rats in group received LS (p<0.05) was found compared with GIO group.
Table 1.
Initial and final weights in the controls and other groups. Values are means (for 8 rats) with their standard deviation.
| Group | initial weights(g) | final weights(g) |
| Control rats | 270 ± 23 | 289 ± 31 |
| GIO rats | 271 ± 28 | 280 ± 26 |
| GIO+LS rats | 273 ± 16 | 309 ± 21* |
| GIO+ALD rats | 271 ± 25 | 291 ± 29 |
| GIO LS+ALD rats | 274 ± 17 | 299 ± 28 |
P<0.05 compared with GIO rats.
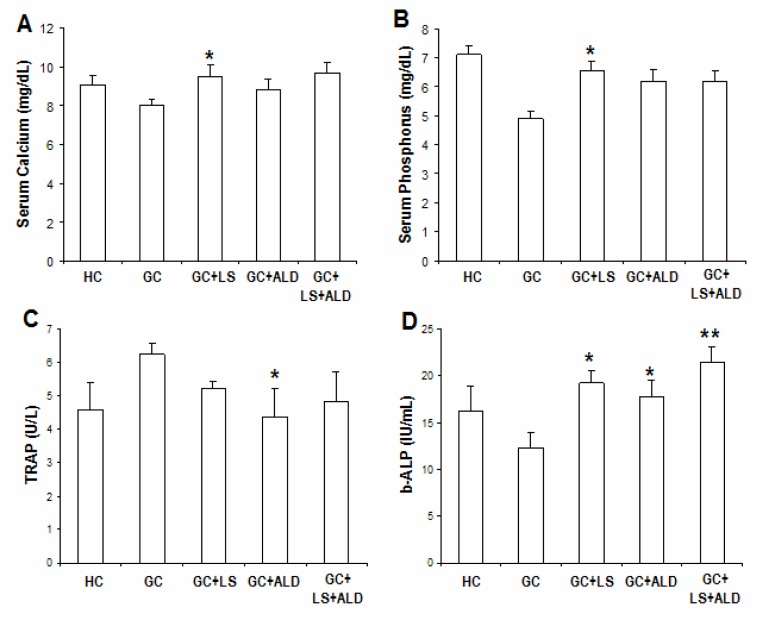
The mean concentrations of serum Ca in GIO group decreased in comparison to other groups; however, it did not reach statistical significance (Figure 1A). In contrast, serum phosphorus (P) concentrations were significantly lower in GIO rats compared with other groups (P<0.0001) (Figure 1B). Treatment with LS significantly raised serum Ca and P levels (P<0.05).
Figure 1.
Effects of different treatments on biochemical parameters studied after 4 weeks of osteoporosis induction by glucocorticoids
Values are expressed as means ± SD (n=8). *P<0.05; **P<0.01 as compared with GIO.
Effect of LS supplementation on bone metabolic markers revealed a significant increase (P<0.05) in the mean concentration of serum TRAP, a parameter of bone resorption in GIO-group when compared to HC and treatment groups. Administration of ALD significantly (P<0.01) decreased serum TRAP. Whereas, no significant difference was found in TRAP between LS and LS+ALD treated groups (Figure 1C).
The mean concentration of serum b-ALP, a parameter of bone formation, in GIO-group was decreased significantly (P<0.05) in comparison with untreated HC group. GIO rats showed significant decrease (P<0.05) in b-ALB compared with HC group. The mean concentration of serum b-ALP was increased significantly in LS-fed and ALD treated groups in comparison with GIO group (bath at P<0.05). The difference in b-ALP concentrations between group treated with LS alone and group treated with LS plus ALD was not significant (Figure 1D).
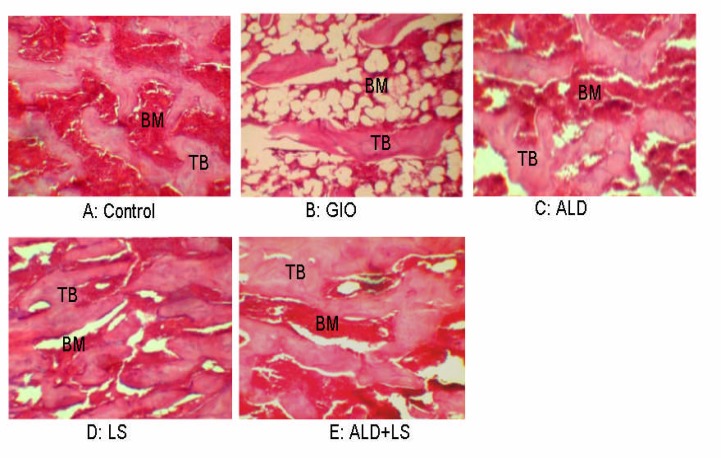
Effects of methylprednisolone acetate and LS supplementation on histological appearance and trabecular bone volume of tibia bone were evaluated too. Abnormal histological appearance of the tibia bone was observed in GIO-rats, where the inner cancellous bone trabeculae lost their normal architecture and appeared as discontinuous bony ossicles separated by widened bone marrow spaces (Figure 2-b). LS-fed rats revealed marked improvement as compared to those of the GIO-rats. The cortical bone thickness was very similar to the HC control group. The cancellous bone trabeculae partially regained near normal structure and appeared more continuous with less widened bone marrow spaces in LS-fed and ALD+LS groups (Figure 2C, 2D).
Figure 2.
Effects of different treatments on the epiphiseal area of the femur A: Osteocytes reside in their lacunae in between the irregular bone lamellae where bone marrow (BM) spaces are seen between the trabeculae (TB); B: loss of the normal architecture of trabeculae bone with widening of bone marrow spaces, (C, D) both ALD and LS treated groups showed denser TB than GIO group, (E) rats treated with both ALD and LS showed the greatest bone volumes, which appeared to be even greater than that of control group (H&E, 20X).
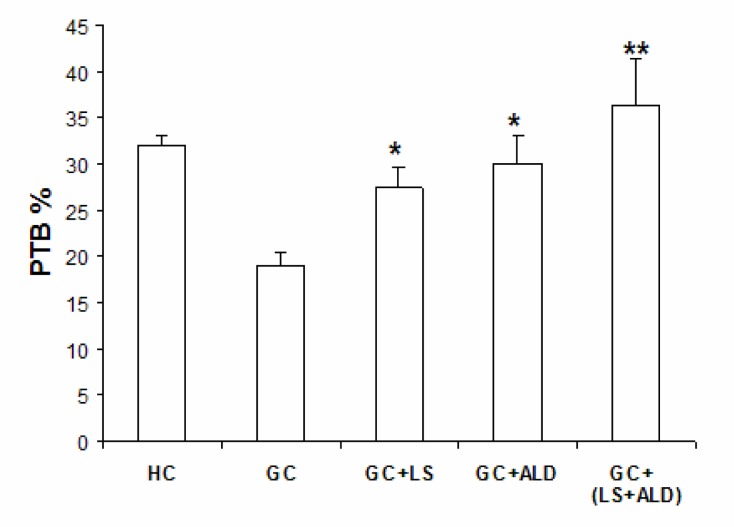
The trabecular bone volume and percentage of trabecular bone (PTB) were calculated and presented in Figure 3. PTB was decreased significantly (P< 0.05) in GIO-group in comparison with HC group. On the other hand, the mean PTB showed a significant increase in LS-group and LS plus ALD groups as compared with the GIO-rat group. In addition, PTB appeared to be even greater than that of control group; however, the difference did not reach statistical significance (P>0.05).
Figure 3.
Effects of different treatments on the percentage of trabecular area/bone marrow area (PTB) of the femur Values are expressed as means ± SD (n=8). *P<0.05; **P<0.01 as compared with GIO.
Discussion
The bone is under constant remodelling, with repeated cycles of bone resorption by osteoclasts followed by deposition of new bone by osteoblasts. This process ensures the repairing of microfractures as well as remodels the bony architecture in response to stress (Manolagas, 2000). Osteoporosis occurs when bone resorption exceeds bone formation, resulting in decreased bone density, degenerated bone microarchitecture and increased risk of fracture (Dempster 2002, Legrand et al., 2000). Previous studies indicated that GCs induced-osteoporosis occurs as a result of an immediate and persistent decrease in bone formation and a rapid and transient increase of bone resorption (Dovio et al., 2004).
Bone turnover and bone mineral homeostasis markers have proven useful in screening for fracture risk in elderly patients, assessing therapeutic response to antiresorptive agents, and identifying patients with high bone turnover to predict rapid bone loss (Bonnick and Shulman 2006, Garnero 2008). Therefore, the present study was designed to compare the efficacy of Alendronate in treating osteoporosis with that of LS in an animal model of GIO by analysing serum markers of bone remodelling (b-ALP and TRAP) as well as mineral homeostasis markers (Ca and P).
In vivo studies using short courses of GCs have shown decreased intestinal absorption of calcium (Reid and Ibbertson, 1987) as well as decreased renal reabsorption of calcium and phosphorus (Cosman et al., 1994, Gram et al., 1998). Meanwhile, GCs administration was reported to enhance renal calcium excretion by an increased filtered calcium load secondary to rapidly decreasing bone formation and increasing bone resorption (Schapira et al., 1995). In the present study, serum Ca concentrations did not show a statistically significant difference between GIO group and control group that might occur as a result of increased release of Ca from bone tissues. These findings are in agreement with previous studies (Cosman et al., 1994; Schapira et al., 1995). In addition, our findings of marked decrease in serum P only and not in serum calcium confirm these data and suggest that the unaltered Ca concentrations, while P levels are decreased, may be caused by a concomitant net increment of bone resorption releasing more Ca from osteoporotic bones.
GIO-group demonstrated significantly increased concentrations of serum TRAP in comparison with HC-rats. TRAP is a lysosomal hydrolyser, which has been shown to be released from osteoclasts during bone resorption (Halleen et al., 2006, Marks and Grolman 1987). This finding suggests an increased bone loss which was supported by the osteoporotic changes we found by histological examination of tibia bones (Figure 2-B) and a marked decrease in PTB weight (Figure 3). In addition, we found a 16% reduction (P<0.05) in serum concentrations of b-ALP in GIO-rats, a marker for bone formation. Treatment with either LS and/or Alendronate regained the concentration of serum b-ALP to levels higher than that in HC group (Figure 1D).
It is interesting to note that significant increase in b-ALP concentrations were seen in rats treated with LS and LS plus Alendronate in comparison with that treated with Alendronate only. The tibia bone weights were also found heavier in LS and LS+ALD groups compared to that of animals treated with Alendronate alone. These results indicate that LS suppressed bone resorption in GIO-rats more efficiently than Alendronate alone and may work in a synergistic manner with Alendronate in lessening bone loss in GIO-rats, as indicated with the augmented serum calcium and b-ALP concentrations along with enhanced histological structure of tibia bones (Figure 2-E) and increased PTB (Figure 3).
The positive effect on bone density of LS is probably due to its rich content of calcium (Gokavi et al., 2004), and on its ability to increase serum and liver alpha linolenic acid (ALA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (Diwakar et al., 2008), which have been shown to have beneficial effects on bone (Kruger et al., 1998). These results are in accordance with previously reported benefits of LS seeds that induced a marked influence on fracture healing in rabbits (Juma 2007). Given the fact that the dose used 2% of LS (w/W) and did not produce any adverse effects or mortality in animals (Adam 1999), our findings clearly support their use by people at risk of developing osteoporosis, including patients on GCs therapy and postmenopausal women.
In conclusion, we have demonstrated that co-treatment of ALN and LS, through a short duration (4 weeks), has significantly improved the biochemical bone indices and restored microarchitecture of femurs and vertebral bones of the GC-rats compared to ALD and LS single treatment groups. The combined use of LS and ALD may be a new treatment strategy for preventing bone loss and reversing bone mass and quality in osteoprotic disorders. That deserves further investigations.
Acknowledgement
The authors are grateful to the Deanship of Scientific Research (DSR), King Abdulaziz University for their financial support to the project (no.: 3-87/430).
References
- 1.Adam S E. Effects of various levels of dietary Lepidium sativum L. seeds in rats. Am J Chin Med. 1999;27:397–405. doi: 10.1142/S0192415X99000458. [DOI] [PubMed] [Google Scholar]
- 2.Bonnick S L, Shulman L. Monitoring osteoporosis therapy: bone mineral density, bone turnover markers, or both? Am J Med. 2006;119:S25–S31. doi: 10.1016/j.amjmed.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Cosman F, Nieves J, Herbert J, Shen V, Lindsay R. High-dose glucocorticoids in multiple sclerosis patients exert direct effects on the kidney and skeleton. J Bone Miner Res. 1994;9:1097–1105. doi: 10.1002/jbmr.5650090718. [DOI] [PubMed] [Google Scholar]
- 4.de Nijs R N, Jacobs J W, Lems W F, Laan R F, Algra A, Huisman A M, et al. Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med. 2006;355:675–684. doi: 10.1056/NEJMoa053569. [DOI] [PubMed] [Google Scholar]
- 5.Dempster D W. The impact of bone turnover and bone-active agents on bone quality: focus on the hip. Osteoporos Int. 2002;13:349–352. doi: 10.1007/s001980200038. [DOI] [PubMed] [Google Scholar]
- 6.Diwakar B T, Dutta P K, Lokesh B R, Naidu K A. Bio-availability and metabolism of n-3 fatty acid rich garden cress (Lepidium sativum) seed oil in albino rats. Prostaglandins Leukot Essent Fatty Acids. 2008;78:123–130. doi: 10.1016/j.plefa.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Dovio A, Perazzolo L, Osella G, Ventura M, Termine A, Milano E, et al. Immediate fall of bone formation and transient increase of bone resorption in the course of high-dose, short-term glucocorticoid therapy in young patients with multiple sclerosis. J Clin Endocrinol Metab. 2004;89:4923–4928. doi: 10.1210/jc.2004-0164. [DOI] [PubMed] [Google Scholar]
- 8.Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther. 2008;12:157–170. doi: 10.1007/BF03256280. [DOI] [PubMed] [Google Scholar]
- 9.Giangregorio L, Dolovich L, Cranney A, Adili A, Debeer J, Papaioannou A, et al. Osteoporosis risk perceptions among patients who have sustained a fragility fracture. Patient Educ Couns. 2009;74:213–220. doi: 10.1016/j.pec.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokavi S S, Malleshi N G, Guo M. Chemical composition of garden cress (Lepidium sativum) seeds and its fractions and use of bran as a functional ingredient. Plant Foods Hum Nutr. 2004;59:105–111. doi: 10.1007/s11130-004-4308-4. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales G F. Ethnobiology and ethnopharmacology of Lepidium meyenii (Maca), a plant from the Peruvian Highlands. Evidence-Based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/193496. 193496; 10 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulding N J. The molecular complexity of glucocorticoid actions in inflammation -a four-ring circus. Curr Opin Pharmacol. 2004;4:629–636. doi: 10.1016/j.coph.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Gram J, Junker P, Nielsen H K, Bollerslev J. Effects of short-term treatment with prednisolone and calcitriol on bone and mineral metabolism in normal men. Bone. 1998;23:297–302. doi: 10.1016/s8756-3282(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 14.Halleen J M, Tiitinen S L, Ylipahkala H, Fagerlund K M, Vaananen H K. Tartrate-resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab. 2006;52:499–509. [PubMed] [Google Scholar]
- 15.Hassen-Zrour S, Korbaa W, Bejia I, Saidani Z, Bergaoui N. Maternal and fetal outcome after long-term bisphosphonate exposure before conception. Osteoporos Int Osteoporos Int. 2009;21(4):709–710. doi: 10.1007/s00198-009-0983-1. [DOI] [PubMed] [Google Scholar]
- 16.Hoes J N, Jacobs J W, Hulsmans H M, De Nijs R N, Lems W F, Bruyn G A, et al. High incidence rate of vertebral fractures during chronic prednisone treatment, in spite of bisphosphonate or alfacalcidol use. Extension of the alendronate or alfacalcidol in glucocorticoid-induced osteoporosis-trial. Clin Exp Rheumatol. 2010;28:354–359. [PubMed] [Google Scholar]
- 17.Hulley P A, Conradie M M, Langeveldt C R, Hough F S. Glucocorticoid-induced osteoporosis in the rat is prevented by the tyrosine phosphatase inhibitor, sodium orthovanadate. Bone. 2002;31:220–229. doi: 10.1016/s8756-3282(02)00807-4. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs J W, de Nijs R N, Lems W F, Geusens P P, Laan R F, Huisman A M, et al. Prevention of glucocorticoid induced osteoporosis with alendronate or alfacalcidol: relations of change in bone mineral density, bone markers, and calcium homeostasis. J Rheumatol. 2007;34:1051–1057. [PubMed] [Google Scholar]
- 19.Juma A H. The effects of Lepidium sativum seeds on fracture-induced healing in rabbits. MedGenMed. 2007;9:23. [PMC free article] [PubMed] [Google Scholar]
- 20.Kasra M, Grynpas M D. The effects of androgens on the mechanical properties of primate bone. Bone. 1995;17:265–270. doi: 10.1016/8756-3282(95)00211-u. [DOI] [PubMed] [Google Scholar]
- 21.Kruger M C, Coetzer H, de Winter R, Gericke G, van Papendorp D H. Calcium, gamma-linolenic acid and eicosapentaenoic acid supplementation in senile osteoporosis. Aging (Milano) 1998;10:385–394. doi: 10.1007/BF03339885. [DOI] [PubMed] [Google Scholar]
- 22.Ledwich L J, Clarke K. Screening and treatment of glucocorticoid-induced osteoporosis in rheumatoid arthritis patients in an urban multispecialty practice. J Clin Rheumatol. 2009;15:61–64. doi: 10.1097/RHU.0b013e31819b65bd. [DOI] [PubMed] [Google Scholar]
- 23.Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, et al. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res. 2000;15:13–19. doi: 10.1359/jbmr.2000.15.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Manolagas S C. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 25.Marks S C, Jr, Grolman M L. Tartrate-resistant acid phosphatase in mononuclear and multinuclear cells during the bone resorption of tooth eruption. J Histochem Cytochem. 1987;35:1227–1230. doi: 10.1177/35.11.3655324. [DOI] [PubMed] [Google Scholar]
- 26.McNicholl D M, Heaney L G. The Safety of Bisphosphonate Use in Pre-menopausal Women on Corticosteroids. Curr Drug Saf. 2009;5(2):182–187. doi: 10.2174/157488610790936178. [DOI] [PubMed] [Google Scholar]
- 27.Meduri G U, Tolley E A, Chrousos G P, Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med. 2002;165:983–991. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- 28.Minsker D H, Manson J M, Peter C P. Effects of the bisphosphonate, alendronate, on parturition in the rat. Toxicol Appl Pharmacol. 1993;121:217–223. doi: 10.1006/taap.1993.1148. [DOI] [PubMed] [Google Scholar]
- 29.Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25-regulatory T cells. J Immunol. 2006;176:4748–4756. doi: 10.4049/jimmunol.176.8.4748. [DOI] [PubMed] [Google Scholar]
- 30.Patschan D, Loddenkemper K, Buttgereit F. Molecular mechanisms of glucocorticoid-induced osteoporosis. Bone. 2001;29:498–505. doi: 10.1016/s8756-3282(01)00610-x. [DOI] [PubMed] [Google Scholar]
- 31.Rackoff P J, Rosen C J. Pathogenesis and treatment of glucocorticoid-induced osteoporosis. Drugs Aging. 1998;12:477–484. doi: 10.2165/00002512-199812060-00005. [DOI] [PubMed] [Google Scholar]
- 32.Reid I R, Ibbertson H K. Evidence for decreased tubular reabsorption of calcium in glucocorticoid-treated asthmatics. Horm Res. 1987;27:200–204. doi: 10.1159/000180820. [DOI] [PubMed] [Google Scholar]
- 33.Sass D A, Bowman A R, Yuan Z, Ma Y, Jee W S, Epstein S. Alendronate prevents cyclosporin A-induced osteopenia in the rat. Bone. 1997;21:65–70. doi: 10.1016/s8756-3282(97)00071-9. [DOI] [PubMed] [Google Scholar]
- 34.Schapira D, Linn S, Sarid M, Mokadi S, Kabala A, Silbermann M. Calcium and vitamin D enriched diets increase and preserve vertebral mineral content in aging laboratory rats. Bone. 1995;16:575–582. doi: 10.1016/8756-3282(95)00088-u. [DOI] [PubMed] [Google Scholar]
- 35.Shahnazari M, Martin B R, Legette L L, Lachcik P J, Welch J, Weaver C M. Diet calcium level but not calcium supplement particle size affects bone density and mechanical properties in ovariectomized rats. J Nutr. 2009;139(7):1308–1314. doi: 10.3945/jn.108.101071. [DOI] [PubMed] [Google Scholar]
- 36.Silverman S L, Lane N E. Glucocorticoid-induced osteoporosis. Curr Osteoporos Rep. 2009;7:23–26. doi: 10.1007/s11914-009-0005-4. [DOI] [PubMed] [Google Scholar]
- 37.Siris E S, Selby P L, Saag K G, Borgstrom F, Herings R M, Silverman S L. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122:S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Yu L, Ao M, Jin W. Effect of ethanol extract of Lepidium meyenii Walp. on osteoporosis in ovariectomized rat. Journal of Ethnopharmacology. 2006;105(1–2):274–279. doi: 10.1016/j.jep.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 39.United Kingdom Guidance on the Operation of the Animals (Scientific Procedures) Act (1986) [20 May 2013]. http://www.archive.official-documents.co.uk/document/hoc/321/321.htm.
- 40.Bethesda, Maryland, USA: U. S. National Institutes of Health; 1997–2012. [20 may 2013]. http://imagej.nih.gov/ij/ [Google Scholar]