Abstract
The antioxidant, phytochemical and nutritional properties of acetone, methanol and aqueous extracts of the leaves of Ocimum gratissimum (Linn) were investigated to evaluate the therapeutic and nutritional potential of the leaves of this plant. The antioxidant of the plant extracts were assessed against 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and ferric reducing agent. Total phenolics, flavonoids, flavonols and proanthocyanidins were determined to assess their corresponding effect on the antioxidant activity of this plant. The extracts exhibited DPPH and ABTS.+ radical scavenging activities, which was comparable to butylated hydroxytoluene (BHT). The phytochemical screening revealed the presence of alkaloids, tannins, saponin, steroids, cardiacglycoside, flavonoid, terpenoids and phenol. The proximate analysis confirms that the leaves contain appreciable amount of ash, crude protein, lipids, fibre and carbohydrates. The macro and micro elements and constituents revealed that the leaves contain significant amount of sodium, potassium, calcium, magnesium, iron, zinc, phosphorus, copper, nitrogen, and manganese. This study shows that the leaf can be used as a therapeutic agent and justifies its application in folkloric medicine.
Keywords: Ocimum gratissimum, oxidative stress, polyphenolic, proximate composition, therapeutic activity
Introduction
Ocimum gratissimum (Linn) is a herbaceous plant which belongs to the Labiatae family. The plant is indigenous to tropical areas, especially India, and it is also found in West Africa. In Nigeria, it is found in the savannah and coastal areas (Nadkarni and Nadkarni, 2000). It is traditionally used in folklore medicine for the treatment of different diseases including upper respiratory tract infections, diarrhoea, headache, conjunctivitis, skin disease, pneumonia, tooth and gum disorder, fever, and as mosquito repellents (Okigbo and Mmeka, 2006). Many studies have reported the compositional evaluation and functional properties of various types of edible plants in use in developing countries (Hassan and Umar, 2006; Edeoga et al., 2006). For example, Amaranthus asper and Rumex sagittatus Thunb, had been studied by Jimoh et al. (2010a,b). However, works reporting compositional evaluation and functional properties of various types of edible plants use in developing countries abound in literature studies need to be carried out. Phytochemicals are naturally occurring and biologically active plant compounds that have potentials disease inhibiting ability (Akubugwo et al., 2007).
Medicinal plants contain several pharmacologically active compounds that may act individually, additively or in synergy to improve health (Azaizeh et al., 2003; Gurib-Fakim, 2006). The use of herbal remedies as alternative medicine plays a significant role in the cultures and beliefs of indigenous populations in Africa (Hutchings et al., 1996). Some important chemical substances found in plants are alkaloids, carbon compounds, hydrogen, nitrogen, glycosides, essential oils, fatty oils, resins, mucilage, tannis, gums and others (Edeoga et al., 2006). Most of these are potent bioactive compounds found in medicinal plant parts that can be used for therapeutic purpose or which are precursors for the synthesis of useful drugs (Sofowora, 1993). Antioxidants protect other molecules from oxidation when they are exposed to free radicals and reactive oxygen species which have been implicated in the aetiology of many diseases (Halliwell and Gutteridge, 1992; Behera et al., 2006). Free radicals in the body contribute to more than one hundred disorders in humans, including atherosclerosis, arthritis, ischemia and reperfusion injury of many tissues, central nervous system injury, gastritis, cancer and acquired immune deficiency syndrome (Pourmorad et al., 2006). The compounds attack the unsaturated fatty acids in the biomembranes resulting in membrane fluidity (Dean and David, 1993). Free radicals have also been implicated in the causation of several degenerative diseases, and compounds that can scavenge free radicals have great potential in ameliorating these disease processes (Behera et al., 2006).
Recently, there has been an upsurge of interest in the therapeutic potential of medical plants as antioxidants in reducing free radical induced tissue damages. Plants containing flavonoids have been reported to possess strong antioxidant properties (Behera et al., 2006; Tripathi et al., 2007). Ocimum gratissimum has been established to provide various culinary and medicinal properties. These medicinal properties exert bacteriostatic and bacteriocidal effects on some bacteria which have been reported by previous authors (Effraim et al., 2000; Okigbo and Mmeka, 2006).Therefore, this study was designed to investigate the nutritional quality, total polyphenolic contents, antioxidant potential of leaves extracts of Ocimum gratissimum and to explore its usefulness in traditional folklore medicine.
Material and Methods
Plant materials
Fresh leaves of O. gratissimum were collected from a local farm in Benin City, Edo State, Nigeria in the month of November and December, 2011, and were identified by the Crop Improvement and Management Department, Rubber Research Institute of Nigeria, Iyanomo, Benin City.
Chemicals
The chemicals used in this study include 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), butylated hydroxytoluene (BHT), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine 4′,4′-disulfonic acid, ferrous chloride, potassium ferricyanide, catechin, ascorbic acid, tannic acid, quercetin, trichloracetic acid (TCA), phosphate buffer, sulfanilic acid, glacial acetic acid, folin-ciocalteu reagent, sodium carbonate, vanillin, aluminium chloride, ascorbic acid and potassium acetate were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). All other chemicals used, including the solvents, were of analytical grade.
Preparation of Extract
The plant leaves were allowed to air-dry at ambient temperature and pulverised using an electric blender (Pye Unicam, Cambridge, England) and stored in an air-tight container for further use. Fifty grams (50 g) of the prepared sample was treated with 500 mL of acetone or methanol separately. Another 50 g of the pulverised leaf was extracted in 500 mL of sterile distilled water with stirring at ambient temperature for 18 to 24 h. Each extract was filtered using Whatman No. 1 filter paper. The acetone and methanol filtrate were separately concentrated to dryness in vacuo using a rotary evaporator (Laborota 4000-efficient, Heldolph, Germany) to remove the solvents. The filtrate obtained from the aqueous extract was frozen at −40°C and dried for 48 h using a freeze dryer Savant Refrigerated Vapour Trap, (RVT 41404, CA, USA).
Determination of total phenolic content
The total phenolic content of the extract were determined by Folin-Ciocalteu method described by Wolfe et al. (2003). A volume of 1.0 mg/mL of the extract was mixed with 5 ml of 10% Folin-Ciocalteu reagent and 4 ml of sodium carbonate (75% w/v). The mixture was vortexed for 15s and incubated at 40°C for 30 min for colour appearance. The absorbance was measured at 765 nm using spectrophotometer. Samples of the extract were evaluated at the final concentration of 1.0 mg/mL. The total phenolic content was expressed as mg/g tannic acid equivalent using the expression obtained from the calibration curve: Y = 0.1216x, R2 = 0.936512, where x is the absorbance and Y is the tannic acid equivalent in mg/g.
Determination of total flavonoid content
The total flavonoid was determined using the method of Ordonez et al. (2006). A volume of 0.5 mL of 2% AlCl3 ethanol solution was added to 0.5 mL of extract solution. The mixture was incubated for 1 h at room temperature for yellow colour appearance; the absorbance was measured at 420 nm. Plant extracts were evaluated at a final concentration of 0.1 mg/mL. Total flavonoids content was calculated as quercetin equivalent (mg/g) using the equation obtained from the curve: Y = 0.255x, R2 = 0.9812, where x is the absorbance and Y is the quercetin equivalent.
Determination of total flavonol content
The total flavonol content was determined using the method of Kumaran and Karunakaran (2007). Two millilitres (2.0 mL) of the sample were mixed with 2.0 mL of AlCl3 prepared in ethanol and 3.0 mL of 50 g/L sodium acetate solution were added. The mixture was incubated at 20°C for 2.5 h after which the absorption was read at 440 nm using spectrophotometer. Plant extracts were evaluated at a final concentration of 0.1 mg/mL. Total flavonols contents were calculated as quercetin (mg/g) using the following equation based on the calibration curve Y = 0.0255x, R2 = 0.9812, where x is the absorbance and Y is the quercetin equivalent.
Determination of proanthocyanidin content
The total proanthocyanidin was determined using the procedure reported by Sun et al. (1998). A volume of 0.5 mL of 0.1 mg/mL of extract solution was mixed with 3.0 mL of 4% vanillin-methanol solution and 1.5 mL hydrochloric acid; the mixture was allowed to stand for 15 min at room temperature, the absorbance was measured at 500 nm. Total proanthocyanidin contents were expressed as catechin (mg/g) using the following equation of the curve: Y= 0.5825x, R2 = 0.9277, where x is the absorbance and Y is the catechin equivalent.
Determination of Antioxidant Activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
The method of Liyana-Pathiana and Shahidi (2005) was used for the determination of scavenging activity of DPPH free radical in the extract solution. A solution of 0.135 mM DPPH in methanol was prepared and 1.0 mL of this solution was mixed with 1.0 mL of extract in methanol containing 0.2–1.0 mg/mL of the extract. The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min. The absorbance of the mixture was measured spectrophotometrically at 517 nm; BHT was used as standard. The scavenging ability of the plant extract was calculated using this equation:
DPPH Scavenging activity (%) = [(Abscontrol − Abssample)] / (Abscontrol)] × 100
Where Abscontrol is the absorbance of DPPH + methanol; Abssample is the absorbance of DPPH radical + sample extract or standard.
2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging assay
The method of Re et al. (1999) was employed for the determination of ABTS activity of the plant extracts. The stock solutions were of 7 mM ABTS.+ and 2.4 mM potassium persulphate solutions. The working solution was then prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12 h at room temperature in the dark. The solution was then diluted by mixing 1 mL ABTS.+ solution with 60 mL of methanol to obtain an absorbance of 0.076 ± 0.001 units at 734 nm. Plant extracts (1 mL) at various concentrations 0.2–1.0 mg/mL of the extract were allowed to react with 1 mL of ABTS.+ solution, and the absorbance was measured at 734 nm after 7 min using spectrophotometer. The ABTS.+ scavenging capacity of the extract was compared with that of BHT and percentage inhibition calculated as: ABTS.+ radical scavenging activity (%) = [(Abscontrol — Abssample)/(Abscontrol)] × 100
Where Abscontrol was the absorbance of ABTS+ radical + methanol; Abssample is the absorbance of ABTS+ radical + sample extract or BHT.
Total antioxidant activity: ferrous reducing antioxidant power (FRAP) assay
The reducing power of the extracts was assayed according to the method of Duh et al. (1999). A volume of 1.0 mL of the extracts, BHT and ascorbic acid at different concentrations 0.2–1.0 mg/mL were mixed individually to the mixture containing 2.5 mL of 0.2 M phosphate buffer pH 6.6 and 2.5 mL potassium ferricyanide (K3Fe(CN)6) (1% w/v). The mixture was incubated at 50 °C for 20 min, followed by the addition of 2.5 mL of trichloroacetic acid (TCA) (10% w/v), centrifuged for 10 min at 1000 × g. The upper layer of the solution was collected and mixed with 2.5 mL of distilled water and 0.5 mL of ferrous chloride (0.1% w/v). The absorbance was measured at 700 nm in a spectrophotometer. The higher absorbance of the reaction mixture indicates strong reducing power of the plant extract.
Phytochemical analysis
The extracts were subjected to phytochemical screening for plant secondary metabolites, tannins, saponins, steroid, alkaloids, terpernoids, flavonoids, phenols and cardiac-glycolsides in accordance with Trease and Evans (1989) and Harborne (1998).
Proximate and mineral analysis
The proximate analysis (carbohydrates, fats, proteins, lipids, iodine, moisture, fibre, and ash) of the plant sample was determined by using Association of Official Analytical Chemists (AOAC) methods. All the proximate values were reported in percentage (AOAC, 1999; Okwu and Morah, 2004). Mineral elements (sodium, potassium, calcium, magnesium, iron, zinc, phosphorus, copper, manganese and nitrogen) were determined using the multiple nutrient extraction method (AOAC, 1999).
Statistical analysis
The experimental results were expressed as mean ± standard deviation (SD) of three replicates and were subjected to one-way analysis of variance (ANOVA) and the significant difference (p < 0.05) using SPSS version 17.0 for windows.
Results and Discussion
Polyphenol content and antioxidant activity
The methanol leaf extract of O. gratissimum possessed relatively high phenolic content when compared with acetone and aqueous extract (Table 1).
Table 1.
Polyphenolic contents of the acetone, methanol and aqueous extracts of the leaves of O. gratissimum
| Extracts | Total phenol (mg tannic acid/g) |
Total flavonoids (mg quercetin/g) |
Total flavonols (mg quercetin/g) |
Total proanthocyanidins (mg catechin/g) |
| Acetone | 10.21 ± 2.20 | 12.03 ± 2.52 | 10.25 ± 1.50 | 12.55 ± 2.02 |
| Methanol | 19.21 ±1.25 | 15.57 ±0.56 | 13.89 ±1.03 | 14.68 ± 2.06 |
| Aqueous | 12.02 ± 2.05 | 11.50 ± 2.51 | 12.85 ± 1.25 | 8.55 ± 1.55 |
Note: ± = Standard derivation of triplicate.
The phenolic content of all the extracts was relatively high, suggesting that the plant extracts have antioxidant potential. Polyphenolic compounds are well known as antioxidant and scavenging agents against free radicals associated with oxidative damage (Fergusion et al., 2006). The study also revealed that all the extracts possessed flavonoids, flavonols and proanthocyanidins. The presence of these compounds in O. gratissimum could promote acceptance for its local usage in the management of oxidative stress induced ailment. Flavonoids are important secondary metabolites of plant modulating lipid peroxidation involved in atherogenesis, thrombosis and carcinogenesis (Shi et al., 2006). The antioxidant activity of proanthocyanidins has been demonstrated to be 50 times greater than vitamin C and 20 times greater than vitamin E (Majo et al., 2008). It has also been shown that proanthocyanidins help to protect body from tissue damage, cancer, and to improve blood circulation by strengthening the capillaries, arteries and veins (Majo et al., 2008; Owolabi et al., 2010; Letelier et al., 2011). Thus, the concentration of this compound as shown in this study could contribute to the significant antioxidant potency of this plant and consequently support its local usage for the treatment of free radical related diseases. There was correlation between total phenols (r = 0.966), total flavonoids (r = 0.988), total flavonols (r = 0.918) and total proanthocyanidins (r = 0.955) respectively. The correlation coefficient between ABTS.+, DPPH assays and polyphenols is (r = 0.989, 0.988, and 0.998) respectively.
DPPH radical scavenging activity
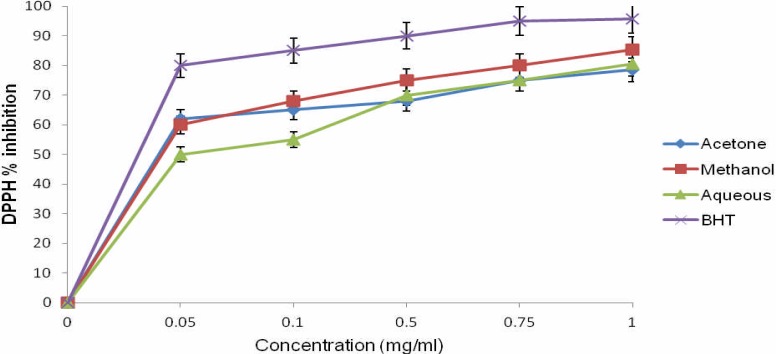
DPPH scavenging activity assay has been utilised to gain the understanding of antioxidant potentials (Liyana-Pathirana and Shahidi, 2005; Igbinosa et al., 2011). The percentage inhibitions of DPPH scavenging activity in all the extracts were dose-dependent (Figure 1).
Figure 1.
DPPH radical scavenging activities of the different extract of O. gratissimum
The highest DPPH scavenging activity was shown by the methanolic extract (85.45%) followed by the acetone (80.5%) and aqueous extracts (78.5%). It was also observed that the scavenging activity of methanol extract was comparable with BHT (95.59%) used as standard drug at 1.0 mg/mL which was the highest concentration tested. The dose-dependent curve of DPPH radical scavenging activity of O. gratissimum extracts compared well with BHT which suggests that the three solvent extracts possess high DPPH scavenging activity at the highest concentration (1.0 mg/mL). The results obtained from this study are in agreement with our previous work (Igbinosa et al., 2011) where it was reported that the antioxidant potential of Jatropha carcus was due to high concentration of phenolic compounds. Consequently, the strong antioxidant activity of O. gratissimum as shown in the present study might be related to high contents of phenolic compounds.
ABTS.+ radical scavenging activity
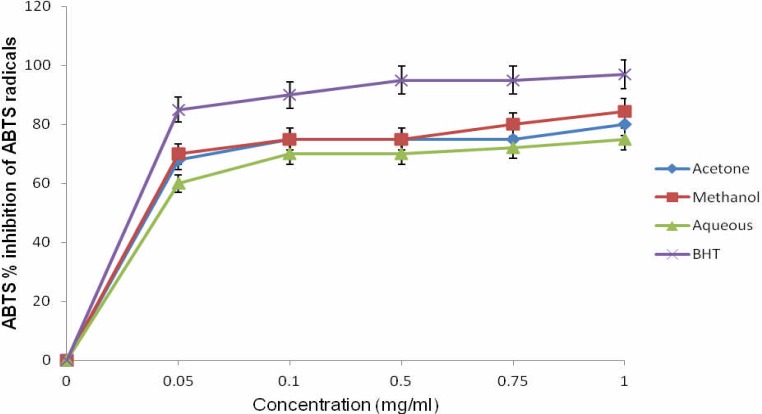
The percentage inhibition of ABTS.+ radical by the plant extracts was dose-dependent. There was increase in ABTS.+ radical scavenging activity with increasing concentration of different solvent extract used in this study (Figure 2).
Figure 2.
ABTS radical scavenging activities of the different extracts of O. gratissimum.
At a concentration of 1.0 mg/mL, the percentage inhibition of methanol extract (84.5%), acetone extract (80.12%), and aqueous extract (75%) showed similar trend but significantly different (P < 0.05, r = 0.985) when compared with that of BHT (97%). Higher concentrations of the extracts were more effective in quenching free radicals in the system, in agreement
With our previous study (Igbinosa et al., 2011). There was a correlation between ABTS.+ radical scavenging activity and total phenolic content (r = 0.989) which indicates that the phenolic compounds may contribute directly to the antioxidant action of these extracts (Afolayan et al., 2007). The scavenging activity of ABTS.+ and DPPH radicals by the extracts was found to be similar at the highest concentration. This result revealed the potential of the extracts to scavenge different free radicals in different systems, indicating that they may be useful therapeutic agents for treating radical related pathologic damage. Antioxidant activities may functions as free radical scavengers, complexes of pro-oxidant metals, reducing agents and quenchers of single-oxygen formations or reactive oxygen species, thereby protecting the body from degenerative diseases (Steenkamp et al., 2005). Although there might be several mechanisms by which this plant effectively acts against free radical implicated diseases, its antioxidant and free radical scavenging properties seem to be significantly high. Consequently, the plant could play an important role in the prevention of oxidative dependent diseases (Afolayan et al., 2007; Igbinosa et al., 2011).
Ferrous reducing antioxidant power (FRAP) assay
The antioxidant potentials of the plant extract was estimated by their ability to reduce Fe3+ to Fe2+. Table 2 shows the ferrous reducing antioxidant power values of the various extracts; methanol extract is highest, followed by acetone and least in aqueous extracts. The observed result obtained showed that the extract possessed antioxidant activity in a concentration dependent manner (data not shown). This effect could suggest the ability of O. gratissimum to minimise oxidative damage to some vital tissues in the body (Kojic et al., 1998; Weighand et al., 1999).
Table 2.
FRAP activity of acetone, methanol and aqueous extracts of O. gratissimum
| Extracts | FRAP* |
| Acetone | 346. 51± 8.54 |
| Methanol | 508.19 ±5.98 |
| Aqueous | 159.83 ± 3.64 |
| Ascorbic acid | 1524.01 ± 23.50 |
| BHT | 75.48 ± 4.51 |
| Catechin | 984.21 ± 0.89 |
| Quercetin | 2435.34 ± 12.05 |
Expressed in units of µmole Fe(II)/g
Phytochemical screening
Investigations on the phytochemical screening of O. gratissimum leaf extract revealed the presence of tannins, saponins, steroid, alkaloids, terpernoids, flavonoids, phenols and cardiac-glycolsides. These compounds are known to be biologically active and therefore aid the antioxidant activities of O. gratissimum. These secondary metabolites exert antioxidant activity through different mechanisms. Tannins have been used traditionally for the treatment of diarrhoea, haemorrhage and detoxification (Okwu and Emenike, 2006). The composition of tannins as observed in this study may justify traditional usage of the plant extract in the management of diarrhoea. Another secondary metabolite compound observed in the leaf extracts of O. gratissimum was alkaloid. One of the most common biological properties of alkaloids is their toxicity against cells of foreign organisms. These activities have been widely studied for their potential use in the elimination and reduction of human cancer cell lines (Nobori et al., 1994). Just et al. (1998) revealed the inhibitory effect of saponins on inflamed cells. Saponin was found to be present in O. gratissimum extracts and has supported the usefulness of this plant in managing inflammation. Flavonoids, another constituent of O. Gratissimum leaf extracts exhibited a wide range of biological activities like antimicrobial, anti-inflammatory, anti-angionic, analgesic, anti-allergic, cytostatic, and antioxidant properties (Hodek et al., 2002).
Proximate composition
This medicinal plant contained appreciable amount of basic food nutrient as shown in Table 4. The moisture content of the leaves concur with definitions of vegetables which were characterised by high water content (Edeoga and Gomina, 2000). The plant provides dietary supplements and may promote bowel regularity and enhance frequent waste elimination, including bile acid. Adequate intake of dietary fibre can lower the serum cholesterol level, risk of coronary heart disease, hypertension, constipation, diabetes, colon and breast cancer (Ishida et al., 2000). The relatively high level of ash, lipid, iodine, carbohydrate and others in this plant is suggestive of its considerable nutritive value.
Table 4.
Mineral elements composition of the leaves of O. gratissimum
| Mineral elements | Value (mg/100g DWB) |
| Sodium | 9.58 ± 0.23 |
| Potassium | 95.35 ± 0.01 |
| Calcium | 75.82 ± 0.02 |
| Magnesium | 88.26 ± 0.01 |
| Iron | 15.46 ± 0.03 |
| Zinc | 6.53 ± 0.01 |
| Phosphorus | 58.21 ± 0.03 |
| Copper | 5.32 ± 0.11 |
| Manganese | 5.41 ± 0.05 |
| Nitrogen | 3.12 ± 0.01 |
DWB - dry weight basis
Mineral elements composition
The macro and micro elements of this plant contained very important nutrients relevant to the well being of humans (Table 4). Sodium is an essential element that is necessary for humans to maintain the balance of the physical fluids system. Potassium is necessary for the function of all living cells and is thus present in all plant and animal tissues (FND, 2002). Calcium, potassium, magnesium, nitrogen observed in the plants are required for repairs of worn out cell, strong bones and teeth in human, building of red blood cells and for body mechanisms (WHO, 1996). The composite levels of these elements show that the leaves of the plants could provide alternative sources of calcium and potassium in diet. Iron is an essential trace element for haemoglobin formation, normal functioning of the central nervous system and the oxidation of carbohydrate, protein and fat (Akubugwo et al., 2007). The Zinc content (6.53 ± 0.01 mg/100g DWB) compares favourably to most values reported for green leafy vegetables in literatures (Hassan and Umar, 2006; Jimoh et al., 2010a,b). Zinc is involved in normal function of immune system. Manganese plays an important role in a number of physiological processes as a constituent of some enzymes and an activator of other enzymes (Idris et al., 2010). Copper has a number of important functions in the human body. It helps to produce red and white blood cells, and triggers the release of iron to form haemoglobin, the substance that carries oxygen around the body (Jimoh et al., 2010a; Idris et al., 2010). Sodium is an essential element that is necessary for humans to maintain the balance of the physical fluids system. It is also required for nerve and muscle functioning (Idris et al., 2010).
In conclusion, the relatively high antioxidant and polyphenolic activities observed in O. gratissimum extracts justify its use in folkloric medicine and suggest that this plant is endowed with natural antioxidant and nutritive constituents, which may be important as a source of nutrients supplement, and in the treatment of radical related diseases.
Table 3.
Proximate composition of the leaves of O. gratissimum
| Constituents | Value (% Dry weight) |
| Lipids | 13.51 ± 0.53 |
| Iodine | 30.25 ± 1.02 |
| Ash | 14.78 ± 1.01 |
| Carbohydrate | 5.85 ± 0.35 |
| Crude fibre | 15.51 ± 0.72 |
| Energy (kcal) | 298.10 ± 1.25 |
| Fat | 2.01 ± 0.12 |
| Moisture | 85.73 ± 0.45 |
| Protein | 16.01 ± 1.50 |
References
- 1.Afolayan AJ, Jimoh FO, Sofidiya MO, Koduru S, Lewu FB. Medicinal potential of the root of Arctotis arctotoides. Pharm Biol. 2007;45:486–493. [Google Scholar]
- 2.Akubugwo IE, Obasi NA, Chinyere GC, Ugbogu AE. Nutritional and chemical value of Amaranthus hybridus L. leaves from Nigeria. Afr J Biotechnol. 2007;6:2833–2839. [Google Scholar]
- 3.AOAC, author. Determination of acetochlor in technical and formulated products, capillary gas chromatography method. 1999 [PubMed] [Google Scholar]
- 4.Azaizeh H, Fulder S, Khalil K, Said O. Ethnobotanical knowledge of local Arab practitioners in the Middle Eastern region. Fitoterapia. 2003;74:98–108. doi: 10.1016/s0367-326x(02)00285-x. [DOI] [PubMed] [Google Scholar]
- 5.Behera BC, Verma N, Sonone A, Makhija U. Determination of antioxidative potential of lichen Usnea ghattensis in vitro. LWT. 2006;39:80–85. [Google Scholar]
- 6.Dean RT, David MJ. Reactive species and their accumulation on radical damaged proteins. Trends Biochem Sci. 1993;18:437–441. doi: 10.1016/0968-0004(93)90145-d. [DOI] [PubMed] [Google Scholar]
- 7.Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) Lebensm Wiss Technol. 1999;32:269–277. [Google Scholar]
- 8.Edeoga HO, Gomina A. Nutritional values of some nonconventional leafy vegetables of Nigeria. J Econ Taxonomic Bot. 2000;24:7–13. [Google Scholar]
- 9.Edeoga HO, Omosun G, Uche LC. Chemical composition of Hyptis sauveolens and Ocimum gratissium hybrids from Nigeria. Afr J Biotechnol. 2006;5:892–895. [Google Scholar]
- 10.Effraim ID, Salami HA, Osewa TS. The effect of aqueous leaf extract of Ocimum gratissium on haematological and biochemical parameters in rabbits. Afr J Biomed Res. 2000;3:175–179. [Google Scholar]
- 11.Ferguson LR, Philpott M, Karunasinghe N. Oxidative DNA damage and repair: significance and biomarkers. J Nutr. 2006;136:2687S–2689S. doi: 10.1093/jn/136.10.2687S. [DOI] [PubMed] [Google Scholar]
- 12.FND, author. Food and Nutrition Board, Institute of Medicine, National Academy of Sciences. Dietary reference Intake for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, protein and Amino acid (micro-nutrients) 2002 doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 13.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs tomorrow. Molecular Aspects of Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Harborne JB. Introduction to Ecological Biochemistry. 3rd edn. Academic Press London; 1988. pp. 10–15. [Google Scholar]
- 15.Halliwell B, Gutteridge JMC. Free radicals, antioxidants and human diseases: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 16.Hassan LG, Umar KJ. Nutritional value of Balsam Apple (Momordica balsamina L.) leaves. Pak J Nutr. 2006;5:522–529. [Google Scholar]
- 17.Hodek P, Trefil P, Stiborova M. Flavonoids Potent and versatile biologically active compounds interacting with cytochrome P450. Chemico-Biol Intern. 2002;139:1–21. doi: 10.1016/s0009-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 18.Hutchings A, Scoth AH, Lewis G, Cunningham AB. Zulu medicinal plants, An inventory. Pietermaritzburg: Natal University Press; 1996. [Google Scholar]
- 19.Idris S, Ndamitso MM, Yisa J, Dauda BEN, Jacob JO. The proximate and mineral composition of the leaves and stems of Balanites aegytiaca. Int'l J Appl Biol Res. 2010;2:76–87. [Google Scholar]
- 20.Igbinosa OO, Igbinosa HI, Chigor VN, Uzunuigbe EO, Oyedemi SO, Odjadjare EE, Okoh AI, Igbinosa EO. Polyphenolic contents and antioxidant potential of stem bark extracts from Jatropha curcas (Linn) Int'l J Mol Sci. 2011;12:2958–2971. doi: 10.3390/ijms12052958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida H, Suzuno H, Sugiyama N, Innami S, Todokoro T, Maekawa A. Nutritional evaluation of chemical component of leaves, stalks and stems of sweet potatoes (Ipomea batatas poir) Food Chem. 2000;68:359–367. [Google Scholar]
- 22.Just MJ, Recio MC, Giner RM, Cueller MJ, Manez S, Bilia AR, Rios JL. Anti-inflammatory activity of unusual lupine saponins from Bupleurum fruticescens. Planta Medica. 1998;64:404–407. doi: 10.1055/s-2006-957469. [DOI] [PubMed] [Google Scholar]
- 23.Jimoh FO, Adedapo AA, Aliero AA, Koduru S, Afolayan AJ. Evaluation of the polyphenolic, nutritive and biological activities of the acetone, methanol and water wxtracts of Amaranthus asper. The Open Complementary Medicine J. 2010a;2:7–14. [Google Scholar]
- 24.Jimoh FO, Adedapo AA, Afolayan AJ. Assessing the polyphenolic, nutritive and biological activities of acetone, methanol and aqueous extracts of Rumex sagittatus Thunb. Afri J Pharm Pharmacol. 2010b;4:629–635. [Google Scholar]
- 25.Kojic G, Vlahonic P, Ravloure D. The possible importance of the cation binding site for the oxidative modification of liver nucleolidase. Arch Physiol Biochem. 1998;106:91–99. doi: 10.1076/apab.106.2.91.4386. [DOI] [PubMed] [Google Scholar]
- 26.Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of Phyllantus species from India. Lebensm Wiss Technol. 2007;40:344–352. [Google Scholar]
- 27.Letelier ME, Rodriquez-Rojas C, Samchez-Jofre S, Aracena-Parks P. Surfactant and antioxidant properties of an extract from Chenopodium quinoa wild seed coats. J Am Ceram Soc. 2011;53:239–243. [Google Scholar]
- 28.Liyana-Pathirana CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433–2440. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- 29.Majo DD, La Guardia M, Giammance S, La Neve L, Giammanco M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008;111:45–49. [Google Scholar]
- 30.Nadkarni KM, Nadkarni AK. Indian Materia Medica-2. 3rd ed. Popular Prakasan, Bombay: 2000. pp. 37–39. [Google Scholar]
- 31.Nobori T, Miurak K, Wu DJ, Takabayashik LA, Carson DA. Deletion of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994;368:753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- 32.Okigbo RN, Mmeka EC. An appraisal of Phytomedicine in Africa. KMITL Science Journal (Thailand) 2006;6:83–93. [Google Scholar]
- 33.Okwu DE, Emenike IN. Evaluation of the phytonutrients and vitamin contents of citrus fruits. Int'l J Mol Med Adv Sci. 2006;2:1–6. [Google Scholar]
- 34.Okwu DE, Morah FN. Mineral and nutritive value of Dennettia ripetala fruits. 2004;59:437–442. [Google Scholar]
- 35.Ordoñez AAL, Gomez JG, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006;97:452–458. [Google Scholar]
- 36.Owolabi MA, Coker HAB, Jaja SI. Bioactivity of the phyto-constituents of the leaves of Persea americana. J Med Plant Res. 2010;53:239–243. [Google Scholar]
- 37.Pourmorad F, Hosseinimehr SJ, Shahabimajd N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr J Biotech. 2006;11:1142–1145. [Google Scholar]
- 38.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 39.Shi J, Yu J, Pohorly J, Young C, Bryan M, Wu Y. Optimization of the extraction of polyphenols from grapes seed meal by aqueous ethanol solution. Food Agric Environ. 2006;1:42–47. [Google Scholar]
- 40.Sofowara AE. Medicinal plants and Traditional Medicine in Africa. 2nd edn. Ibadan: Spectrum Book Ltd; 1993. [Google Scholar]
- 41.Steenkamp V, Stewart MJ, Chimuka L, Cukrowska E. Uranium concentrations in South African herbal remedies. Health Physiol. 2005;89:79–83. doi: 10.1097/01.hp.0000168614.97386.43. [DOI] [PubMed] [Google Scholar]
- 42.Sun JS, Tsuang YW, Chen JJ, Huang WC, Hang YS, Lu FJ. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns. 1998;24:225–231. doi: 10.1016/s0305-4179(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 43.Trease GE, Evans WC. Pharmocognosy. 11edn. Braillier Tiridel and Macmillan Publishers; 1989. [Google Scholar]
- 44.Tripathi R, Mohan H, Kamat JP. Modulation of oxidative damage by natural products. Food Chem. 2007;100:81–90. [Google Scholar]
- 45.Weighand MA, Laipple A, Plascke K. Concentration changes of malondialdehyde across the cerebral vascular bed and shedding of L selectin during carolide endarterectomy. Stroke. 1999;30:306–311. doi: 10.1161/01.str.30.2.306. [DOI] [PubMed] [Google Scholar]
- 46.WHO, author. World Health Organization Technical Series: Trace elements in Human Nutrition and Health. Geneva: World Health Organization; 1996. pp. 199–205. [Google Scholar]
- 47.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]