Abstract
Introduction
More than 3.5 million women and children under five die each year in poor countries due to underlying undernutrition. Many of these are associated with concomitant micronutrient deficiencies. In the last decade point of use or home fortification has emerged to tackle the widespread micronutrient deficiencies. We in this review have estimated the effect of Micronutrient Powders (MNPs) on the health outcomes of women and children.
Methods
We systematically reviewed literature published up to November 2012 to identify studies describing the effectiveness of MNPs. We used a standardized abstraction and grading format to estimate the effect of MNPs by applying the standard Child Health Epidemiology Reference Group (CHERG) rules.
Results
We included 17 studies in this review. MNPs significantly reduced the prevalence of anemia by 34% (RR: 0.66, 95% CI: 0.57-0.77), iron deficiency anemia by 57% (RR: 0.43, 95% CI: 0.35-0.52) and retinol deficiency by 21% (RR: 0.79, 95% CI: 0.64, 0.98). It also significantly improved the hemoglobin levels (SMD: 0.98, 95% CI: 0.55-1.40). While there were no statistically significant impacts observed for serum ferritin and zinc deficiency. Our analysis shows no impact of MNPs on various anthropometric outcomes including stunting (RR: 0.92, 95% CI: 0.81, 1.04), wasting (RR: 1.13, 95% CI: 0.91, 1.40), underweight (RR:0.96, 95% CI: 0.83, 1.10), HAZ (SMD: 0.04, 95% CI: -0.13, 0.22), WAZ (SMD: 0.05, 95% CI: -0.12, 0.23) and WHZ (SMD: 0.04, 95% CI: -0.13, 0.21), although showing favorable trends. MNPs were found to be associated with significant increase in diarrhea (RR: 1.04, 95% CI: 1.01, 1.06) with non-significant impacts on fever and URI.
Conclusion
Our analysis of the effect of MNPs in children suggests benefit in improving anemia and hemoglobin however the lack of impact on growth and evidence of increased diarrhea requires careful consideration before recommending the intervention for implementing at scale.
Introduction
More than 3.5 million women and children under five die each year in poor countries due to underlying undernutrition [1]. An estimated 178 million children under five are stunted and 55 million children are wasted [2]. Of these stunted children, 160 million (90%) live in just 36 countries, representing almost half of the children in those countries [2] and many of these children have concomitant micronutrient deficiencies. Deficiencies in vitamin A, iron, zinc and iodine are the most prevalent, accounting for 11% of global disease burden [3]. The World Health Organization (WHO) estimates that of the roughly two billion people suffering from micronutrient deficiencies, 85% live in resource poor settings [4]and these often occur as multiple rather than single micronutrient deficiencies [5]. The prevalence is especially high in Southeast Asia and sub-Saharan Africa.
Iron deficiency is widespread and globally about 1.62 billion people are anemic with the highest prevalence among preschool children (47%) followed by pregnant women (42%) [6]. Iodine deficiency (IDD) is a public health problem in 130 countries and affects 13% of world's population [7]. Globally about 740 million people are affected by goiter, and over two billions are considered at risk of IDD. It is estimated that one-third of the world population live in countries with a high prevalence of zinc deficiency. Clinical Vitamin A Deficiency (VAD) affects at least 2.80 million preschool children in over 60 countries, and sub clinical VAD is considered a problem for at least 251 million that includes school-age children and pregnant women [8].
Micronutrients play a critical role in cellular and humoral immune responses, cellular signaling and function, learning and cognitive functions, work capacity, reproductive health and even in the evolution of microbial virulence [9,10]. Infants, children and pregnant women have high demands for vitamins and minerals because of increased growth and metabolic requirements and yet their dietary intake often fails to meet these requirements [3,11]. In children these micronutrient deficiencies can cause anemia [12], restrict growth [13] and hamper motor and cognitive development [14] and also effect the immune function [15]. Under nutrition in children and women leaves a long term impact on population health and productivity.
Several strategies have been employed to supplement micronutrients to women and children [16-19]. These include nutrition education, dietary modification, food provision, supplementation and fortification. In the last decade point of use or home fortification of maternal and child diets has emerged to tackle the widespread micronutrient deficiencies. Multiple Micronutrient Powders (MNPs) or Sprinkles are powdered encapsulated vitamins and minerals that can be added to prepared foods with little change to the food’s taste or texture. MNPs are designed to provide the recommended daily nutrient intake of 2 or more vitamins and minerals to their target populations.
Despite the wide body of primary research on MNP interventions, there are few syntheses of the existing data. A recent Cochrane review has established that MNPs appear effective for reducing anemia and iron deficiency for children under 2 years of age [20]. We in this review have estimated the effect of these MNPs on the health of women and children. We have reviewed the available literature and evaluated the quality of included studies according to the Child Health Epidemiology Group (CHERG) adaptation of Grading of Recommendations, Assessments, Development and Education (GRADE) criteria [21].
Methods
We systematically reviewed literature published up to November 2012 to identify studies describing the effectiveness of MNPs. Following CHERG Systematic Review Guidelines [21], we searched PubMed, Cochrane Libraries, Embase, and WHO Regional Databases to identify all published and unpublished trials. Additional studies were identified by hand searching references from included studies. Search terms included combinations of Micronutrient* OR ‘multiple micronutrient” OR “multi-vitamin” OR “multi-mineral” OR “micronutrient powder” OR MNP OR sprinkle AND Fortifi* OR “food fortifi*” OR “point of use” OR “home fortification”. No language or date restrictions were applied in the searches.
Inclusion criteria
MNPs were identified as point-of-use powders with two or more micronutrients in their formulation. Studies were included that provided MNPs either in the home or at designated centers, using different multiple micronutrient formulations, with different dosages and duration. Studies that included supporting interventions such as nutrition education were included only if the supporting interventions were given to both the intervention and comparison groups, so that the difference between the two groups was solely of MNPs. Because of the unique nature of this intervention and a need to do a separate analysis specifically for this intervention, we excluded studies examining the impact of supplementary food provision, lipid-based supplements, micronutrient crushable tablets or foodlets, fortified milk or complementary foods and other fortified foods and beverages including fortified seasoning powders.
Abstraction, analysis and summary measure
We abstracted data describing study identifiers and context, study design and limitations, intervention specifics and outcome effects into a standardized abstraction form for studies that met the final inclusion criteria as detailed in the CHERG Systematic Review Guidelines [21]. Outcomes of interest included hematological; anemia, hemoglobin levels, serum micronutrient levels, anthropometric; stunting, wasting, underweight, weight for age z-score (WAZ), height for age z- score (HAZ), weight for height z-score (WHZ), head circumference and morbidity; diarrhea, upper respiratory infections (URI), fever and mortality among women and children. Each study was assessed and graded according to the CHERG adaptation of the GRADE technique [21].
Quantitative data synthesis
We conducted a meta-analysis for individual studies and pooled statistics were reported as the relative risk (RR) for categorical variables and standard mean difference (SMD) for continuous variables between the experimental and control groups with 95% confidence intervals (CI). Mantel–Haenszel pooled RR and corresponding 95% CI were reported or the DerSimonian–Laird pooled RR and corresponding 95% CI where there was an unexplained heterogeneity. All analyses were conducted using the software Review Manager 5.1. Heterogeneity was quantified by Chi2 and I2, which can be interpreted as the percentage of the total variation between studies that is attributable to heterogeneity rather than to chance, a low p-value (less than 0.1) or a large chi-squared statistic relative to its degree of freedom and I2 values greater than 50% were taken as substantial and high heterogeneity. In situations of high heterogeneity, causes were explored by sensitivity analysis and random effect models were used.
We summarized the evidence by outcome, including qualitative assessments of study quality and quantitative measures, according to the standard guidelines. A grade of “high”, “moderate”, “low” and “very low” was used for grading the overall evidence indicating the strength of an effect on specific health outcome according to the CHERG Rules for Evidence Review [21].
Results
We identified 2556 titles from search conducted in all databases. After screening titles and abstracts, we reviewed 26 papers for the identified outcome measures of interest of which 11 papers investigated either multiple micronutrient spreads or seasonings and were excluded from this review and 17 [22-38] studies were finally selected for inclusion which evaluated the impact of MNP versus no intervention or control and reported the outcomes of interest (Figure 1). Most of the studies were done on children aged 6 months to 6 years of age, while two studies had children up to 11 years of age. All studies were conducted in developing countries. There were no studies identified which were on women and met our inclusion criteria. None of the included studies reported on the outcome of mortality. Table 1 shows the characteristics of the included studies.
Figure 1.

Search Flow Diagram
Table 1.
Characteristics of included studies
| Study | Country | Target Group | MNP Composition | Duration |
|---|---|---|---|---|
| Adu-Afarwuah 2007 | Ghana | 6-12 month olds | Β-Carotene-300 µg RE, Vitamin C-50 mg, Vitamin D3-7.5 µg, Folic acid- 150 µg, Iron (Fumarate)- 12.5 mg, Zinc (Gluconate)- 5 mg | 1 year |
| Adu-Afarwuah 2008 | Ghana | 6-12 month olds | Β-Carotene-300 µg RE, Vitamin C-50 mg, Vitamin D3- 7.5 µg, Folic acid- 150 µg, Iron (Fumarate)-12.5 mg, Zinc (Gluconate)- 5 mg | 1 year |
| Agostoni 2007 | Cambodia | 6 month olds | Fe- 12.5mg (fumarate), Zn- 5 mg (gluconate), Vitamin C - 50mg, Vitamin A - 300mg, Vitamin D3-7.5mg, Folic acid - 150mg, Potato maltodextrins SQ to 1 g. | 1 year |
| Kounnavong 2011 | Lao People’s Democratic Republic | 6-52 month olds | Vitamin A - 400μg RE, Vitamin D- 35 μg, Vitamin E - 5 mg TE, Vitamin B1, B2, B6 - each 0.5 mg, Folic acid- 150 μg, Niacin- 6 mg, Vitamin B12- 0.9 μg, Vitamin C- 30 mg, Iron- 10 mg, Zinc- 4.1 mg, Selenium- 17 μg, Copper- 0.56 mg, Iodine- 90 μg | 6 months |
| Kumar 2007 | India | 7-11 year olds | Vitamin A 1500 IU/g, Vitamin B2, B6, B12 each -1 mg/g, Calcium pentothenate- 1 mg/g, Niacin -15 mg/g, Folic acid-100 mcg/g, Vitamin E -30 IU/g, Vitamin C- 30 mg/g, Iron - 10 mg/g, Lysine - 250 mg/g, Calcium - 15.63 % | 1 year |
| Lundeen 2010 | Kyrgyzistan | 6-36 months old | Elemental iron (fumarate)- 12.5 mg, Vitamin A - 300 μg, Zinc (gluconate)- 5 mg, Vitamin C (ascorbic acid) - 30 mg, Folic acid- 160 μg | 2 months |
| Macharia-Mutie 2012 | Kenya | 1-5 year olds | Retinyl palmitate - 100 mg RE, Cholecalciferol- 5 mg, 1-a tocopheryl acetate- 5 mg TE , Phylloquinone - 30 mg, Thiamin- 0.5 mg, Riboflavin - 0.5 mg, Pyridoxine - 0.5 mg, Folic acid - 90 mg, Niacin - 6 mg, Vitamin B-12 - 0.9 mg, Vitamin C - 60 mg, Iron (as NaFeEDTA) - 2.5 mg, Zinc - 2.5 mg, Selenium - 17 mg, Copper - 0.34 mg, Iodine- 30 mg | 4 months |
| Menon 2007 | Haiti | 9-24 month olds | Iron- 12.5 mg, Zinc- 5mg, Vitamin A- 400mg, Folic acid - 160mg, Vitamin C- 30mg | 2 months |
| Osei 2010 | India | 6-10 year olds | Iron (NaFeEDTA)- 10 mg, Vitamin A (retinyl acetate)- 375 mg, Zinc (zinc gluconate) -4.2 mg, Folic acid - 225 mg, Iodine (potassium iodide) - 90 mg, Vitamin C (ascorbic acid) - 26.25 mg, Thiamine (thiamine mononitrate)- 0.68 mg, Riboflavin- 0.68 mg, Niacin (nicotinamide) - 9 mg, Vitamin B-12- 1.35 mg, Vitamin B-6- 0.75 mg, Vitamin D (ergocalciferol) - 3.75 mg, Vitamin E - 5.25 mg, Copper [CuSO4.(H2O)5]- 0.45 mg | 8 months |
| Sharieff 2006 | Pakistan | 6-12 month olds | Zinc gluconate- 5 mg, Ferrous fumarate - 30 mg, Vitamin C - 50 mg, Vitamin A - 300 mg, Vitamin D3 - 7.5 mg, Folic acid- 150 mg | 2 months |
| Varma 2007 | India | 36-66 month olds | Ferrous fumarate- 14 mg, Vitamin A- 500 IU, Folic acid- 0.05 mg | 6 months |
| Giovannini 2006 | Cambodia | 6 month olds | Fe (iron II fumarate) - 12.5 mg, Zn (gluconate) - 5 mg, Vitamin C - 50 mg, Vitamin A - 300 µg, Vitamin D3 - 7.5 µg, Folic acid 50- 150 µg, Potato maltodextrins - SQ to 1 g | 1 year |
| Sharieff 2007 | China | 3-6 years | Iron (ferrous fumarate)- 30 mg, Zinc gluconate- 5mg, Vitamin C- 50 mg, Vitamin A- 300 mg, Vitamin D3 - 7.5mg, Folic acid- 150mg | 3 months |
| Suchdev 2010 | Kenya | 6-35 month olds | Ferrous fumarate- 12.5mg, Vitamin A- 375 µg, Zinc- 5 mg, Folic acid- 150 µg, Vitamin C-35 mg, Vitamin D3 - 5 µg, Vitamin E- 6 mg, Niacin- 6 mg, Copper- 0.6 mg, Iodine - 50 µg, Thiamine, riboflavin and vitamin B-6 - 0.5 mg, Vitamin B-12- 0.9 mg | 1 year |
| Jack 2012 | Cambodia | 6 month olds | Iron (ferrous fumarate) - 12.5 mg, Zinc gluconate - 10 mg, Vitamin A - 300μg, Iodine - 90 μg, Vitamin B1 - 0.5 mg, Vitamin B2 - 0.5 mg, Vitamin B6 - 0.5 mg, Vitamin B12 - 0.9 μg, Niacin - 6 mg, Folate, folic acid - 160 μg, Vitamin C - 30 mg, Copper - 0.3 mg, Vitamin D - 5 μg, Vitamin E - 6 IU | 18 months |
| Bhutta (unpublished) | Pakistan | 6-18 months | Ferrous fumarate- 12.5 mg, Vitamin C - 50 mg, Vitamin A (retinol acetate)- 300 μg, Vitamin D - 5 μg, Folic acid - 150 μg, Zinc gluconate- 10 mg | 24 months |
In Table 2 and 3, we report the quality assessment of studies by outcomes. All the evidence was of moderate outcome specific quality. For the hematologic indicators (Table 2), the findings were based on 15 studies. MNPs significantly reduced the prevalence of anemia by 34% (RR: 0.66, 95% CI: 0.57-0.77) (Figure 2), iron deficiency anemia by 57% (RR: 0.43, 95% CI: 0.35-0.52) and retinol deficiency by 21% (RR: 0.79, 95% CI: 0.64, 0.98). It also significantly improved the hemoglobin levels (SMD: 0.98, 95% CI: 0.55-1.40) (Figure 3). MNPs did not show a significant improvement in serum ferritin concentration and zinc deficiency.
Table 2.
Quality Assessment by Hematologic Outcome
| Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Directness | No of events | |||||||
| No of studies | Design | Limitations | Consistency | Generalizability to population of interest | Generalizability to intervention of interest | Intervention | Control | RR / SMD (95% CI) |
| Anemia: Moderate outcome specific quality of evidence | ||||||||
| Eleven | RCT | Significant heterogeneity, random effect model used | Six of ten studies suggest benefit | All studies from the developing countries | The duration of the studies ranged from 2-12 months and the age of the children from 6 months to 10 years. | 1081 | 1443 | RR: 0.66 [0.57, 0.77] |
| Iron deficiency Anemia: Moderate outcome specific quality of evidence | ||||||||
| Seven studies (six data sets) | RCT | Significant heterogeneity, random effect model used | Four of six studies suggest benefit | All studies from the developing countries | The duration of the studies ranged from 2-12 months | 404 | 986 | RR: 0.43 [0.35, 0.52] |
| Hemoglobin: Moderate outcome specific quality of evidence | ||||||||
| Fourteen studies (Fifteen data sets) | RCT | Significant heterogeneity, random effect model used | Nine studies suggest benefit | All studies from the developing countries | Studies ranged in duration from 2-12 months. | 4571 | 3783 | SMD: 0.98 [0.55, 0.40] |
| Serum Zinc: Moderate outcome specific quality of evidence | ||||||||
| Three | RCT | Significant heterogeneity so a random effect model used | One study suggested benefit | All studies from developing countries | One study was conducted in school | 761 | 788 | SMD: -0.22 [-0.52, 0.09] |
| Serum Retinol: Moderate outcome specific quality of evidence | ||||||||
| Two | RCT | Significant heterogeneity so a random effect model used | One study suggest benefit | Both studies from India | Study duration ranged from 6-8 months. | 464 | 504 | SMD: 1.66 [-1.60, 4.92] |
| Serum Ferritin: Moderate outcome specific quality of evidence | ||||||||
| Four | RCT | Significant heterogeneity, random effect model used | Three studies suggest benefit | All studies from developing countries | Studies ranged in duration from 6 months to 12 months | 850 | 884 | SMD: 1.78 [-0.31, 3.88] |
| Zinc Deficiency: Moderate outcome specific quality of evidence | ||||||||
| Two | RCT | No significant heterogeneity, fixed effect model used | None of the study suggests benefit | All studies from the developing countries | The study duration ranged from 6-8 months. | 258 | 272 | RR: 1.02 [0.87, 1.19] |
| Retinol Deficiency: Moderate outcome specific quality of evidence | ||||||||
| Three | RCT | No significant heterogeneity, fixed effect model used | None of the study suggests benefit | All studies from the developing countries | The study duration ranged from 6-12 months. | 111 | 145 | RR: 0.79 [0.64, 0.98] |
Table 3.
Quality assessment by anthropometric and morbidity outcomes
| Stunting: Moderate outcome specific quality of evidence | ||||||||
|---|---|---|---|---|---|---|---|---|
| Two study (three data sets) | RCT | No significant heterogeneity, fixed effect model used | None of the study suggests benefit | All studies from the developing countries | 810 | 838 | RR: 0.92 [0.81, 1.04] | |
| Wasting: Moderate outcome specific quality of evidence | ||||||||
| Two study (three data sets) | RCT | No significant heterogeneity, fixed effect model used | None of the study suggests benefit | All studies from the developing countries | 263 | 234 | RR: 1.13 [0.91, 1.40] | |
| Underweight: Moderate outcome specific quality of evidence | ||||||||
| Three studies (four data sets) | RCT | No significant heterogeneity, fixed effect model used | None of the studies suggest significant benefit | All the studies from developing countries | All studies included more than 3 micronutrients. The studies ranged in duration from 4-12 months. | 671 | 679 | RR: 0.96 [0.83, 1.10] |
| HAZ: Moderate outcome specific quality of evidence | ||||||||
| Three | RCT | No significant heterogeneity, fixed effect model used | None of the studies suggest significant benefit | All studies are from Africa | All studies included more than 3 micronutrients. The studies ranged in duration from 6-12 months. | 271 | 253 | SMD: 0.04 [-0.13, 0.22] |
| WAZ: Moderate outcome specific quality of evidence | ||||||||
| Three | RCT | No significant heterogeneity, fixed effect model used | None of the studies suggest significant benefit | All studies from Africa | All studies included more than 3 micronutrients. The studies ranged in duration from 6-12 months. | 271 | 253 | SMD: 0.05 [-0.12, 0.23] |
| WHZ: Moderate outcome specific quality of evidence | ||||||||
| Three | RCT | No significant heterogeneity, fixed effect model used | None of the studies suggest significant benefit | All studies from Africa | All studies included more than 3 micronutrients. The studies ranged in duration from 6-12 months. | 271 | 253 | SMD: 0.04 [-0.13, 0.21] |
| Diarrhea: Moderate outcome specific quality of evidence | ||||||||
| Four studies (five data sets) | RCT | No significant heterogeneity, fixed effect model used | Direction of evidence is consistent across studies | All the studies from developing countries | Number of micronutrients ranged from 5-15. Duration varied from 2-12 months. One of the studies targeted children in school. | 1692 | 1679 | RR: 1.04 [1.01, 1.06] |
| Recurrent Diarrhea: Moderate outcome specific quality of evidence | ||||||||
| One | RCT | Only one study | Study was conducted in Cambodia | Study was conducted over a 12 month period | 1 | 0 | RR: 2.86 [0.12, 69.00] | |
| URI: Moderate outcome specific quality of evidence | ||||||||
| Two | RCT | No significant heterogeneity, fixed effect model used | None of the studies suggest benefit | All the studies from developing countries | Number of micronutrients ranged from 5-14. Duration varied from 8-12 months. One of the studies targeted children in school. | 30 | 26 | RR: 1.17 [0.71, 1.92] |
| Fever: Moderate outcome specific quality of evidence | ||||||||
| One | RCT | Only one study | Study was conducted in India | 41 | 41 | RR: 1.03 [0.70, 1.51] | ||
Figure 2.

Forest Plot for the impact of MNPs on anemia in children
Figure 3.
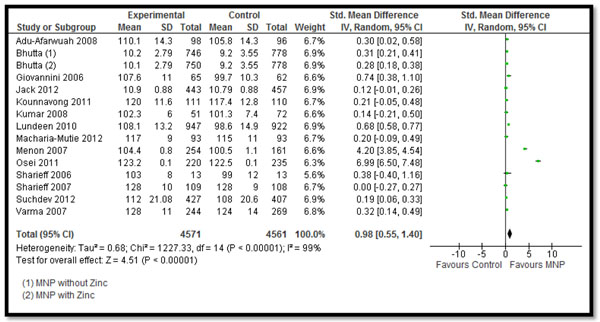
Forest Plot for the impact of MNPs on hemoglobin in children
For the anthropometric outcomes (Table 3), data was pooled for six studies. MNPs did not show a significant improvement in any of the anthropometric outcomes including stunting (RR: 0.92, 95% CI: 0.81, 1.04), wasting (RR: 1.13, 95% CI: 0.91, 1.40), underweight (RR:0.96, 95% CI: 0.83, 1.10), HAZ (SMD: 0.04, 95% CI: -0.13, 0.22), WAZ (SMD: 0.05, 95% CI: -0.12, 0.23) and WHZ (SMD: 0.04, 95% CI: -0.13, 0.21), although showing favorable trends, as the direction of effect was on the positive side although non-significant.
For the morbidity outcomes (Table 3), data from four studies was pooled. MNPs were associated with significant increase in the incidence of diarrhea (RR: 1.04, 95% CI: 1.01, 1.06) (Figure 4), while there was no significant rise in recurrent diarrhea (RR: 2.86, 95% CI: 0.12-69.0), fever (RR: 1.03, 95% CI: 0.70, 1.51) and URI (RR: 1.17, 95% CI: 0.71, 1.92).
Figure 4.

Forest Plot for the impact of MNPs on diarrhea in children
Recommendation for the LiST model
Of the outcomes assessed for the effect of MNPs in children, we applied the CHERG rules for evidence review to these outcomes. There was no data on mortality and the evidence on anthropometric outcomes is weak. With the current available evidence, we suggest that MNPs in children is associated with a 34% decrease in the incidence of anemia. The evidence of increased diarrhea suggests careful evaluation of the associated risks.
Discussion
In this systematic review our objective was to summarize the effect of MNPs on the health outcomes of women and children. We did not find any study reporting outcomes on women and seventeen studies were included that reported on various outcomes on children. The studies contributing data in this review were conducted in developing countries hence increasing the generalizability of the studies to children in low and middle income countries with the highest undernutrition rates. Most of the studies were effectiveness trials evaluating the impact of MNPs in community settings. All of the studies were on children less than six years of age, except two studies [30,39] that included children over 6 years of age although the subgroup analysis for children under five did not show any difference in the findings. Clinical heterogeneity was observed due to variations in type of intervention (number of micronutrients used ranged from 3 to 15), duration of the intervention (2-24 months), target population and different time intervals for follow-up. All the MNPs used contained iron in their composition.
The intervention was mostly reported to be acceptable by the mothers and children and there was no major loss to follow-up reported due to the intervention in any of the included studies. There have been no adverse events identified by any study except one [38] that reported increased diarrhea in the intervention group compared to control.
This review shows that MNPs raise serum hemoglobin levels and reduce anemia significantly, but the evidence on growth is weak, as relatively few studies have evaluated this outcome. Improved hemoglobin and anemia status could be attributable to the iron component in all the MNPs used. Some studies have reported benefits on other developmental outcomes like walking by 12 months but not on growth [22]. This could be due to relatively shorter duration of the intervention to show actual long term impacts. These findings also suggest that multiple micronutrient interventions alone might not improve growth outcomes. To ensure long term impacts and sustainability, health education that aims to modify food habits would be necessary to improve child growth rates. Also, if the intervention initiation coincides with the child’s diet transition from breast feeding to complementary feeding, the results may show improved growth.
The finding of significantly increased diarrhea is potentially alarming. It is mainly based on the significant increase in diarrhea observed in one large trial [38]. The association between increased diarrhea with iron supplementation is well recognized in the literature and is also reported in a review on iron supplementation by Gera [40]. However, our finding of excess morbidity and negligible growth benefit cannot be ignored in settings where large scale use of MNPs is being considered. The increased diarrhea burden could be one of the potential explanations for reduced growth benefits of MNPs.
The evidence is weak for any effect of MNPs on growth, as there were very few studies pooled for each outcome. More research is needed and studies need to report the outcomes of stunting, wasting, morbidity and mortality consistently to strengthen the evidence and evaluate its actual impact on growth and morbidity. A major research gap identified was that there were no studies evaluating the impact on women as all the studies targeted children only.
Conclusion
Our analysis of the effect of MNPs in children suggests benefit in improving anemia and hemoglobin however there is lack of impact on growth. Evidence of increased diarrhea requires careful consideration before recommending the intervention for implementation at scale.
Competing interests
We do not have any financial or non-financial competing interests for this review.
Authors' contributions
Dr. ZAB was responsible for designing the review and coordinating the review. RAS, CM and JKD were responsible for: data collection, screening the search results, screening retrieved papers against inclusion criteria, appraising quality of papers, abstracting data from papers, entering data into RevMan, analysis and interpretation of data and writing the review. ZAB and RAS critically reviewed and modified the manuscript.
Contributor Information
Rehana A Salam, Email: rehana.salam@aku.edu.
Ceilidh MacPhail, Email: ceilidh.macphail@mail.utoronto.ca.
Jai K Das, Email: jai.das@aku.edu.
Zulfiqar A Bhutta, Email: zulfiqar.bhutta@aku.edu.
Acknowledgment
This work was supported in part by a grant from the Bill & Melinda Gates Foundation.
Declarations
The publication costs for this supplement were funded by a grant from the Bill & Melinda Gates Foundation to the US Fund for UNICEF (grant 43386 to "Promote evidence-based decision making in designing maternal, neonatal, and child health interventions in low- and middle-income countries”). The Supplement Editor is the principal investigator and lead in the development of the Lives Saved Tool (LiST), supported by grant 43386. He declares that he has no competing interests.
This article has been published as part of BMC Public Health Volume 13 Supplement 3, 2013: The Lives Saved Tool in 2013: new capabilities and applications. The full contents of the supplement are available online at http://www.biomedcentral.com/bmcpublichealth/supplements/13/S3.
References
- WHO. Severe malnutrition: report of a consultation to review current literature. Geneva: World Health Organization; 2000. [Google Scholar]
- Bhutta ZA. Micronutrient needs of malnourished children. Curr Opin Clin Nutr Metab Care. 2008;11(3):309–314. doi: 10.1097/MCO.0b013e3282fbf5a0. [DOI] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, De Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- WHO. World health report. Geneva: World Health Organization; 2000. [Google Scholar]
- Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiologic Reviews. 2010;32(1):5–25. doi: 10.1093/epirev/mxq001. [DOI] [PubMed] [Google Scholar]
- Benoist B, McLean E, Egll I, Cogswell M. Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia. Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia. 2008.
- Vir SC. Current status of iodine deficiency disorders (IDD) and strategy for its control in India. Indian journal of pediatrics. 2002;69(7):589–596. doi: 10.1007/BF02722687. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Ottesen EA. Global malnutrition. Parasitology. 2000;121 Suppl:S5–S22. doi: 10.1017/s0031182000006478. [DOI] [PubMed] [Google Scholar]
- Guerrant RL, Lima AAM, Davidson F. Micronutrients and infection: interactions and implications with enteric and other infections and future priorities. Journal of Infectious Diseases. 2000;182(Supplement 1):S134–S138. doi: 10.1086/315924. [DOI] [PubMed] [Google Scholar]
- Kapil U, Bhavna A. Adverse effects of poor micronutrient status during childhood and adolescence. Nutr Rev. 2002;60(5 Pt 2):S84–S90. doi: 10.1301/00296640260130803. [DOI] [PubMed] [Google Scholar]
- Black RE. Micronutrients in pregnancy. British Journal of Nutrition. 2001;85(Suppl 2):S193–197. doi: 10.1079/bjn2000314. [DOI] [PubMed] [Google Scholar]
- Le HT, Brouwer ID, Verhoef H, Nguyen KC, Kok FJ. Anemia and intestinal parasite infection in school children in rural Vietnam. Asia Pacific Journal of Clinical Nutrition. 2007;16(4):716–723. [PubMed] [Google Scholar]
- Lawless JW, Latham MC, Stephenson LS, Kinoti SN, Pertet AM. Iron supplementation improves appetite and growth in anemic Kenyan primary school children. The Journal of nutrition. 1994;124(5):645. doi: 10.1093/jn/124.5.645. [DOI] [PubMed] [Google Scholar]
- Black RE. Zinc deficiency, infectious disease and mortality in the developing world. Journal of Nutrition. 2003;133(5 Suppl 1):1485S–1489S. doi: 10.1093/jn/133.5.1485S. [DOI] [PubMed] [Google Scholar]
- Thurnham DI. Micronutrients and immune function: some recent developments. Journal of clinical pathology. 1997;50(11):887–891. doi: 10.1136/jcp.50.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best C, Neufingerl N, Del Rosso JM, Transler C, van den Briel T, Osendarp S. Can multi micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review. Nutrition Reviews. 2011;69(4):186–204. doi: 10.1111/j.1753-4887.2011.00378.x. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HPS. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- Dewey KG, Yang Z, Boy E. Systematic review and meta analysis of home fortification of complementary foods. Maternal Child Nutrition. 2009;5(4):283–321. doi: 10.1111/j.1740-8709.2009.00190.x. [DOI] [Google Scholar]
- Serdula M. Maximizing the impact of flour fortification to improve vitamin and mineral nutrition in populations. Food and nutrition bulletin. 2010;31(1 Suppl):S86–93. doi: 10.1177/15648265100311s108. [DOI] [PubMed] [Google Scholar]
- De-Regil LM, Suchdev PS, Vist GE, Walleser S, Peña-Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst Rev. 2011. p. CD008959. [DOI] [PubMed]
- Walker N, Fischer-Walker C, Bryce J, Bahl R, Cousens S. Standards for CHERG reviews of intervention effects on child survival. International journal of epidemiology. 2010;39(suppl 1):i21–i31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. American Journal of Clinical Nutrition. 2007;86(2):412–420. doi: 10.1093/ajcn/86.2.412. [DOI] [PubMed] [Google Scholar]
- Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. American Journal of Clinical Nutrition. 2008;87(4):929–938. doi: 10.1093/ajcn/87.4.929. [DOI] [PubMed] [Google Scholar]
- Agostoni C, Riva E, Giovannini M. Functional ingredients in the complementary feeding period and long-term effects. Nestle Nutrition Workshop Series Paediatric Programme. 2007;60:123–135. doi: 10.1159/000106365. discussion 135-128. [DOI] [PubMed] [Google Scholar]
- Kounnavong S, Sunahara T, Mascie-Taylor CG, Hashizume M, Okumura J, Moji K, Boupha B, Yamamoto T. Effect of daily versus weekly home fortification with multiple micronutrient powder on haemoglobin concentration of young children in a rural area, Lao People's Democratic Republic: a randomised trial. Nutrition Journal. 2011;10:129. doi: 10.1186/1475-2891-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MV, Rajagopalan S. Multiple micronutrient fortification of salt and its effect on cognition in Chennai school children. Asia Pacific Journal of Clinical Nutrition. 2007;16(3):505–511. [PubMed] [Google Scholar]
- Lundeen E, Schueth T, Toktobaev N, Zlotkin S, Hyder SM, Houser R. Daily use of Sprinkles micronutrient powder for 2 months reduces anemia among children 6 to 36 months of age in the Kyrgyz Republic: a cluster-randomized trial. Food & Nutrition Bulletin. 2010;31(3):446–460. doi: 10.1177/156482651003100307. [DOI] [PubMed] [Google Scholar]
- Macharia-Mutie CW, Moretti D, Van den Briel N, Omusundi AM, Mwangi AM, Kok FJ, Zimmermann MB, Brouwer ID. Maize porridge enriched with a micronutrient powder containing low-dose iron as NaFeEDTA but not amaranth grain flour reduces anemia and iron deficiency in Kenyan preschool children. Journal of Nutrition. 2012;142(9):1756–1763. doi: 10.3945/jn.112.157578. [DOI] [PubMed] [Google Scholar]
- Menon P, Ruel MT, Loechl CU, Arimond M, Habicht JP, Pelto G, Michaud L. Micronutrient Sprinkles reduce anemia among 9- to 24-mo-old children when delivered through an integrated health and nutrition program in rural Haiti. Journal of Nutrition. 2007;137(4):1023–1030. doi: 10.1093/jn/137.4.1023. [DOI] [PubMed] [Google Scholar]
- Osei AK, Rosenberg IH, Houser RF, Bulusu S, Mathews M, Hamer DH. Community-level micronutrient fortification of school lunch meals improved vitamin A, folate, and iron status of schoolchildren in Himalayan villages of India. Journal of Nutrition. 2010;140(6):1146–1154. doi: 10.3945/jn.109.114751. [DOI] [PubMed] [Google Scholar]
- Sharieff W, Yin SA, Wu M, Yang Q, Schauer C, Tomlinson G, Zlotkin S. Short-term daily or weekly administration of micronutrient Sprinkles has high compliance and does not cause iron overload in Chinese schoolchildren: a cluster-randomised trial. Public Health Nutrition. 2006;9(3):336–344. doi: 10.1079/PHN2006841. [DOI] [PubMed] [Google Scholar]
- Varma JL, Das S, Sankar R, Mannar MG, Levinson FJ, Hamer DH. Community-level micronutrient fortification of a food supplement in India: a controlled trial in preschool children aged 36-66 mo. American Journal of Clinical Nutrition. 2007;85(4):1127–1133. doi: 10.1093/ajcn/85.4.1127. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Sala D, Usuelli M, Livio L, Francescato G, Braga M, Radaelli G, Riva E. Double-blind, placebo-controlled trial comparing effects of supplementation with two different combinations of micronutrients delivered as sprinkles on growth, anemia, and iron deficiency in Cambodian infants. Journal of pediatric gastroenterology and nutrition. 2006;42(3):306–312. doi: 10.1097/01.mpg.0000189363.07040.4b. [DOI] [PubMed] [Google Scholar]
- Sharieff W, Yin S, Wu M, Yang Q, Schauer C, Tomlinson G, Zlotkin S. Short-term daily or weekly administration of micronutrient Sprinkles has high compliance and does not cause iron overload in Chinese schoolchildren: a cluster-randomised trial. Public Health Nutr. 2006;9(3):336–344. doi: 10.1079/PHN2006841. [DOI] [PubMed] [Google Scholar]
- Suchdev PS, Ruth L, Obure A, Were V, Ochieng C, Ogange L, Owuor M, Ngure F, Quick R, Juliao P. Monitoring the marketing, distribution, and use of Sprinkles micronutrient powders in rural western Kenya. Food & Nutrition Bulletin. 2010;31(Supplement 2):168–178. doi: 10.1177/15648265100312S209. [DOI] [PubMed] [Google Scholar]
- Suchdev PS, Ruth LJ, Woodruff BA, Mbakaya C, Mandava U, Flores-Ayala R, Jefferds MED, Quick R. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: a cluster-randomized controlled trial. The American journal of clinical nutrition. 2012;95(5):1223–1230. doi: 10.3945/ajcn.111.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack SJ, Ou K, Chea M. Effect of micronutrient Sprinkles on reducing anemia: a cluster-randomized effectiveness trial. Arch Pediatr Adolesc Med. 2012;166(9):842–850. doi: 10.1001/archpediatrics.2012.1003. [DOI] [PubMed] [Google Scholar]
- Soofi S, Cousens S, Iqbal SP, Akhund T, Ahmed I, Zaidi AKM, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet. 2013;382(9886):29–40. doi: 10.1016/S0140-6736(13)60437-7. [DOI] [PubMed] [Google Scholar]
- Vinod Kumar M, Rajagopalan S. Trial using multiple micronutrient food supplement and its effect on cognition. Indian Journal of Pediatrics. 2008;75(7):671–678. doi: 10.1007/s12098-008-0127-1. [DOI] [PubMed] [Google Scholar]
- Gera T, Sachdev HPS. Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ. 2002;325(7373):1142. doi: 10.1136/bmj.325.7373.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]


