Abstract
The urachus is a duct connecting the allantois with the fetal bladder, forming the median umbilical ligament; it usually obliterates during fetal life. Carcinomas arising from urachal remnants are rare but associated with a poor prognosis. We present one case of non-invasive urachal papillary urothelial carcinoma, and through a systematic literature search, we identified 12 additional cases of urachal urothelial carcinoma reported in English literature in the past 20 years. The cases were compared according to the Sheldon Staging System and the Mayo Staging System presented by Ashley et al in 2006, and both Staging Systems tend to predict clinical outcome. The urachal carcinoma is an important differential diagnosis in patients presenting with haematuria or an infraumbilical mass, because the symptoms may be sparse and diagnosis at an early stage is essential for successful treatment.
Background
The urachus is a duct connecting the allantois to the early fetal bladder. It is a three-layered structure with a luminal layer formed of cuboidal or transitional epithelium, an intermediate submucosal connective tissue layer and an outer smooth muscle layer. As the bladder descends to the pelvis at the fourth and fifth months of normal fetal development, the urachus is progressively stretched and the lumen obliterated. The remaining fibromuscular cord forms the median umbilical ligament.1 Urachal remnants can give rise to pathology, such as infections or cancer. The most common carcinoma arising from urachal remnants is adenocarcinoma, but urothelial carcinoma, squamous cell carcinoma and neuroendocrine tumours have also been reported.2 3 Urachal cancers constitute less than 1% of all bladder associated cancers,2 and of all urachal carcinomas, 5–10% are of urothelial origin.2–4 Several staging systems for grading urachal cancers are available, with the most used being the Sheldon Staging System 3 and the Mayo Staging System presented by Ashley et al4 (table 1). Usually, urachal cancers are detected late due to sparse symptoms, and urachal carcinoma is associated with a poor prognosis if not diagnosed at an early stage.5 The symptoms of urachal cancers, if present, are most commonly haematuria, mucosuria or a palpable infraumbilical mass. To our knowledge, non-invasive urachal urothelial carcinoma has not been reported previously.
Table 1.
The Urachal Cancer Staging System
| Stage | Definition |
|---|---|
| Defined by Sheldon et al | |
| Stage I | Urachal cancer confined to the urachal mucosa |
| Stage II | Urachal cancer with invasion confined to the urachus itself |
| Stage IIIA | Local urachal cancer extension to the bladder |
| Stage IIIB | Local urachal cancer extension to the abdominal wall |
| Stage IIIC | Local urachal cancer extension to the peritoneum |
| Stage IIID | Local urachal cancer extension to the viscera other than bladder |
| Stage IVA | Metastatic urachal cancer to the lymph node |
| Stage IVB | Metastatic urachal cancer to the distant sites |
| Defined by Ashley et al | |
| Stage I | Tumors confined to the urachus and/or bladder |
| Stage II | Tumors extending beyond the muscular layer of the urachus and/or the bladder |
| Stage III | Tumors infiltrating the regional lymph nodes |
| Stage IV | Tumors infiltrating the non-regional lymph nodes or other distant sites |
Case presentation
A 49-year-old woman developed a palpable, painless mass near the umbilicus that was resected by a general physician. She had no episodes of gross haematuria or umbilical secretion. Histological examination revealed a cystic process, containing a papillary lesion covered by dysplastic transitional-cell epithelium, suspicious of an urachal cyst (figures 1 and 2). No sign of invasive growth was seen. Immunohistochemical analysis showed a positive reaction with cytokeratin 7, cytokeratin 20 and P63, supportive of the diagnosis of papillary urothelial carcinoma (figures 3 and 4). The patient was then referred to the Department of Urology, Roskilde Hospital.
Figure 1.
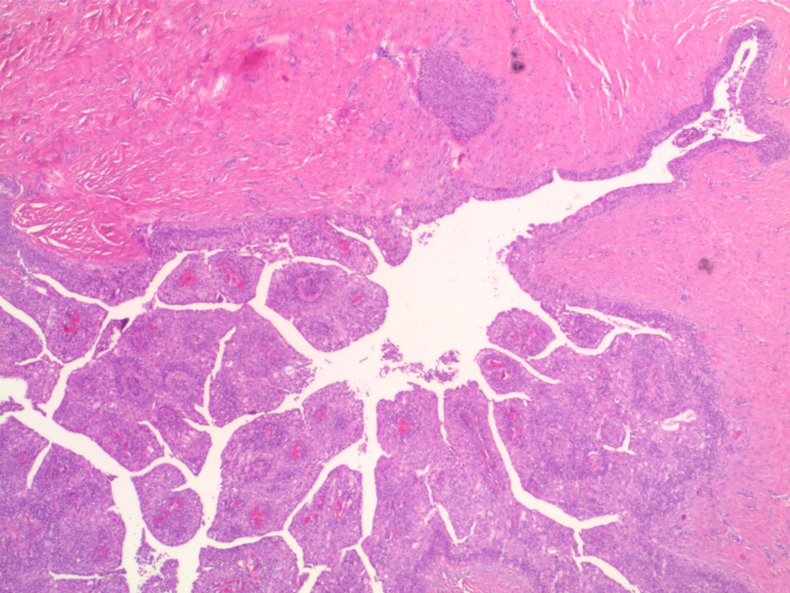
Subcutaneous cyst with papillary tumour—H&E×2.5.
Figure 2.
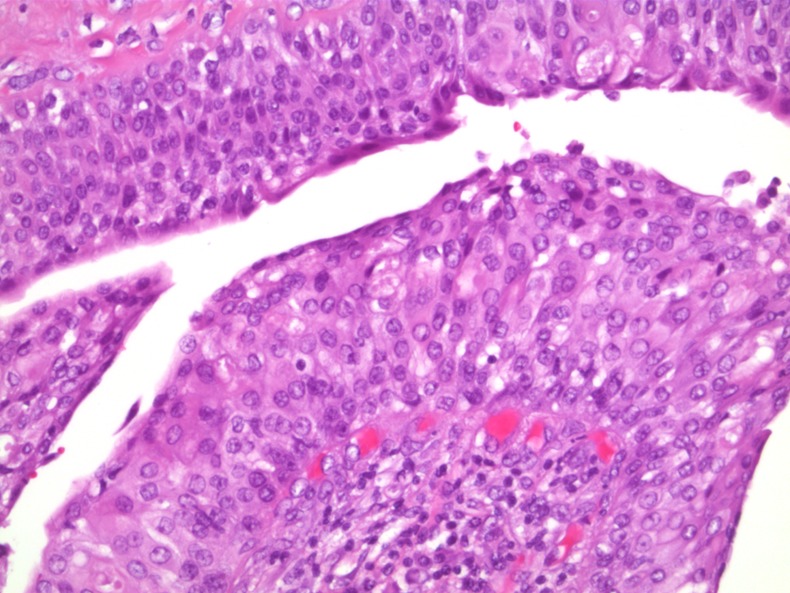
Hyperplastic epithelium with low-grade atypia—H&E×20.
Figure 3.
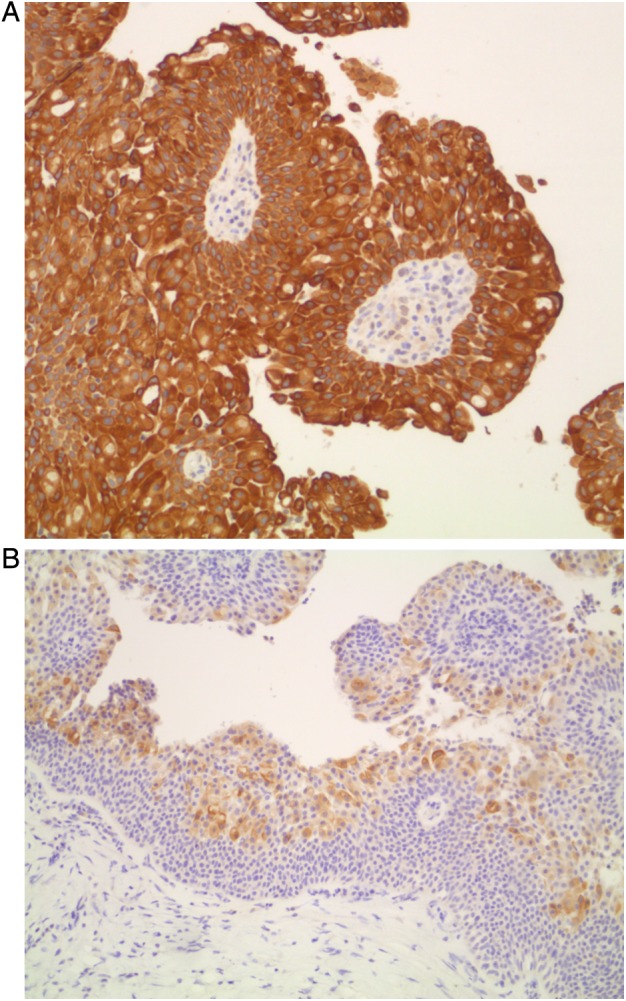
(A) Papillary tumour, positive reaction with cytokeratin 7×10 (brown cytoplasmic reaction). (B) Papillary tumour, positive reaction with cytokeratin 20×10 (superficial cells with brown cytoplasmic reaction).
Figure 4.
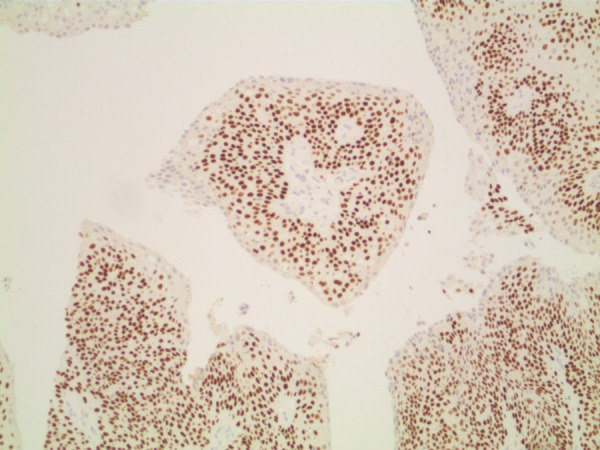
Papillary tumour, positive reaction with P63×10 (brown reaction in nuclei).
Investigations
CT revealed an urachal remnant but an otherwise normal configuration of the urinary tract.
Cystoscopy revealed a small area on the left side of the bladder, where the epithelium appeared slightly thickened but not obviously suspicious of malignancy.
Urine cytology was normal.
Gynaecological examination was normal.
Differential diagnosis
The most important differential diagnoses for urachal cancer include the following:
Non-neoplastic urachal remnants (sinus, diverticulum or cyst) with or without infection;
Tumour in the upper or lower urinary tract;
Lipoma or atheroma near or below the umbilicus;
Metastatic tumours, for example, from the ovaries.
Treatment
The patient underwent a laparoscopic partial cystectomy with en bloc resection of the urachus and umbilicus. The specimen contained mucosal surface, bladder muscle, subcutaneous tissue and skin from the abdominal wall. The bladder urothelium was hyperplastic but not dysplastic. A sinus/cyst extended continuously with the bladder mucosa (figure 5). There was no sign of carcinoma in the bladder mucosa. In the deep intramural tissue of the bladder wall, a small cyst was located; the cyst was lined with a two-layered epithelium, typical for an urachal remnant (figure 6). This cyst contained a papillary lesion of dysplastic urothelium that resembled the papillary lesion in the primarily resected subcutaneous tissue. The surgical margins were negative.
Figure 5.
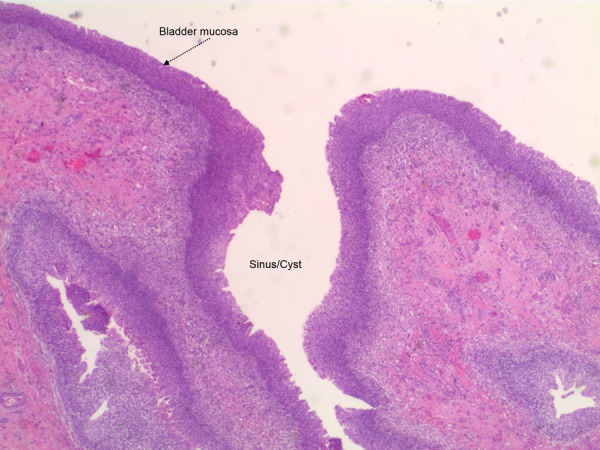
Bladder dome specimen, mucosal surface continuous with sinus.
Figure 6.
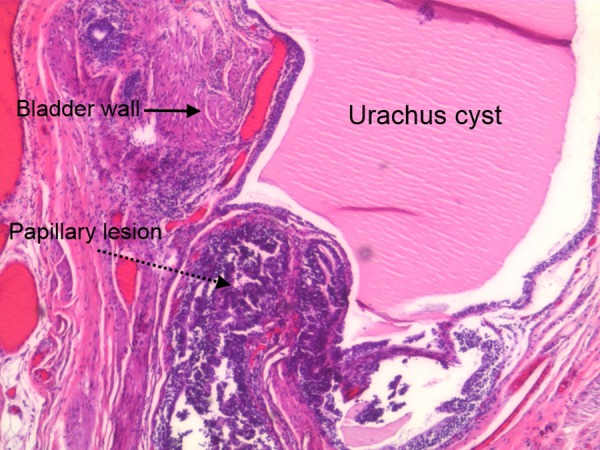
The remnant of urachus cyst with papillary lesion and bladder wall.
Outcome and follow-up
At 6 and 12 months we performed the following:
CT revealed no sign of recurrence or metastases.
Flexible cystoscopy at 6 months was normal, and at 12 months showed a red, thickened and irritated area at the dome of bladder and recurrence was suspected.
Urine cytology was normal at 6 and 12 months.
Bladder biopsies at 12 months showed inflammatory changes and no sign of recurrence of disease.
Discussion
In addition to the case presented, we identified 12 cases of urachal urothelial carcinoma published during the past 20 years in English language literature2 6–13 (table 2).
Table 2.
Distribution of cases
| Reference | Age | Sex | Sheldon stage | Mayo stage | Treatment | Follow-up | Out-come |
|---|---|---|---|---|---|---|---|
| Pedersen, 2013 | 49 | F | I | I | pc+en bloc | 12 months | Aned |
| Satake, et al12 | 42 | M | II | I | pc+en bloc | 8 months | Aned |
| Lin et al8 | 50 | M | II | I | pc+en bloc | 19 months | Dod |
| Rubin et al11 | 21 | F | II* | I | cp+en bloc | None | |
| Maletic et al9 | 49 | M | IIIa | I | pc+en bloc | 12 months | Aned |
| Paner et al2 | 68 | M | IIIa | I | cp+pelvic lnd | 60 months | Aned |
| Ichiyanagi et al6 | 48 | M | IIIa | I | pc+en bloc, at recurrence ct | 10 years+13 months | Awd |
| Nese et al10 | 66 | M | IIIa | I | pc+rt, at recurrence ct | 6 months | Awd |
| Soni et al13 | 33 | M | IIIb | II | Resection | None | |
| Paner et al2 | 45 | M | IVa | III | cp+pelvic lnd+en bloc+ct, palliative rt | 6 months | Dod |
| Paner et al2 | 85 | M | IVa | III | pc+pelvic lnd+ct | 12 months | Dod |
| Paner et al2 | 59 | M | IVb | IV | ct+rt, pc+en bloc+left inguinal mass resection | 9 months | Dod |
| Paner et al2 | 72 | M | IVb | IV | cp+pelvic lnd | 3 months | Dod |
*Estimated from informations available.
aned, Alive, no evidence of disease; awd, alive with disease; cp, cystoprostatectomy; ct, chemotherapy; dod, dead of disease; en bloc, en bloc resection of urachus and umbilicus; lnd, lymph node dissection; pc, partial cystectomy; rt, radiation therapy.
There was a male predominance with a male/female ratio of 5.5:1, and the mean age at diagnosis was 52.8 years (range 21–85). Follow-up data were available for 11 cases. The mean follow-up period was 25.5 months (range 3–133). Of these 11 patients, 4had no sign of recurrence and 7 had recurrence of the disease of them 5 died of the disease, in the follow-up period. Of the 11 cases with follow-up data, only 3 were diagnosed at an early stage (I and II) using the Sheldon system; using the Mayo system, 7 of the patients were diagnosed at stages I and II. Of the patients diagnosed at Sheldon stages I and II, 66% had good outcomes, compared with 57% for patients diagnosed at Mayo stages I and II. The patients who were diagnosed at stages III and IV, according to the Sheldon Staging System and the Mayo Staging System had generally poor outcomes, that is, 75% vs 100%, respectively.
Eight patients, our case included, had a partial cystectomy with en bloc resection of the urachus and umbilicus performed. In four patients, total cystoprostatectomy with en bloc resection of the urachus and umbilicus was the treatment of choice. In one case, the surgical procedure was not described. Four of the 13 patients also had pelvic lymph node dissections. In one patient (Ref. 2, 59-year-old man) the tumour was inoperable at the time of diagnosis, and the initial treatment choices were chemotherapy and radiation therapy; when these treatments failed, surgery was performed.
We identified but excluded three other cases reported in Japanese, Chinese and Spanish.14–16
Several studies and case reports of urachal carcinoma are published based on the surgical pathology files and the tumour registry of the Mayo Clinic, and some cases of the urachal urothelial carcinoma seems to be subjects to more than one publication.2 4 5 17 Another problem with collecting and comparing the case reports from the literature is the differences in the diagnostic criteria upon which the diagnosis was based; the former diagnostic criteria were based on urachal adenocarcinoma. Paner et al suggested a set of criteria for the pathological confirmation of urachal carcinoma other than adenocarcinoma. For the diagnosis to be confirmed, their system requires 1–3 and one of 4–6 of the following:
Located at the bladder dome or anterior wall and/or midline supravesical to the umbilicus.
Tumour epicentre away from the bladder surface.
No primary tumour of similar morphology elsewhere, except for a urothelial carcinoma in the genitourinary tract.
Close relationship with urachal remnant structures.
If no remnant urachal structure is identifiable, the tumour does not involve the intact bladder surface.
If no remnant urachal structure is identifiable and the tumour involves the bladder surface—for urothelial carcinoma only, a cavitary or cystic tumour with intraluminal papillary structures, or all non-glandular urachal carcinomas, the presence of a reverse invasive front.
Our case fulfils points 1–4 (figures 5 and 6).
Early and correct diagnosis of urachal carcinoma is essential because the prognosis of these cancers seems poor, especially when detected at a late stage.4 5 18 Follow-up data were available for 11 of 13 patients and of these, 7 (64%) had recurrence of disease of them 5 (45%) died. Even though the number of patients is small, this is in overall concordance with previously reported data. Paner et al2 reported an overall recurrence rate of 47% with a 27% mortality rate in their literature review of urachal urothelial carcinomas. In another series by Ashley et al4, different histological types of urachal carcinoma were pooled together, which resulted in an overall 5-year cancer-specific survival rate of 49%. The most commonly used staging system for all histological types of urachal carcinoma is the Sheldon system, but the Mayo system has also been used in the resent literature.9 Both systems seems to predicts cancer-specific mortality equally well.4 High tumour stage and positive surgical margin are the most consistent predictors of poor outcome of urachal carcinoma of all histological types.4 19
Learning points.
Urachal carcinoma is an important differential diagnosis in patients presenting with haematuria or an infraumbilical mass.
The gold standard of treatment is a partial cystectomy with en bloc resection of the urachal remnants and umbilicus.
A high tumour stage at diagnosis and positive surgical margin are the most consistent predictors of poor outcome.
Owing to the high recurrence rate and mortality, we have decided that follow-up procedure after surgery at our institution has to include: CT, flexible cystoscopy and urine cytology every 6 months for at least 5 years.
Acknowledgments
Pilt, Anette Pedersen MD, Department of pathology, Roskilde Hospital, Roskilde.
Footnotes
Contributors: NHA and GLP were involved in the conception and design of the manuscript. CD contributed in the drafting of the article and revising it critically for important intellectual content.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Nix JT, Menville JG, Albert M, et al. Congenital patent urachus. J Urol 1958;2013:264–73 [DOI] [PubMed] [Google Scholar]
- 2.Paner GP, Barkan GA, Mehta V, et al. Urachal carcinomas of the nonglandular type: salient features and considerations in pathologic diagnosis. Am J Surg Pathol 2012;2013:432–42 [DOI] [PubMed] [Google Scholar]
- 3.Sheldon CA, Clayman RV, Gonzalez R, et al. Malignant urachal lesions. J Urol 1984;2013:1–8 [DOI] [PubMed] [Google Scholar]
- 4.Ashley RA, Inman BA, Sebo TJ, et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer 2006;2013:712–20 [DOI] [PubMed] [Google Scholar]
- 5.Molina JR, Quevedo JF, Furth AF, et al. Predictors of survival from urachal cancer: a Mayo Clinic study of 49 cases. Cancer 2007;2013:2434–40 [DOI] [PubMed] [Google Scholar]
- 6.Ichiyanagi O, Sasagawa I, Suzuki Y, et al. Successful chemotherapy in a patient with recurrent carcinoma of the urachus. Int Urol Nephrol 1998;2013:569–73 [DOI] [PubMed] [Google Scholar]
- 7.Isotalo PA, Robertson SJ, Futter NG. Urinary bladder urachal remnants underlying papillary urothelial carcinoma. Arch Pathol Lab Med 2002;2013:1252–3 [DOI] [PubMed] [Google Scholar]
- 8.Lin CN, Lu NM, Chiang HS, et al. Urachal carcinoma: a report of two cases. Zhonghua Yi Xue Za Zhi (Taipei) 1995;2013:436–9 [PubMed] [Google Scholar]
- 9.Maletic V, Cerovic S, Lazic M, et al. Synchronous and multiple transitional cell carcinoma of the bladder and urachal cyst. Int J Urol 2008;2013:554–6 [DOI] [PubMed] [Google Scholar]
- 10.Nese N, Kesici G, Lekili M, et al. Urachal urothelial carcinoma diagnosed at a radical prostatectomy operation: a case report. Anal Quant Cytol Histol 2010;2013:174–7 [PubMed] [Google Scholar]
- 11.Rubin JP, Kasznica JM, Davis CA, IIIet al. Transitional cell carcinoma in a urachal cyst. J Urol 1999;2013:1687–8 [PubMed] [Google Scholar]
- 12.Satake I, Nakagomi K, Tari K, et al. Metachronous transitional cell carcinoma of the urachus and bladder. Br J Urol 1995;2013:244. [DOI] [PubMed] [Google Scholar]
- 13.Soni HC, Marda S, Goswami KG, et al. Transitional cell carcinoma in urachal cyst. Abdom Imaging 2010;2013:764–6 [DOI] [PubMed] [Google Scholar]
- 14.Abe K, Wada T, Ueda M, et al. [Transitional cell carcinoma of urachus: a case report]. Hinyokika Kiyo 2000;2013:631–4 [PubMed] [Google Scholar]
- 15.Lara C, Porras V, Jurado P, et al. [Papillary urothelial carcinoma of the urachus]. Arch Esp Urol 2006;2013:914–16 [DOI] [PubMed] [Google Scholar]
- 16.Tian J, Ma JH, Li CL, et al. [Urachal mass in adults: clinical analysis of 33 cases]. Zhonghua Yi Xue Za Zhi 2008;2013:820–2 [PubMed] [Google Scholar]
- 17.Binkovitz LA. Case of the month. Urachal carcinoma. Mayo Clin Proc 1993;2013:393–4 [DOI] [PubMed] [Google Scholar]
- 18.Gopalan A, Sharp DS, Fine SW, et al. Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol 2009;2013:659–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herr HW, Bochner BH, Sharp D, et al. Urachal carcinoma: contemporary surgical outcomes. J Urol 2007;2013:74–8 [DOI] [PubMed] [Google Scholar]


