Abstract
Background
Although a declining trend in age at menarche has been observed in developed countries over decades commonly attributed to childhood excessive weight gain and sedentary life, little is known about this case in the developing countries.
Methods
A cross-sectional study design and multistage sampling was used to include 660 school adolescents for analysis. Data collection included weight and height measurements. Multinomial logistic regression analyses were done for early and late age of menarche, in reference to average age at menarche, to measure the association of age at menarche with some socio-demographic variables and body habits.
Results
The mean age at menarche was 13.9±1.2 years (95%CI, 13.8–14.0). The menarche ages ranged between 10 and 12 years for 10.5%, 13 and 14 years for 54.5%, and 15+ years for 35%. Low menarche age was independently associated with high calorie consumption, high protein diet, more coffee intake, low physical activity and parents' low educational background. Low body mass index, low parents' income, exercise, and Amhara ethnic background were associated with late menarche age.
Coclusion
The mean menarche age found in this study was higher than the report from developed countries. But, the proportion of adolescents with low menarche age was comparable with reports from developed countries. Inactive adolescents were more likely to see menarche earlier than average age. Healthy eating habits, regular exercise and nutrition education need to be promoted among school children.
Keywords: adolescent, cross sectional, menarche age, Ethiopia
Introduction
As a menopause (the last menses) declares the end, menarche (the first menses) is a signal that indicates a girl is entering into a reproductive age. The average age of the onset of menarche is 11.75 years; typically occurs about two years after the onset of the larche (breast lump) which is the first physical sign of puberty occurring at an average age of 10.5 years (1). The world health organization defines the age group of 10–19 and 15–24 years as adolescents and youth, respectively (2).
Individual differences in timing of menarche are associated with age differences in the onset of sexual activity and first pregnancy. Late menarche is associated with a decreased risk of operative delivery by decreasing the interval between menarche and first birth (3). Younger age at menarche is also a well known risk for unplanned pregnancy, unsafe abortion, breast cancer, endometrioses and STIs including HIV (4–9). Above all, a recent meta-analysis using data from 35 countries and 117 epidemiological studies demonstrated a statistically significant association of breast cancer with younger age at menarche (10). Another recent meta-analysis also concluded that early menarche is strongly associated with ovarian cancer (11).
Furthermore, early menarche is reported to be a risk factor for asthma, cardiovascular disease, diabetes and hypertension (12, 13). Several population-based studies from Asia and Europe showed that age at menarche was inversely associated with metabolic syndrome, particularly with hypertension, type II diabetes and cardiovascular diseases (14–17). Various factors like the socioeconomic status, diet, exercise, environment, genetic, heredity, ethnicity, psychological stress, and chronic illnesses have been postulated to affect the age at menarche (17–20).
From the early 1800s to the mid 1950s, studies done on developed urban populations showed the occurrence of menarche at increasingly younger ages (21), but inconsistent results were found in the last 40 to 50 years. In some developed countries, it remained stable and in others started to increase (22–24). In some others, however, the downward trend still continues (18, 25).
A cross-sectional study done in Northwest Ethiopia showed that the mean age at menarche was 15.8 ±1 years with 0.3 years earlier for urban females (26). In general, a declining trend is observed in many parts of the world (19, 20, 27–30). It ranges from about 12.5 years in contemporary western countries to more than 15 years in poorly developed countries (31).
It is known that genetics, hormone and environment are some of the factors that have an effect on the timing of puberty and the onset of menarche (32–37). Additionally, various studies done in different European countries and in the USA have identified the effects of body habits and their relation with the onset of menarche and suggested the importance of appropriate level of physical activity, weight gain, and normal dieting behavior and sleep patterns in the normal timing of puberty (38).
Most analysis considers nutritional status to be the dominant determinant of age of menarche. Diet having high contents of calories and rich in protein causes better physical maturation and early menarche (39). A family-based cross-sectional study conducted in 2004/5 in two towns of China showed that early menarche was associated with increase in body fat in adolescents and young adult women (40). There are emerging data suggesting that the rate of weight increase in childhood determines the age at puberty through complex mechanisms involving leptin. Two large scale studies confirmed that a higher gain in body mass index (BMI) during childhood is related to an earlier onset of puberty (41, 42). The aim of this study was to assess the age at menarche in adolescents and its association with body habits (diet, exercise and weight) and socioeconomic factors.
Methods
Using a cross-sectional study design, data on age of menarche and its association with body habits and socioeconomic factors were collected from adolescents in school between October and December 2011. The study participants were selected from one primary and one secondary public school in Sawla Town (Gammogofa Zone, Southern Ethiopia). The students were coming from the town and nearby rural villages within about 1–2 hours of walking distance.
The sample size was determined using a single proportion formula considering a proportion of 65% of school going adolescents having an ideal age of menarche (12–14 years of age) (38), a sampling error of 5% and design effect of 2.0. To accommodate refusal and non-response, 5% of the above was added. Of the total sampled (734) adolescents, 660 (93%) had seen their menarche and were eligible for analysis. Multistage sampling technique was used to identify the study participants. One of the primary schools was selected randomly and the only secondary school was included. In order to get the desired sample size, adolescent students (aged 10–19 years) from grade 7–12 were stratified. Then, sampling fraction was used to determine the number of study units from each grade. Finally, after dividing grades into sections and selecting each section randomly by lottery method, data were collected from all adolescents of that section until the determined sample size of that grade was attained.
The dependent variable was age at menarche. Some of the independent variables included were socio-demographic characteristics of study participants (age, residence, ethnicity, and religion) and their parents (marital status, survival, educational level, occupation and income); number of siblings, class achievement and body habits (exercise/physical activity, diet and eating, sleep) and anthropometry (weight and height).
Their family income was divided into five: < 30, 30–59, 60–89, 90–119 and > 120 USD. To measure exercise/physical activity, questions such as household activity commonly performed by the subjects, estimate of hours spent walking to school daily, number of times competitive/noncompetitive sports performed in a week, active outdoor games played with friends and history of participation in formal active sport competition were asked. Using principal component analysis method, factor score was generated for each of the questions and added across each case. Using visual binning, this summed value was divided by three cut points to generate four percentile categories each containing 25% of the cases. Individuals with value ≤25th percentiles were grouped as inactive/not exercising, >25th and ≤50th as mildly active/exercising, >50th and ≤75th as moderately active/exercising and with a value >75th as highly active/exercising group.
To measure diet and eating habits, the most commonly used food variety as a source of energy and protein is asked. Using Mike's Calorie and fat gram chart for 1000 foods from online database (21), the amount of energy in calorie and protein in gram per serving size was determined. To calculate the average amount of energy and protein intake of the subjects, it was classified into >75th as high, >25th–≤75th as medium and ≤25th as low percentile groups. Additionally, the variety of food commonly used from major food groups (carbohydrate, protein, fats, vegetables and fruits), share of vegetable servings per week, the regularity and frequency of meals used in 24 hours were used to assess eating habit.
After measuring weight using a digital weighing scale and approximating it to the nearest 1kg and standing height from head to the base of the foot to the nearest 10 mm, the BMI of each girl was calculated. The average amount of hours spent in sleep during the night time was used to assess the sleep habit. Adolescents who spent ≤ 6 hours were categorized as insufficient sleep, 7–9 hours as adequate sleep and ≥ 10 hours as excess sleep (43).
The questionnaire was modified and translated to the Amharic language after pretested in a high school in the same Woreda, which was not included as a study site. Using the SPSS for Window, multinomial logistic regression analyses were done for early age menarche and late age menarche in reference to average age menarche. P-value < 0.05 was considered statistically significant.
After Institutional Review Board of Hawassa University approved the proposal, official letter was written to the Woreda Council and school managers of the area. Then a letter of approval was obtained from the respective officials. Data were collected from adolescents who gave consent/assent to participate. Furthermore, anonymity was secured by analyzing the data in aggregate.
Results
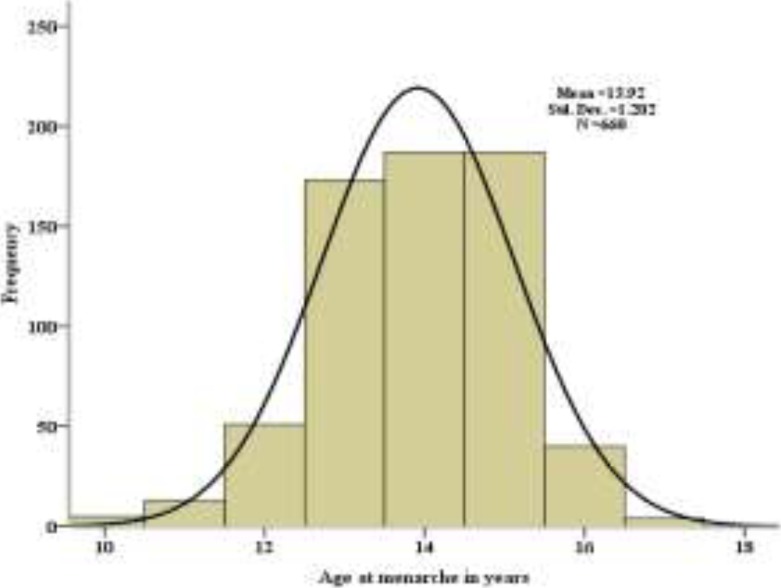
Fig 1 shows the age distribution of adolescents by the time of their menarche. The mean age of menarche was 13.9 ± 1.2 years (95% CI, 13.8 – 14.0 years). The menarche ages ranged between 13 and 14 years for about 55%, 10–12 years for 11% and 15+ years for 35%. They had an average weight and height of 54.4 ± 7.5 kg and 1.6 ± 0.1m, respectively, and their mean BMI was 21.2 ± 2.6 kg/m2. The average ages at menarche for overweight, normal weight and underweight adolescents were 14.1, 13.9 and 13.3 years, respectively. More than 60% of the adolescents who got adequate sleep during the night had an earlier mean age at menarche.
Figure 1.
Distribution of age of adolescents at menarche in Sawla Town, South Ethiopia, 2011.
Table 1 shows the mean age of adolescents at menarche stratified by sociodemographic characteristics and body habits (physical activity, diet and sleep pattern). Three hundred fifty eight (54.3%) of the participants were from urban areas. Adolescents who had been living in urban areas, good in school performance, have high family income and girls who had fathers who own business had seen their menarche at about a mean age of less than 14 years. The trend of age at menarche by birth year also showed a reduction in both rural and urban residence in recent years with a more steeping down in rural girls.
Table 1.
Mean age of adolescents at menarche in relation to sociodemographic characteristics and body habits in Sawla town, South Ethiopia, 2011.
| Mean age (SD) at | |||
| Variables | N0 (%) | menarche | |
| Residence | Urban | 358(54.3) | 13.8 (1.16) |
| Rural | 302(45.8) | 14.0 (1.24) | |
| Ethnicity | Goffa | 215(32.6) | 13.9 (1.27) |
| Amhara | 138(20.9) | 13.9 (1.18) | |
| Others | 307(46.5) | 13.9 (1.16) | |
| Religion | Orthodox | 251(38.0) | 13.8 (1.25) |
| Protestant | 339(51.4) | 13.9 (1.14) | |
| Others | 70(10.6) | 13.8 (1.30) | |
| Father occupation | Business owner | 104(16.2) | 13.8 (1.19) |
| Employee | 212(33.1) | 13.9 (1.12) | |
| Self employed | 325(50.7) | 13.9 (1.27) | |
| Mother occupation | Business owner | 119(19.2) | 13.9 (1.18) |
| Employee | 86(13.8) | 13.8 (1.15) | |
| Self employed | 416(66.9) | 13.9 (1.23) | |
| Parents' marriage | Married | 470(71.2) | 13.9 (1.22) |
| Divorced | 70(10.6) | 14.1 (1.03) | |
| Widowed | 104(15.7) | 13.8 (1.23) | |
| Others | 16(2.4) | 14.2 (1.22) | |
| Parents' life | Both alive | 536(81.2) | 13.9 (1.19) |
| One died | 108(16.4) | 13.7 (1.24) | |
| Both died | 16(2.4) | 14.2 (1.22) | |
| Parents' income, USD | >120 | 141(24.5) | 13.6 (1.15) |
| 90-120 | 27(4.7) | 13.7 (1.29) | |
| 60-89 | 116(20.1) | 14.0 (1.20) | |
| 30-59 | 99(17.2) | 14.1 (1.22) | |
| < 30 | 193(33.5) | 14.0 (1.21) | |
| Class rank in the previous year | 1-10 | 158(26.4) | 13.7 (1.19) |
| 11-20 | 220(36.8) | 13.9 (1.21) | |
| 21-30 | 128(21.4) | 14.1 (1.28) | |
| >=31 | 92(15.4) | 14.0 (1.05) | |
| Physical activity & | Inactive | 166 (25.2) | 13.1 (1.14) |
| exercise | Mildly active | 163 (24.7) | 13.9 (1.07) |
| Moderately active | 166 (25.1) | 14.1 (1.01) | |
| Highly active | 165 (25.0) | 14.6 (1.02) | |
| Eat regularly (≥ 3meals 24 hrs) | Yes | 212 (32.1) | 13.9 (1.17) |
| No | 448 (67.9) | 13.9 (1.22) | |
| Commonly used food | One variety | 72 (10.9) | 13.7 (1.36) |
| Variety | Two food variety | 363 (55.0) | 14.0 (1.18) |
| Three and more | 225 (34.0) | 13.8 (1.16) | |
| Average weekly servings of | <=1/3 | 117 (17.7) | 13.6 (1.28) |
| vegetables | ½ of the servings | 359 (54.3) | 14.0 (1.20) |
| >=2/3 | 184 (27.8) | 14.0 (1.13) | |
| Calorie content | High calorie | 185 (28.0) | 13.6 (1.21) |
| Medium calorie | 273 (41.3) | 14.0 (1.22) | |
| Low calorie | 202 (30.6) | 14.2 (1.10) | |
| Protein content | High | 172 (26.0) | 13.6 (1.18) |
| Medium | 283 (42.8) | 13.9 (1.20) | |
| Low | 205 (31.0) | 14.2 (1.16) | |
| Number of cup (50cc) of coffee | One or less | 383 (58.0) | 13.9 (1.20) |
| consumed daily | Two cup | 223 (33.7) | 13.9 (1.17) |
| Three or more | 54 (8.1) | 13.9 (1.39) | |
| BMI in kg/m2 | Underweight | 54 (8.1) | 14.1 (1.01) |
| Normal weight | 561 (85.0) | 13.9 (1.20) | |
| Overweight | 45 (6.8) | 13.3 (1.29) | |
| Hours of sleep during the night time | Excess (>=10hrs) | 21 (3.2) | 14.0 (1.26) |
| Adequate (7-9hrs) | 391 (60.7) | 13.8 (1.17) | |
| Insufficient (≤6hrs) | 232 (36.0) | 14.1 (1.23) |
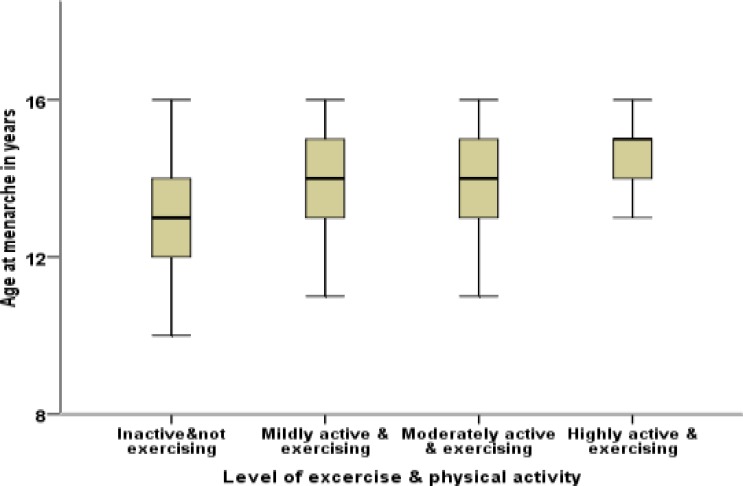
There was an increase in the mean age at menarche with increased level of physical activity. Mean age of menarche ranged from 13.1 years for relatively inactive to 14.6 years for highly active adolescents. The Whisker and Box plot (Figure 2) also showed that there was an increase in the level of exercise/physical activity in adolescents who saw their first menses in later age. In other words, there was a marked difference in both median and interquartile age ranges between physically inactive and highly active girls; they had menarche at median ages of 13 and 15 years, respectively.
Figure 2.
Whisker and Box plot showing age at menarche with level of exercise & Physical activity of the adolescents at Sawla town in 2011.
Adolescents that took their meal regularly account for more than 32% of the observation and had menarche at a lower mean age than those adolescents who were not taking meal regularly. About 34% of the adolescents who had seen their menarche were using three or more food variety of the major dietary source (carbohydrate, protein and fat) but there was little difference in mean age of menarche with regard to the major food varieties. However, the mean age at menarche decreased with an increase in the amount of calorie available in their common diet: 14.2 years for low calorie content, 13.9 years for medium calorie content and 13.6 years for high calorie content. Similarly, those who had consumed with high, medium and low protein content had seen their menarche at a mean age of 13.6 years, 13.9 years and 14.2 years, respectively. On the other hand, adolescents who used vegetables in about one-third or less of the weekly servings had seen their menarche at less mean age than those who used vegetables in about two-thirds of their weekly meals.
Table 2 shows the results of the multinomial logistic regression model for adolescents by early age at menarche in reference to the average age at menarche. In the crude odds ratio, high calorie food intake, lack of parents' education, physically inactive and high protein food showed statistically significant association. After adjusting for potential confounders, high calorie food intake was associated with low menarche age (adjusted OR = 1.1; 95%CI, 1.01 – 1.18). In the adjusted odds ratio, adolescents who had drunk ≤ 2 cups of coffee per day were about 80% less likely to see their first menses at an earlier age than those who took more cups of coffee per day. As compared to daughters of parents who took tertiary education, daughters of uneducated parents were more likely to see their menarche at an early age (AOR = 4.2; 95%CI, 1.13 – 15.54). Inactive adolescents had increased relative odds to see menarche at an earlier age (AOR = 31.8; 95%CI, 3.70 – 273.5). The odds ratio of early age onset of menarche for adolescents who used foods containing medium and high amount of protein was about 3.3 and 3.9 times higher than those adolescents served with low amount of protein.
Table 2.
Results of multinomial logistic regression for adolescents of early age at menarche in reference to adolescents of average age at menarche, 2011
| Crude | Adjusted | ||
| Predictors | Classification | OR (95%CI) | OR (95%CI) |
| Age | Scale | 1.9 (1.58 – 2.22)*** | 2.2 (1.74 – 2.68)*** |
| BMI | Scale | 0.9 (0.86 – 1.01) | 0.8 (0.76 – 0.94) |
| Calorie | Scale | 0.9 (0.88 – 0.97)** | 0.9 (0.86 – 0.98)** |
| Coffee drink/day | One cup | 0.9 (0.42 – 1.85) | 1.0 (0.43 – 2.5) |
| Two cup | 0.8 (0.37 – 1.72) | 0.8 (0.31 – 1.96) | |
| ≥3 cup Rc | |||
| Parents life | Both alive | 1.9 (0.38 – 9.72) | 1.4 (0.24 – 8.14) |
| Mother died | 2.3 (0.33 – 15.33) | 0.5 (0.05 – 4.8) | |
| Father died | 1.7 (0.28 – 9.86) | 1.1 (0.15 – 7.57) | |
| Both diedRc | |||
| Parents educational | No education | 1.0 (0.55 – 1.93) | 0.4 (0.17 – 1.10) |
| status | Primary | 1.2 (0.70 – 1.96) | 0.6 (0.28 – 1.35) |
| Secondary | 1.0 (0.57 – 1.82) | 0.6 (0.27 – 1.27) | |
| Tertiary Rc | |||
| Ethnicity | Gofa | 1.2 (0.75 – 1.89) | 0.9 (0.46 – 1.62) |
| Amhara | 1.4 (0.81 – 2.33) | 2.2 (1.10 – 4.58)* | |
| OthersRc | |||
| Religion | Orthodox | 0.7 (0.35 – 1.54) | 0.4 (0.14 – 1.08) |
| Protestant | 0.7 (0.32 – 1.37) | 0.3 (0.12 – 0.84)* | |
| OthersRc | |||
| Monthly income | >120 | 0.6 (0.32 – 0.94) | 0.4 (0.17 – 0.83) |
| 90–120 | 0.3 (0.08 – 1.04) | 0.2 (0.03 – 0.82) | |
| 60–89 | 0.9 (0.51 – 1.57) | 1.1 (0.51 – 2.26) | |
| 30–59 | 0.8 (0.46 – 1.52) | 0.9 (0.42 – 1.85) | |
| < 30Rc | |||
| Exercise | Inactive | 0.1 (0.04 – 0.19)* | 0.1 (0.02 – 0.15)* |
| Mildly active | 0.4 (0.2 – 0.61) | 0.3 (0.14 – 0.55)* | |
| Moderately active | 0.6 (0.33 – 0.94) | 0.7 (0.37 – 1.29) | |
| Highly activeRc | |||
| Protein content | High | 0.5 (0.31 – 0.80)* | 0.6 (0.30 – 1.12) |
| Medium | 0.6 (0.34 – 0.96)* | 0.6 (0.32 – 1.18) | |
| LowRc | |||
| Hours of sleep | Insufficient (<=6) | 1.1 (0.37 – 2.99) | 1.1 (0.31 – 3.9) |
| Adequate ( 7–9) | 0.8 (0.51 – 1.17) | 0.7 (0.44 – 1.26) | |
| Excess (>=10)Rc |
RC-reference category.
P < 0.05;
P < 0.01;
P < 0.0001
The multinomial logistic regression model results for adolescents of late age at menarche in reference to the average age at menarche is presented in Table 3. Every one year increase in age of adolescents increases the crude and adjusted odds of seeing menarche at a late age by about 2-folds. After adjustment for other covariates, a 1kg/m2 increase in BMI made adolescents about 20% less likely to see menarche at a later than at average age. An additional one unit increase in calorie in foods made adolescents approximately 10% less likely to see menarche at a later age than average age. Adolescents with Amhara ethnic background became 2.2 times more likely to see their menarche late in their age (AOR = 2.2; 95%CI, 1.74 –2.68). Parents' monthly income of ≥ 90 USD was found to decrease the likelihood of adolescents' menarche age by 60%-80%. Being physically inactive decreased the crude and adjusted odds of adolescents to see menarche at a late age by about 90%.
Table 3.
Results of multinomial logistic regression for adolescents of late age at menarche in reference to adolescents of average age at menarche, 2011.
| Crude | Adjusted | ||
| Predictors | Classification | OR (95%CI) | OR (95%CI) |
| Age | Scale | 0.9 (0.67 – 1.08) | 0.8 (0.58 – 1.10) |
| BMI | Scale | 1.1 (0.99 – 1.26) | 1.1 (0.97 – 1.33) |
| Calorie | Scale | 1.1 (1.01 – 1.14)* | 1.1 (1.01 – 1.18)* |
| Coffee drink/day | One cup | 0.6 (0.22 – 1.46) | 0.2 (0.06 – 0.80)* |
| Two cup | 0.4 (0.14 – 1.15) | 0.2 (0.05 – 0.70)* | |
| ≥3 cup Rc | |||
| Parents life | Both alive | 1.0 (0.12 – 8.87) | 0.5 (0.05 – 5.65) |
| Mother died | 0.8 (0.04 – 14.58) | 0.2 (0.005 – 6.31) | |
| Father died | 3.3 (0.35b – 31.74) | 1.7 (0.14 – 21.04) | |
| Both diedRc | |||
| Parents education | No education | 2.7 (1.12 – 6.50)* | 4.2 (1.13 – 15.54)* |
| Primary | 1.0 (0.41 – 2.45) | 0.6 (0.17 – 1.72) | |
| Secondary | 1.4 (0.57 – 3.62) | 1.4 (0.42 – 4.83) | |
| Tertiary Rc | |||
| Ethnicity | Gofa | 1.0 (0.51 – 2.05) | 1.1 (0.42 – 3.01) |
| Amhara | 0.9 (0.41 – 2.16) | 0.6 (0.23 – 1.69) | |
| OthersRc | |||
| Religion | Orthodox | 0.7 (0.26 – 2.06) | 1.4 (0.37 – 4.92) |
| Protestant | 0.5 (0.16 – 1.26) | 0.7 (0.17 – 2.55) | |
| OthersRc | |||
| Monthly income | >120 | 0.7 (0.33 – 1.60) | 0.5 (0.17 – 1.65) |
| in USD | 90–120 | 0.9 (0.24 – 3.71) | 0.9 (0.157 – 4.88) |
| 60–89 | 0.5 (0.19 – 1.40) | 0.3 (0.08 – 1.06) | |
| 30–59 | 1.0 (0.42 – 2.40) | 0.8 (0.26 – 2.53) | |
| < 30Rc | |||
| Exercise | Inactive | 22.6 (2.98 – 171.0)** | 31.8 (3.70–273.5)** |
| Mildly active | 7.8 (0.97 – 62.16) | 6.4 (0.71 – 56.98) | |
| Moderately active | 3.9 (0.44 – 34.24) | 3.0 (0.30 – 29.07) | |
| Highly activeRc | |||
| Protein content | High | 2.4 (1.08 – 5.12)* | 3.9 (1.35 – 11.33)* |
| Medium | 2.1 (0.91 – 4.86) | 3.3 (1.10 – 10.03) | |
| LowRc | |||
| Hours of sleep | Insufficient (<=6) | 0.6 (0.08 – 5.46) | 0.3 (0.02 – 3.35) |
| Adequate ( 7–9) | 1.3 (0.66 – 2.48) | 2.2 (0.92 – 5.18) | |
| Excess (>=10)Rc |
RC -reference category.
P < 0.05;
P < 0.01;
P < 0.0001
Table 4 shows the results of multinomial logistic regression model for adolescents of early age menarche in reference to late age menarche. Every 1 year increase in age of adolescents decreased the relative probability to see menarche at an early age by 60% (AOR = 0.4; 95%CI, 0.25 –0.53). Daughters of uneducated parents were about 10 times more likely to see menarche at earlier than late age. Adolescents with Amhara ethnic background were about 70% less likely to see menarche at earlier than late age. The AOR for inactive adolescents to see menarche at earlier than late age was extremely high. Furthermore, mildly active adolescents were about 22 times more likely to see menarche earlier than late age (AOR = 22.7; 95%CI, 2.40 –214.80). Every 1kg/m2 increase in BMI was associated with about 1.4 times increase to see menarche earlier than late age. Adolescents who drunk a cup of coffee per day relative to ≥ 3 cup of coffee per day were less likely to see menarche earlier than late age. Adolescents who got adequate sleep at night were about 3 times more likely to see menarche earlier than late age.
Table 4.
Results of multinomial logistic regression for adolescents of early age at menarche in reference to adolescents of late age at menarche, 2011.
| Crude | Adjusted | ||
| Predictors | Classification | OR (95%CI) | OR (95%CI) |
| Age | Scale | 0.5 (0.35 – 0.59)*** | 0.4 (0.25 – 0.53)*** |
| BMI | Scale | 1.2 (1.06 – 1.37)** | 1.4 (1.12 – 1.62)** |
| Calorie | Scale | 1.2 (1.08 – 1.24)*** | 1.2 (1.10 – 1.32)*** |
| Coffee drink/day | One cup | 0.6 (0.23 – 1.7) | 0.2 (0.05 – 0.93)* |
| Two cup | 0.5 (0.17 – 1.52) | 0.2 (0.05 – 1.08)* | |
| ≥3 cup Rc | |||
| Parents survival | Both alive | 0.5 (0.05 – 6.1) | 0.4 (0.02 – 6.14) |
| status | Mother died | 0.3 (0.01 – 8.18) | 0.4 (0.01 – 20.86) |
| Father died | 2.0 (0.15 – 25.75) | 1.6 (0.08 – 32.50) | |
| Both diedRc | |||
| Parents educ | No education | 2.6 (1.03 – 6.67)* | 9.7 (2.10 – 45.13)** |
| status | Primary | 0.8 (0.33 – 2.15) | 0.9 (0.23 – 3.45) |
| Secondary | 1.4 (0.53 – 3.73) | 2.4 (0.60 – 9.72) | |
| Tertiary Rc | |||
| Ethnicity | Gofa | 0.9 (0.41 – 1.78) | 1.3 (0.42 – 4.00) |
| Amhara | 0.7 (0.29 – 1.6) | 0.3 (0.08 – 0.90)* | |
| OthersRc | |||
| Religion | Orthodox | 1.0 (0.35 – 2.9) | 3.4 (0.73 – 16) |
| Protestant | 0.7 (0.23 – 1.95) | 2.1 (0.43 – 10.26) | |
| OthersRc | |||
| Monthly income | >120 | 1.3 (0.57 – 3.06) | 1.4 (0.37 – 5.35) |
| 90–120 | 3.4 (0.62 – 18.16) | 5.6 (0.55 – 57.30) | |
| 60–89 | 0.6 (0.21 – 1.6) | 0.3 (0.07 – 1.16) | |
| 30–59 | 1.2 (0.49 – 2.95) | 0.9 (0.25 – 3.36) | |
| < 30Rc | |||
| Exercise | Inactive | 268.0 (32 – 2235.2)*** | 577.6(55.80–5982)*** |
| Mildly active | 22.3 (2.80 – 180.4)** | 22.7 (2.40 – 214.80)** | |
| Moderately active | 7.0 (0.79 – 61.66) | 4.3 (0.43 – 43.7) | |
| Highly activeRc | |||
| Protein content | High | 4.7 (2.097 – 10.67)*** | 6.7 (2.00 – 22.5)** |
| Medium | 3.7 (1.50 – 8.80)** | 5.4 (1.59 – 18.46)** | |
| LowRc | |||
| Hours of sleep | Insufficient (<=6) | 0.6 (0.07 – 5.33) | 0.3 (0.02 – 3.94) |
| Adequate ( 7–9) | 1.7 (0.83 – 3.3) | 2.9 (1.10 – 7.82)* | |
| Excess (>=10)Rc |
RC -reference category.
P < 0.05;
P < 0.01;
P < 0.0001
Discussion
This study has shown that body habits and socioeconomic status significantly affected age at menarche, and the age at menarche seems declining in the study area. The mean age at menarche was earlier than the previously reported ones in Ethiopia, some African countries and other less developed countries of the world (18, 26–28), but later than a report from one of the rural districts of Bangladesh (19) and from developed countries (20). As it was found in different studies (20, 27, 29–35), there may be a declining trend of age at menarche in Ethiopia as well, which may be a proxy indicator of an ongoing improvement in the socioeconomic status of the population in the study area.
Age at menarche was directly related to the current age, level of exercise/physical activity, use of vegetables, and educational status of parents and inversely related to socioeconomic status, weight gain, increased intake of calorie and protein as well as increase in hours of sleep. This means that adolescents who were daughters of educated parents were having better exercise/physical activity, used vegetables adequately, took a wide variety of food, had adequate sleep, modified their energy and protein intake according to their energy expenditure and were less likely to see menses at an earlier age as well as in very late age.
The adjusted odds ratio also demonstrated that the level of exercise/physical activity, use of vegetables, the amount of calorie and protein content, eating habits, BMI and sleep habit of the adolescents were some of the body habits which were significantly associated with age at menarche. Specific to physical activity/exercise, the finding of this study is consistent with other studies (36, 37). These studies related their findings with higher energy expenditure in highly active girls to be the primary factor affecting the pulsatile secretion of gonadotropin releasing hormone that will result in hyperandrogenism. Other literature also pointed out that hypothalamic dysfunction associated with strenuous exercise results in disturbance of gonadotrophin releasing hormone, which in turn can delay age of menarche (44). The increase in the mean age at menarche with increase use of vegetables is higher than the study finding in India, which showed the mean age of vegetarian to be about 3.2 months higher than those adolescents taking mixed diet (38).
The other factor that affected age at menarche was an intake of calorie and protein. There was a decrease in age at menarche when there was an increase in amount of calorie and protein intake. A previous study in Britain also showed that the mean age at menarche for those girls whose diet was rich in animal protein was lower than that of those girls whose diet was not enriched with animal protein (45). There was also a report from South Africa that found adolescents who had a large intake of animal proteins experienced menarche at least four to five months earlier than adolescents who were not taking animal source protein (46) and this was also supported by a cross-sectional study conducted in one of the rural districts of Bangladesh (19).
The inverse association of age at menarche with BMI found in this study is similar to other studies (47–51). The underlying mechanism for such association was stated as still unclear. Some authors speculated that high BMI during childhood (especially 9–11 years) is directly associated with high BMI later in their lives, and can reflect the changes in growth and body composition in the prepubertal period (42, 48). As a result, it has been suggested that childhood high BMI drives the age at onset of sexual maturation and hence the age at menarche.
Similar to other reports (27, 29, 51), current ages of the participants, monthly income, address, academic achievement and ethnicity were among the socioeconomic factors that are significantly associated with age at menarche. This may be explained by the fact that the increase in socioeconomic status is likely to improve the nutritional and health status of adolescents and thereby contributing to the early pubertal onset including age at menarche. Unlike another study (where 98% of females reached menarche by age 15 years) (20), 35% of this study participants do not reach menarche until 15 years, which can be a clue indicating the possibility of primary amenorrhea in some of the study participants as the definition of primary amenorrhea is no menarche by the age of 16 years regardless of secondary sexual characteristics (44, 52). But, there is no literature that demonstrates the association of late menarche with any of the long term medical problems.
On the other hand, 10.5% of the adolescents had menarche at the age of 12 years which should not be underestimated when we compare with reports from well developed nations like USA where only 10% of females are menstruating at age 11.11 years (20, 44). In other words, in this study, this much proportion of adolescents with early menarche is more worrying than the high proportion of late menarche. This is because several studies have reported that early menarche is strongly associated with breast cancer, ovarian cancer, hypertension, diabetes, asthma, and metabolic syndrome in later age (10–17). The implication is that early menarche is highly associated with childhood weight gain, physical inactivity, high protein and high calorie nutrition, which all these are in turn likely to be associated with the above metabolic disorders and malignancies.
The implication is that literature review has shown how risky early menarche is. But, we do not think that parents and policy makers in this country are aware of the risks of early menarche and risk factors for early menarche. Particularly in urban areas, where childhood obesity is observed here and there, the issue of early menarche and its long term complications is probably going to be a serious public health issue in the future unless preventive actions are implemented. Therefore, parents have to know that several non-infectious diseases during adulthood are strongly linked with early menarche, which is again strongly associated with childhood obesity and physical inactivity. In short, a history of early menarche is probably a strong signal for several medical disorders and malignancies to come during adulthood.
This study is not without limitations. From our day to day observation, there are several school children in urban areas who started menstruating early in their age and physically appear overweight and obese. Thus, the findings in this study may not reflect the adolescent population in big towns where more adolescents are probably entering early into their puberty age. Therefore, in Ethiopia, assessing menarche age and its risk factors in a large scale study warrants urgent due consideration. Because of the lengthy data collection, mainly due to time-taking anthropometric measurements, information contamination was somehow unavoidable. The too wide 95% CI in some variables also indicate a low level of precision of the OR. Since the majority of the data were based on the adolescents' recent memory, there is a possibility of recall bias. Secondly, since the age of menarche is dependent on age of puberty, which is in turn influenced by several factors (32–37) (not all included in this analysis), the findings should be interpreted cautiously.
This study has shown that the mean menarche age was higher than the report from developed countries. But, the proportion of adolescents with a low menarche age was comparable with reports from developed countries. The majorities of the adolescents with early age at menarche were overweight, inactive, had unhealthy dieting and eating habits, and were daughters of uneducated parents. The finding of more than one third of adolescents having experienced late menarche may indicate the other extreme (probably malnutrition, strenuous work and stress). Therefore, we recommend promoting healthy dieting and eating habits, emphasizing the importance of regular exercise/physical activity, and provision nutrition education for parents and growing children. These findings can serve as a baseline, 1) to estimate the mean age of menarche for the nation, 2) to assess the perception of the people about early menarche and childhood obesity.
References
- 1.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. The Journal of pediatrics. 1985;107(3):317–329. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 2.World health organization (WHO), author The health of youth. Geneva: WHO; 1989. Document A42/Technical discussions//2. Available from: whqlibdoc.who.int/hq. [Google Scholar]
- 3.Smith G. Age at menarche & the risk of operative first delivery. BJOG. 2009;116:1613–1621. doi: 10.1111/j.1471-0528.2009.02340.x. [DOI] [PubMed] [Google Scholar]
- 4.Marston C, Cleland J. The effect of contraception on Obstetric Outcomes. Geneva: WHO; 2004. [Google Scholar]
- 5.Tewodros A, Jemal H, Dereje H. Determinants of Adolescents fertility in Ethiopia. Ethiop J Health Dev. 2010;24(1):30–38. [Google Scholar]
- 6.Central statistics Agency, author. Ethiopia Demography and Health Survey. Addis Ababa, Ethiopia: 2011. Available from: measuredhs.com/pubs/pdf/FR255/FR255.pdf. [Google Scholar]
- 7.Golub MS, Collman GW, Foster PM, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121(3):218–230. doi: 10.1542/peds.2007-1813G. [DOI] [PubMed] [Google Scholar]
- 8.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 9.Laurie B. Late Age of Menarche Linked to Lower Risk for Endometriosis. American Journal of Obstetrics and Gynecology. 2010 Available from: http://www.medscape.com/viewarticle/714499. [Google Scholar]
- 10.Ferenc M. Early Age at Menarche, Lung Function, and Adult Asthma. Am J Respir Crit Care Med. 2011;183(1):8–14. doi: 10.1164/rccm.200912-1886OC. [DOI] [PubMed] [Google Scholar]
- 11.Remsberg KE, Demerath EW, Schubert CM, Chumlea WC, Sun SS, Siervogel RM. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab. 2005;90:27:18–24. doi: 10.1210/jc.2004-1991. [DOI] [PubMed] [Google Scholar]
- 12.Mike, author. Mike's calorie and fat gram chart for 1000 foods, health advantage. 1996–2009. Available from: http//www.nutricounter.com.
- 13.Ninemsn, author. Health and wellbeing. Family health, adolescents eating habit. Ninemsn Pty Ltd; 2005. Available from: http//www.ninemsn.com. [Google Scholar]
- 14.Chris E. Good habits for healthy body. EzineArticles RSS; 2010. Available from: http://EzineArticles.com/expert. [Google Scholar]
- 15.Gavin ML. Growth and Puberty. What is the right weight for my height? Nemours foundation; 2008. Available from: http://www.teenshealth.com. [Google Scholar]
- 16.Collins A. Healthy Eating and Weight Loss for Teenagers. 2007. Available from: http://www.annecollins.com.
- 17.Thomas F, Renaud F, Benefice E, de Meeüs T, Guegan JF. International variability of ages at menarche and menopause: patterns and main determinants. Human Biology. 2001;73(2):271–290. doi: 10.1353/hub.2001.0029. [DOI] [PubMed] [Google Scholar]
- 18.Zegeye DT, Megabiaw B, Mulu A. Age at menarche and the menstrual pattern of secondary school adolescents in northwest Ethiopia. BMC women health. 2009;9:29. doi: 10.1186/1472-6874-9-29. Available from: www.biomedcentral.com/bmcwomenshealth/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouj U, Ve E. Age at menarche & the menstrual pattern of women Igbo state southwest Nigeria. Afric J Reprod Health. 2008;1(2):90–95. [PubMed] [Google Scholar]
- 20.Barnes-Josiah D, Augustin A. Secular trend in the age at menarche in Haiti. Am J Hum Biol. 1997;7:357–362. doi: 10.1002/ajhb.1310070311. [DOI] [PubMed] [Google Scholar]
- 21.Rah JH, Shamim AA, Arju UT, Labrique AB, Rashid M, Christian P. Age of Onset, Nutritional Determinants, and Seasonal Variations in Menarche in Rural Bangladesh. J Health Popul Nutr. 2009;27(6):802–807. doi: 10.3329/jhpn.v27i6.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chumlea WC, Shubert CM, Roche AF, et al. Age at menarche and racial comparisons in US girls. Pediatrics. 2003;111:110–113. doi: 10.1542/peds.111.1.110. [DOI] [PubMed] [Google Scholar]
- 23.Okasha M, McCarron P, McEwen J, et al. Age at menarche: secular trends and association with adult anthropometric measures. Ann Hum Biol. 2001;28:68–78. doi: 10.1080/03014460150201896. [DOI] [PubMed] [Google Scholar]
- 24.Clavel-Chapelon F, E3N-EPIC group Evolution of age at menarche and at onset of regular cycling in a large cohort of French women. Hum Reprod. 2002;17:228–232. doi: 10.1093/humrep/17.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanner JM. Trends towards earlier menarche in London, Oslo, Copenhagen, the Netherlands and Hungary. Nature. 1973;243:95–96. doi: 10.1038/243095a0. [DOI] [PubMed] [Google Scholar]
- 26.Whincup PH, Gilg JA, Odaki K, Taylor SJ, Cook DG. Age at menarche in contemporary British teenagers. BMJ. 2001;322:1095–1096. doi: 10.1136/bmj.322.7294.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 28.Andersen SE, Dallal GE, Must A. Relative weight and race influences average age at menarche: results from two internationally representative surveys of US Adolescentsstudied 25 years apart. Pediatrics. 2003;111:844–850. doi: 10.1542/peds.111.4.844. [DOI] [PubMed] [Google Scholar]
- 29.Styne DM. Puberty, obesity and ethnicity. Trends Endocrinol Metab. 2004;15:472–478. doi: 10.1016/j.tem.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Sidhu L, Grewal R. Age of menarche in various categories of Indian Sportswomen. British Journal of Sports Medicine. 1980;14:199–203. doi: 10.1136/bjsm.14.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren MP, Perlroth NE. The effects of intense exercise on the female reproductive system. Journal Endocrinology. 2001;170:3–11. doi: 10.1677/joe.0.1700003. [DOI] [PubMed] [Google Scholar]
- 32.Rokade S, Mane A. A Study of Age at Menarche, The Secular Trend And Factors Associated With It. The Internet Journal of Biological Anthropology. 2009;3:2. [Google Scholar]
- 33.St George IM, Williams S, Silva PA. Body size and menarche: the Dunedin Study. J Adolesc Health. 1994;15:573–576. doi: 10.1016/1054-139x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- 34.Biro FM, McMahon RP, Striegel-Moore, et al. Impact of timing of pubertal maturation on growth in black and white female adolescent: The National Heart, Lung and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–643. doi: 10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- 35.Laitinen J, Power C, Jarvelin MR. Family social class, maternal body mass index, childhood body mass index and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74:287–294. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- 36.Freedman DS, Kettel Khan L, Serdula MK, et al. The relation of age at menarche to obesity in childhood and adulthood: the Bogalusa Heart Study. BMC Pediatr. 2003;3:3. doi: 10.1186/1471-2431-3-3. Available from: www.biomedcentral.com/bmcpediatrics/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ketheleen M, Rosalind B, Penny G. Healthy habits Among Adolescents: Sleep, Exercise, Diet and Body Image Child trends. 2003. For Indicators of Positive Development Conference. Available from: www.childtrends.org.
- 38.Padmavati V, Poosha DVR, Busi BR. A note on age at menarche and its relationship to diet, economic class, sibship size and birth order in 300 Andhra girls. Man in India. 1984;64(2):175–180. [PubMed] [Google Scholar]
- 39.Roger IS, Northstone K, Dunger DB, Cooper AR, Ness AR, Emmett PM. Diet throughout childhood and age at menarche in a contemporary cohort of British girls. Public Health Nutr. 2010;13(12):2052–2063. doi: 10.1017/S1368980010001461. [DOI] [PubMed] [Google Scholar]
- 40.Kark E. Menarche in South African Bantu girls. South African J Med Sce. 1943;8:35–40. [Google Scholar]
- 41.Van Lenthe FJ, Kemper HCG, van Mechelen W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. American J Clin Nutr. 1996;64:18–24. doi: 10.1093/ajcn/64.1.18. [DOI] [PubMed] [Google Scholar]
- 42.Power C, Lake JK, Cole TJ. Body mass index and height from childhood to adulthood in the 1958 British born cohort. American J Clin Nutr. 1997;66:1094–1101. doi: 10.1093/ajcn/66.5.1094. [DOI] [PubMed] [Google Scholar]
- 43.Biro FM, McMahon RP, Striegel-Moore, et al. Impact of timing of pubertal maturation on growth in black and white female adolescent: The National Heart, Lung and Blood Institute Growth and Health Study. J Pediatr. 2001;138:636–643. doi: 10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- 44.Laitinen J, Power C, Jarvelin MR. Family social class, maternal body mass index, childhood body mass index and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74:287–294. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- 45.Muhammad N. Age at menarche and the related issues. Journal of Youth and Adolescent. 1984;13(6):559–567. doi: 10.1007/BF02088599. [DOI] [PubMed] [Google Scholar]
- 46.The American College of Obstetricians and Gynecologists, author. Menstruation in adolescents: using the menstrual cycle as a vital sign. Vol. 349. ACOG committee opinion Washington DC; 2006. pp. 49–51. Available from: www.acog.org/Committee/AdolescentHealth. [Google Scholar]