Abstract
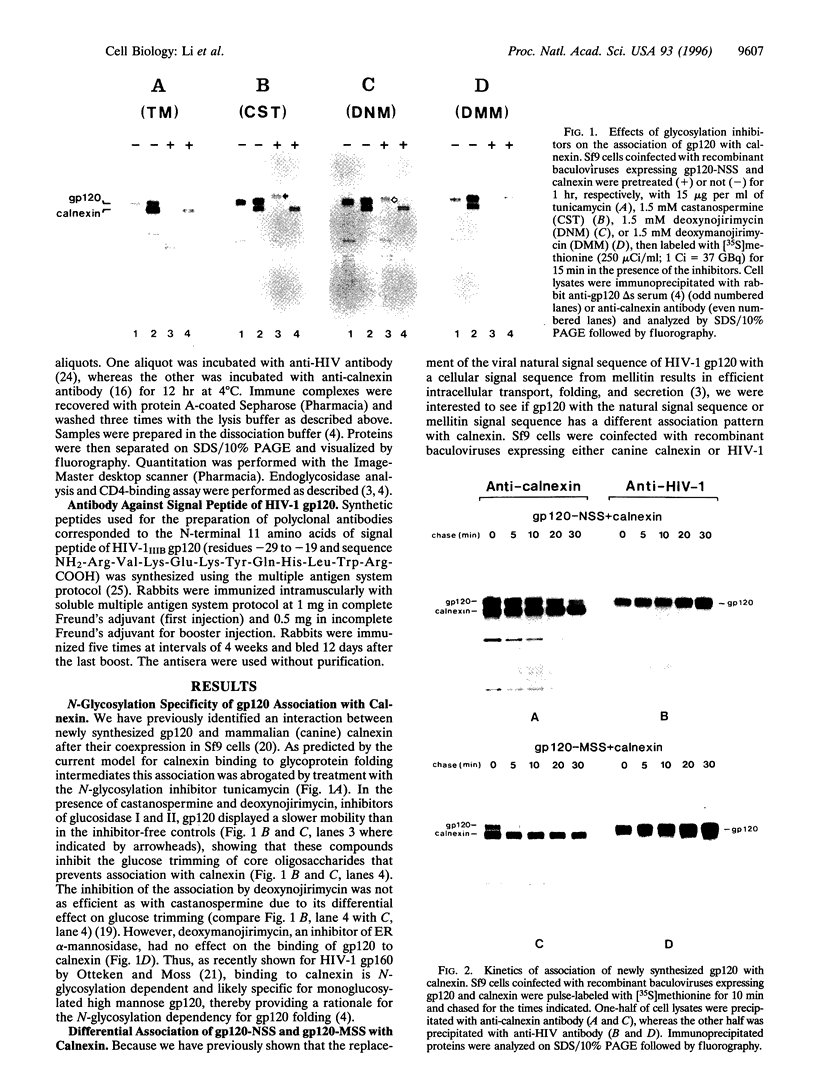
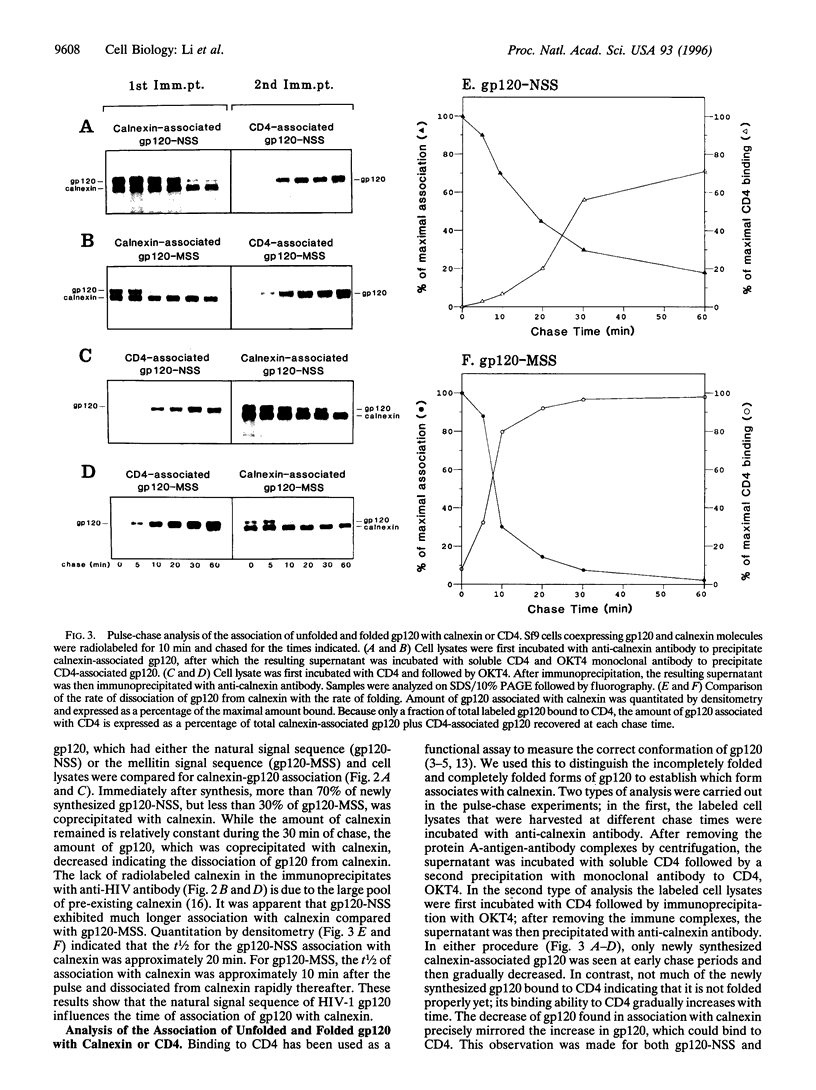
The HIV-1 envelope glycoprotein gp120 displays inefficient intracellular transport, which is caused by its retention in the endoplasmic reticulum. Coexpression in insect cells (Sf9) of HIV-1 gp120 with calnexin has shown that their interaction was modulated by the signal sequence of HIV-1 gp120. gp120, with its natural signal sequence, showed a prolonged association with calnexin with a t1/2 of greater than 20 min. Replacement of the natural signal sequence with the signal sequence from mellitin led to a decreased time of association of gp120 with calnexin (t1/2 < 10 min). These different times of calnexin association coincided both with the folding of gp120 as measured by the ability of bind CD4 and with endoplasmic reticulum to Golgi transport as analyzed by the acquisition of partial endoglycosidase H resistance. Using a monospecific antibody to the HIV-1 gp120 natural signal peptide, we showed that calnexin associated with N-glycosylated but uncleaved gp120. Only after dissociation from calnexin was gp120 cleaved, but very inefficiently. Only the small proportion of signal-cleaved gp120 molecules acquired transport competence and were secreted. This is the first report demonstrating the effect of the signal sequence on calnexin association.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Horst S. A., Gardella T. J., Baba H., Levine M. A., Kronenberg H. M. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest. 1990 Oct;86(4):1084–1087. doi: 10.1172/JCI114811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D., Bost S., Vassalli J. D., Strub K. A two-step recognition of signal sequences determines the translocation efficiency of proteins. EMBO J. 1996 Feb 1;15(3):468–478. [PMC free article] [PubMed] [Google Scholar]
- Berman P. W., Nunes W. M., Haffar O. K. Expression of membrane-associated and secreted variants of gp160 of human immunodeficiency virus type 1 in vitro and in continuous cell lines. J Virol. 1988 Sep;62(9):3135–3142. doi: 10.1128/jvi.62.9.3135-3142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Helenius J., Braakman I., Helenius A. Cotranslational folding and calnexin binding during glycoprotein synthesis. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6229–6233. doi: 10.1073/pnas.92.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp J. S., Johansen H., Hellmig B., Beck J., Matthews T. J., Delers A., Rosenberg M. Regulated expression allows high level production and secretion of HIV-1 gp120 envelope glycoprotein in Drosophila Schneider cells. Biotechnology (N Y) 1991 Feb;9(2):173–177. doi: 10.1038/nbt0291-173. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Degen E., Cohen-Doyle M. F., Williams D. B. Efficient dissociation of the p88 chaperone from major histocompatibility complex class I molecules requires both beta 2-microglobulin and peptide. J Exp Med. 1992 Jun 1;175(6):1653–1661. doi: 10.1084/jem.175.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar R. L., Natarajan V., Vasudevachari M. B., Salzman N. P. Synthesis and processing of human immunodeficiency virus type 1 envelope proteins encoded by a recombinant human adenovirus. J Virol. 1989 Jan;63(1):129–136. doi: 10.1128/jvi.63.1.129-136.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Doms R. W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991 Apr;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennie C., Lasky L. A. Model for intracellular folding of the human immunodeficiency virus type 1 gp120. J Virol. 1989 Feb;63(2):639–646. doi: 10.1128/jvi.63.2.639-646.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley E. M., Kornfeld S. Subcellular localization and targeting of cathepsin E. J Biol Chem. 1994 Dec 9;269(49):31259–31266. [PubMed] [Google Scholar]
- Hallenberger S., Tucker S. P., Owens R. J., Bernstein H. B., Compans R. W. Secretion of a truncated form of the human immunodeficiency virus type 1 envelope glycoprotein. Virology. 1993 Mar;193(1):510–514. doi: 10.1006/viro.1993.1156. [DOI] [PubMed] [Google Scholar]
- Hammond C., Braakman I., Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton D. J., Watowich S. S., Murray P. J., Lodish H. F. Increased cell surface expression and enhanced folding in the endoplasmic reticulum of a mutant erythropoietin receptor. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):190–194. doi: 10.1073/pnas.92.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach F., David V., Watkins S., Brenner M. B. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4734–4738. doi: 10.1073/pnas.89.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Kosowski S. G., Schaaf K. F. Expression of envelope glycoproteins of human immunodeficiency virus by an insect virus vector. J Virol. 1987 Nov;61(11):3617–3620. doi: 10.1128/jvi.61.11.3617-3620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Oiso Y., Murase T., Kondo K., Saito H., Chinzei T., Racchi M., Lively M. O. Possible involvement of inefficient cleavage of preprovasopressin by signal peptidase as a cause for familial central diabetes insipidus. J Clin Invest. 1993 Jun;91(6):2565–2571. doi: 10.1172/JCI116494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., Cohen-Doyle M. F., Peterson P. A., Williams D. B. Regulation of MHC class I transport by the molecular chaperone, calnexin (p88, IP90). Science. 1994 Jan 21;263(5145):384–387. doi: 10.1126/science.8278813. [DOI] [PubMed] [Google Scholar]
- Jungnickel B., Rapoport T. A. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995 Jul 28;82(2):261–270. doi: 10.1016/0092-8674(95)90313-5. [DOI] [PubMed] [Google Scholar]
- Karaplis A. C., Lim S. K., Baba H., Arnold A., Kronenberg H. M. Inefficient membrane targeting, translocation, and proteolytic processing by signal peptidase of a mutant preproparathyroid hormone protein. J Biol Chem. 1995 Jan 27;270(4):1629–1635. doi: 10.1074/jbc.270.4.1629. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Groopman J. E., Fennie C. W., Benz P. M., Capon D. J., Dowbenko D. J., Nakamura G. R., Nunes W. M., Renz M. E., Berman P. W. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science. 1986 Jul 11;233(4760):209–212. doi: 10.1126/science.3014647. [DOI] [PubMed] [Google Scholar]
- Li H., Takeuchi K. H., Manly K., Chapman V., Swank R. T. The propeptide of beta-glucuronidase. Further evidence of its involvement in compartmentalization of beta-glucuronidase and sequence similarity with portions of the reactive site region of the serpin superfamily. J Biol Chem. 1990 Sep 5;265(25):14732–14735. [PubMed] [Google Scholar]
- Li Y., Luo L., Rasool N., Kang C. Y. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J Virol. 1993 Jan;67(1):584–588. doi: 10.1128/jvi.67.1.584-588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luo L., Thomas D. Y., Kang C. Y. Control of expression, glycosylation, and secretion of HIV-1 gp120 by homologous and heterologous signal sequences. Virology. 1994 Oct;204(1):266–278. doi: 10.1006/viro.1994.1531. [DOI] [PubMed] [Google Scholar]
- Luo L., Li Y., Cannon P. M., Kim S., Kang C. Y. Chimeric gag-V3 virus-like particles of human immunodeficiency virus induce virus-neutralizing antibodies. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10527–10531. doi: 10.1073/pnas.89.21.10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre S. S. Regulated export of a secretory protein from the ER of the hepatocyte: a specific binding site retaining C-reactive protein within the ER is downregulated during the acute phase response. J Cell Biol. 1992 Jul;118(2):253–265. doi: 10.1083/jcb.118.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteken A., Moss B. Calreticulin interacts with newly synthesized human immunodeficiency virus type 1 envelope glycoprotein, suggesting a chaperone function similar to that of calnexin. J Biol Chem. 1996 Jan 5;271(1):97–103. doi: 10.1074/jbc.271.1.97. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Bergeron J. J., Li Y., Kang C. Y., Thomas D. Y. Conformational changes induced in the endoplasmic reticulum luminal domain of calnexin by Mg-ATP and Ca2+. J Biol Chem. 1995 Jul 28;270(30):18051–18059. doi: 10.1074/jbc.270.30.18051. [DOI] [PubMed] [Google Scholar]
- Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993 Aug 26;364(6440):771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Horowitz B., Baker L., Shulman R. W., Ralph H., Valinsky J., Cundell A., Brotman B., Boehle W., Rey F. Failure of a human immunodeficiency virus (HIV) immune globulin to protect chimpanzees against experimental challenge with HIV. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6944–6948. doi: 10.1073/pnas.85.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racchi M., Watzke H. H., High K. A., Lively M. O. Human coagulation factor X deficiency caused by a mutant signal peptide that blocks cleavage by signal peptidase but not targeting and translocation to the endoplasmic reticulum. J Biol Chem. 1993 Mar 15;268(8):5735–5740. [PubMed] [Google Scholar]
- Rajagopalan S., Xu Y., Brenner M. B. Retention of unassembled components of integral membrane proteins by calnexin. Science. 1994 Jan 21;263(5145):387–390. doi: 10.1126/science.8278814. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Lynn D. L., Robert-Guroff M., Langlois A. J., Lyerly H. K., Carson H., Krohn K., Ranki A., Gallo R. C., Bolognesi D. P. Humoral immune response to the entire human immunodeficiency virus envelope glycoprotein made in insect cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6924–6928. doi: 10.1073/pnas.84.19.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E., Compans R. W. Expression and characterization of a functional human immunodeficiency virus envelope glycoprotein in insect cells. Virology. 1990 Jun;176(2):575–586. doi: 10.1016/0042-6822(90)90028-p. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Bonifacino J. S., Potts B. J., Martin M. A., Klausner R. D. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9580–9584. doi: 10.1073/pnas.85.24.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]