Abstract
ACTA2 mutations have recently been shown to cause a multisystem smooth muscle dysfunction syndrome that may result in pediatric stroke. We report a case of ACTA2 mutation in a 3-year-old girl presenting with acute ischemic stroke and provide high resolution imaging of the cerebral arteries demonstrating novel findings of multiple tiny aneurysms (particularly in the posterior circulation), as well as the more characteristic imaging phenotype of straightened and narrowed proximal intracranial vessels, dilated cervical vessels and occlusion of the M1 MCA segment without lenticulostriate collateral formation. This newly identified disease should be added to the differential diagnosis of pediatric stroke and cerebral vasculopathy. Neuroradiologists, interventionalists, surgeons and neurologists should become familiar with this rare disease and its clinical sequelae.
Keywords: Brain, Congenital, Genetic, Pediatrics, Vasculitis
Background
Arg179His ACTA2 mutation has recently been identified as a cause of multisystem smooth muscle dysfunction. ACTA2 mutations were first identified in cases of familial thoracic aortic aneurysms1 and later expanded to account also for a pediatric syndrome of stroke, congenital mydriasis and other smooth muscle dysfunctions.2 Imaging features of this syndrome in pediatric patients have been defined as predominantly involving the white matter, possibly indicating small vessel involvement, as well as large vessel abnormalities including occlusions without the lenticulostriate collaterals typically identified in Moyamoya disease and straightening and narrowing of intradural vessels and dilation of the extradural internal carotid artery (ICA).
This case expands our understanding of the neuroradiologic appearance of this syndrome. MRI demonstrates a purely cortical involvement of the stroke in our case. Cerebral angiography (both digital subtraction angiography (DSA) and MR angiography (MRA)) demonstrates the previously seen findings, but also expands upon them to identify several tiny aneurysms more numerous in the posterior circulation. Additionally, small vessel abnormalities including corkscrew appearance and beading are also noted in the fourth order middle cerebellar artery (MCA) and third order posterior cerebellar artery (PCA) branches.
As an arteriopathy with a known genetic cause that presents in childhood, ACTA2 mutation arteriopathy is important to recognize so that appropriate counseling can be offered to patients and their parents. With the increasing availability of gene sequencing even from single cells, the discovery of systemic or regional mosaic genetic abnormalities underpinning several of the diseases we encounter (including cerebral aneurysms,3 arteriovenous malformations4 and brain tumors,5) is increasingly likely and familiarity with such abnormalities is crucial for practicing neurointerventionalists and our colleagues.
Case presentation
A 3-year-old girl with a history of urinary incontinence, congenital mydriasis, patent ductus arteriosus (PDA) and no contributory family history presented with slurred speech, clumsiness, sleepiness and falling that progressively worsened over days. CT demonstrated acute right MCA infarction which was confirmed by MRI. MRA identified several abnormalities of the intracranial vasculature that were confirmed and further elucidated on DSA.
DSA demonstrated dilation of the cervical ICAs with a marked straightened course and abrupt tapering of the intracranial vessels proximally. Distal small vessel abnormalities included corkscrew appearance of third and fourth order MCA and PCA, leptomeningeal collaterals and small aneurysms at the distal branch points predominantly in the posterior circulation. Right M1 MCA occlusion explained the acute MCA infarction. Lenticulostriate collaterals (the key finding in Moyamoya disease) were conspicuously absent. Angiography of the abdominal vasculature was unremarkable.
The patient also had a chest x-ray with a PDA clip and a prior voiding cystourethrogram (VCUG) which demonstrated a markedly dilated urinary bladder.
ACTA2 mutation was suspected clinically and ACTA2 genetic testing confirmed heterozygous Arg179His substitution.
Investigations
Imaging
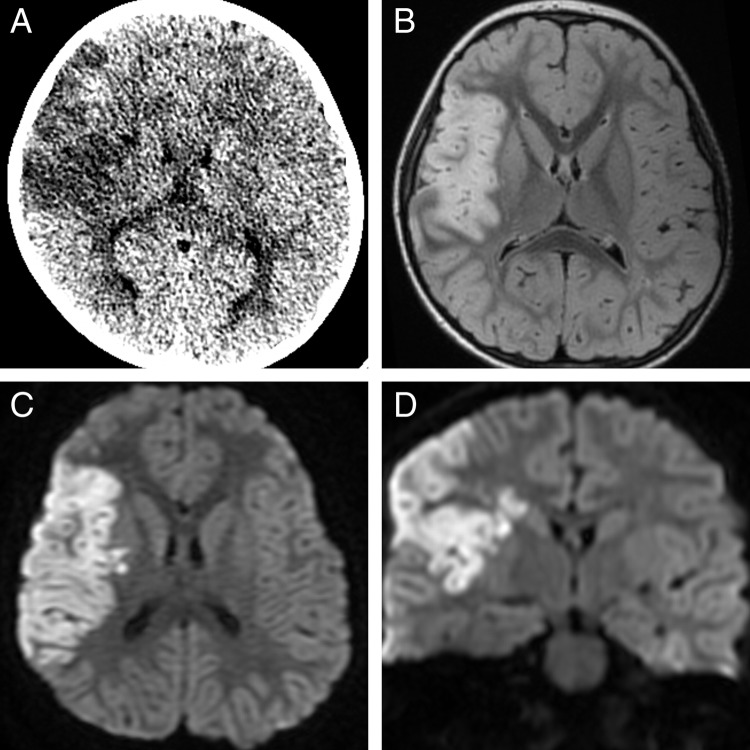
Non-contrast head CT demonstrated right MCA territory confluent low density consistent with acute infarction (figure 1A). The chest x-ray was notable for PDA clip (PDA is described in the syndrome caused by ACTA2), clear lungs (no evidence of infection) and a normal cardiomediastinal silhouette (figure 2A). An image from a prior VCUG showed marked dilation of the contrast-filled urinary bladder (urinary dysfunction with urinary retention is reported to be associated with ACTA2 mutation) (figure 2B). A transthoracic echocardiogram identified the aortic root as mildly dilated (images not shown).
Figure 1.
Initial diagnostic imaging demonstrates right middle cerebral artery confluent low density on non-contrast head CT (A) that is confirmed to be infarction on MRI with T2 prolongation on T2 FLAIR (B) and reduced diffusion in both the axial and coronal diffusion-weighted images (C and D). There was no evidence of hemorrhage on gradient recalled echo imaging (not shown).
Figure 2.
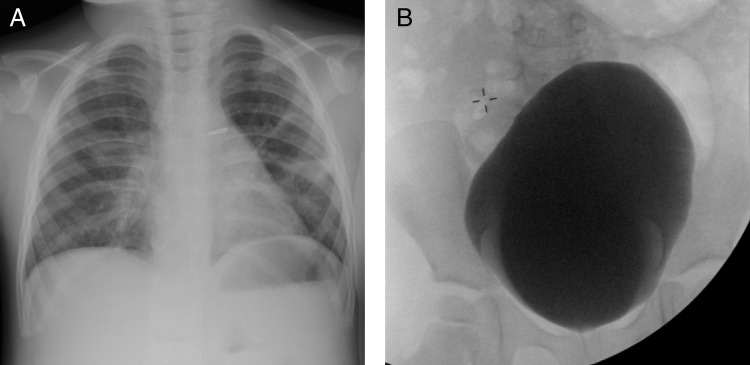
Review of the patient's prior x-rays demonstrates (A) patent ductus arteriosus (PDA) clip on prior chest x-ray (note the infiltrate in the left mid lung zone) and a markedly distended urinary bladder from a prior voiding cystourethrogram (B). PDA and dilated urinary bladder are also attributed to smooth muscle dysfunction in patients with ACTA2 mutation.
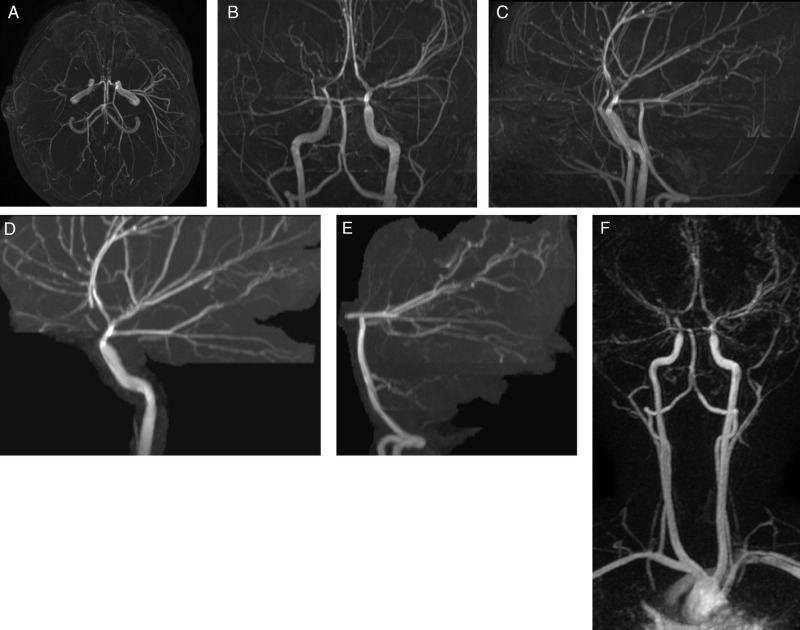
MRI confirmed infarct with cortical diffusion-weighted imaging reduced diffusion (figure 1C,D), T2 hyperintensity on FLAIR (figure 1B), mild mass effect and no evidence of hemorrhage (gradient recalled echo images not shown). MRA (figure 3) showed multiple abnormalities of the intracranial vessels with abnormal enlargement of the proximal ICAs, straightening and narrowing of the intradural vessels near the skull base and a suggestion of beading of the distal vessels. MRA was also notable for right M1 MCA without lenticulostriate collateral vessels.
Figure 3.
Time-of-flight MR angiography shows multiple abnormalities of the intracranial arteries with abnormal enlargement of the proximal internal carotid arteries, straightening and narrowing of the intradural arteries near the skull base and a suggestion of beading of the distal arteries. MR angiography is also notable for distal right M1 middle cerebral artery non-filling without lenticulostriate collateral vessels. (A) Collapsed view of the intracranial arterial circulation viewing from inferior. (B) Anteroposterior view. (C) Lateral view of both vessels collapsed. (D) Selective left anterior oblique view of the left internal carotid circulation. (E) Left anterior oblique view of the posterior circulation. (F) Anteroposterior view of a contrast-enhanced MR angiogram.
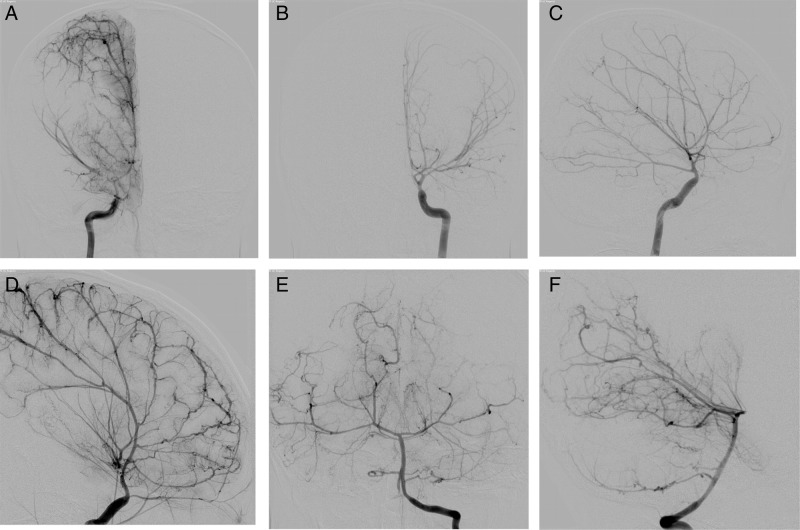
DSA (figure 4) confirmed a filling defect in the right M1 MCA and also identified the corkscrew appearance of the distal vessels, multiple tiny aneurysms at distal vessel branch points that were more numerous in the posterior circulation, and narrowing with near occlusion of the ophthalmic artery origins bilaterally.
Figure 4.
(A) Selective injection of the right internal carotid artery (ICA) in the anteroposterior projection in the late arterial phase confirms non-filling in the distal right M1 middle cerebral artery (MCA) without leptomeningeal collateral vessels (the main distinguishing feature of Moyamoya disease). Also note the dilated extradural ICA and marked narrowed and straightened appearance of the intradural ICA well seen on the left ICA anteroposterior (B) and lateral (C) projections in the early arterial phase. (D) A higher magnification view of the distal ACA and MCA branches on the lateral left ICA injection demonstrates the distal arterial corkscrew appearance. (E) Anteroposterior and (F) lateral projections with selective injection in the left vertebral artery show that the posterior circulation is also affected by the ACTA 2 mutation. Note the distal posterior cerebral artery vessels have a beaded appearance caused by multiple small aneurysms at branch points.
A broad investigation for causes of CNS vasculitis was carried out but no infectious, autoimmune or inflammatory etiologies were identified. Lumbar puncture demonstrated bland cerebrospinal fluid.
Differential diagnosis
The differential diagnosis of a diffuse cerebral vasculopathy includes genetic, autoimmune and infectious etiologies. Taking into account the congenital mydriasis limits the differential diagnosis. One might initially consider Moyamoya disease or syndrome, but the lack of characteristic lenticulostriate collateral vessels excludes those entities.
Treatment
The patient was treated with aspirin 3–5 mg/kg to decrease the risk of recurrent ischemic stroke and improved with supportive care. Revascularization surgery was considered because of her vaso-occlusive cerebral arteriopathy. However, no revascularization surgery was performed because the risk of postoperative stroke was considered to be too great. In a limited series of three pediatric patients with ACTA2 mutation, two of the children suffered arterial ischemic stroke after revascularization. In comparison, three of 73 children (4.1%) with Moyamoya disease at the same institution had a postoperative ischemic stroke.6 7
Outcome and follow-up
The patient recovered well from her initial stroke but presented 4 months later with a transient ischemic attack causing 1 h of left hemiparesis in the setting of hot weather and relative dehydration. Five months after the initial stroke a neurologic examination demonstrated persistent mydriasis (unchanged) but normal speech and no residual weakness.
Overall, with fewer than 20 patients reported in the literature (age of presentation ranging from 3 months to 27 years), the natural history of this disease has not been fully delineated.
Discussion
Heterozygous mutations in ACTA2 cause a diffuse and diverse smooth muscle disease including the cerebral arteries, thoracic aorta, dermal arteries and cardiac arteries.2 6 7 The cerebral arteriopathy is characterized by dilated extradural arteries, straightening and narrowing of the intradural arteries, large arterial occlusions without lenticulostriate collaterals seen in Moyamoya disease, distal small vessel corkscrewing and distal artery small aneurysms more predominant in the posterior circulation. These imaging characteristics, particularly the appearance of the distal arteries, multiple small aneurysms and posterior circulation involvement, have not been previously described. Previous descriptions of the neuroimaging findings in these patients focused on the large artery abnormalities and inferred small vessel involvement by the predominance of white matter signal changes on MRI.6 In support of the hypothesis of Munot et al6 that younger patients with cerebrovascular involvement are less likely to have white matter involvement, our patient had no definable signal changes in the white matter. To our knowledge, this is the first reported case of ACTA2 cerebrovascular involvement with purely gray matter signal abnormalities and normal white matter imaging.
ACTA2 mutation was initially identified in patients with familial thoracic aortic aneurysms and aortic dissections.1 In addition to the thoracic aorta and cerebral vessels, ACTA2 mutation may also result in early onset coronary artery disease.2 6 8 These patients commonly have PDA, congenital mydriasis and urinary bladder and gastrointestinal dystonia secondary to smooth muscle dysfunction.1 9 10
While ACTA2 mutation was initially identified in cases of familial thoracic aortic aneurysms, patients with cerebrovascular ACTA2 arteriopathy have all been reported to have de novo mutations (either by direct testing or negative phenotype).
ACTA2 mutation seems to have different effects on vessels resulting in aorta ectasia, dilation of extradural vessels and abrupt narrowing of these same vessels in their intradural segment. More distally in the arterial tree, vessels are narrowed but also develop aneurysms. Site-specific manifestations of ACTA2 mutation have previously been attributed to whether the affected artery is muscular or elastic.6 9
A genetic etiology for cerebrovascular disease is not unique to ACTA2 mutations. Ehlers–Danlos syndrome (mutations in COL3A1 gene [MIM 130050] in EDS type IV), neurofibromatosis 1 (NF1 gene (MIM 162200)) and cerebral amyloid angiopathy (APP gene [MIM 605714]) are the most well-known vasculopathies caused by single gene mutations. Rarer vasculopathies with known genetic causes involving the CNS include cerebral arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (NOTCH3 gene [MIM 125310]), retinal vessel with cerebral leukodystrophy (TREX1 gene [MIM 192315]), proliferative vasculopathy and hydranencephaly-hydrocephaly syndrome (PVHH) (FLVCR2 gene [MIM 225790]).
Improved imaging resolution, increased awareness of possible genetic etiologies and greater access to gene mapping is likely to lead to the discovery of new genetic cerebrovascular diseases.
Learning points.
ACTA2 mutation results in a multisystem smooth muscle dysfunction that may lead to diffuse cerebral arteriopathy presenting as pediatric stroke.
- Angiographic imaging of ACTA2 cerebral arteriopathy identifies:
- Dilated extradural arteries.
- Straightening and narrowing of the intradural arteries.
- Large artery occlusions.
- No lenticulostriate collaterals (as would be seen in Moyamoya disease).
- Distal small artery corkscrewing and distal artery small aneurysms more predominant in the posterior circulation.
Footnotes
Contributors: All authors contributed to the manuscript generation, manuscript revision, care of the patient clinically and in generating the images/figures for the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Milewicz DM, Guo DC, Tran-Fadulu V, et al. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet 2008;9:283–302 [DOI] [PubMed] [Google Scholar]
- 2.Guo DC, Papke CL, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet 2009;84:617–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruigrok YM, Rinkel GJ. Genetics of intracranial aneurysms. Stroke 2008;39:1049–55 [DOI] [PubMed] [Google Scholar]
- 4.Nishida T, Faughnan ME, Krings T, et al. Brain arteriovenous malformations associated with hereditary hemorrhagic telangiectasia: gene-phenotype correlations. Am J Med Genet A 2012;158A:2829–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salnikova LE, Belopolskaya OB, Zelinskaya NI, et al. The potential effect of gender in CYP1A1 and GSTM1 genotype-specific associations with pediatric brain tumor. Tumour Biol 2013;34:2709–19 [DOI] [PubMed] [Google Scholar]
- 6.Munot P, Saunders DE, Milewicz DM, et al. A novel distinctive cerebrovascular phenotype is associated with heterozygous Arg179 ACTA2 mutations. Brain 2012;135(Pt 8):2506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng J, Thompson D, Lumley JP, et al. Surgical revascularisation for childhood moyamoya. Childs Nerv Syst 2012;28:1041–8 [DOI] [PubMed] [Google Scholar]
- 8.Guo DC, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 2007;39:1488–93 [DOI] [PubMed] [Google Scholar]
- 9.Milewicz DM, Kwartler CS, Papke CL, et al. Genetic variants promoting smooth muscle cell proliferation can result in diffuse and diverse vascular diseases: evidence for a hyperplastic vasculomyopathy. Genet Med 2010;12:196–203 [DOI] [PubMed] [Google Scholar]
- 10.Milewicz DM, Østergaard JR, Ala-Kokko LM, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A 2010;152A:2437–43 [DOI] [PMC free article] [PubMed] [Google Scholar]