Abstract
Erythrocytic malaria parasites degrade hemoglobin in an acidic food vacuole to acquire free amino acids and maintain parasite homeostasis. Hemoglobin hydrolysis appears to be a cooperative process requiring cysteine proteases (falcipains) and aspartic proteases (plasmepsins), but the specific roles of different enzymes in this process are unknown. We previously showed that falcipain-2 is a major trophozoite food vacuole cysteine protease. To characterize the specific role of falcipain-2, we disrupted the falcipain-2 gene and assessed the effect of this alteration. Falcipain-2-knockout trophozoites had markedly diminished cysteine protease activity and swollen, dark staining food vacuoles, consistent with a block in hemoglobin hydrolysis, as caused by cysteine protease inhibitors. However, more mature stages of knockout parasites were indistinguishable from wild-type parasites and developed normally. The knockout parasites had decreased and delayed expression of falcipain-2, which appeared to be directed by increased transcription of a second copy of the gene (falcipain-2′). Expression of other falcipains and plasmepsins was similar in wild-type and knockout parasites. Compared with wild-type, knockout parasites were about 3 times more sensitive to the cysteine protease inhibitors E-64 and leupeptin, and over 50-fold more sensitive to the aspartic protease inhibitor pepstatin. Our results assign a specific function for falcipain-2, the hydrolysis of hemoglobin in trophozoites. In addition, they highlight the cooperative action of cysteine and aspartic proteases in hemoglobin degradation by malaria parasites.
Malaria, particularly disease caused by Plasmodium falciparum, causes several hundred million illnesses and over a million deaths each year (1). Control of malaria has been difficult, in part because of increasing resistance to available drugs (2, 3). New antimalarial drugs, ideally directed against new targets, are greatly needed. Among potential new targets for antimalarial drugs are parasite proteases. Proteases of multiple classes appear to be involved in critical processes during parasite development (4–7). One of these processes is hemoglobin degradation. Malaria parasites hydrolyze hemoglobin in an acidic food vacuole to obtain free amino acids for protein synthesis and possibly to maintain osmotic stability (8–10). Plasmodial aspartic, cysteine, and metallo proteases all appear to participate in hemoglobin hydrolysis, and cooperative functions of different classes of proteases in this process have been proposed (7, 11–15).
Four genes encode cysteine proteases of P. falciparum, known as falcipains. Falcipain-1, a fairly distant relative of the other falcipains, is encoded on chromosome 14 and has been implicated in erythrocyte invasion by merozoites (16, 17). The other three falcipains, two nearly identical copies of falcipain-2 (18) and a single falcipain-3 (19), are encoded within a 12.5-kb stretch of chromosome 11 (http://PlasmoDB.org) and appear to play key roles in hemoglobin hydrolysis by parasites, as supported by the following data. First, cysteine protease inhibitors block the hydrolysis of hemoglobin by cultured parasites, causing the food vacuole to swell and fill with undegraded hemoglobin (11, 20–22). Second, cysteine protease inhibitors block the development of cultured P. falciparum parasites (11, 23) and cure mice infected with murine malaria parasites (24). Third, falcipain-2 and falcipain-3 localize to the food vacuole, are active at the acidic pH of the food vacuole, and readily hydrolyze native and denatured hemoglobin (18, 19). The two copies of falcipain-2 are nearly identical in sequence (97.5% amino acid identity between the mature proteases), but only falcipain-2 was identified by sequencing of the purified native enzyme (18), and the other copy (falcipain-2′) has not yet been biochemically characterized. Falcipain-2 and falcipain-3 are 66.6% identical in their catalytic domains, but they differ in stage-specificity, with maximal expression of falcipain-2 in early trophozoites, and of falcipain-3 in more mature parasites (19). Considering aspartic proteases, plasmepsin I, plasmepsin II, plasmepsin III [histoaspartic protease (HAP)], and plasmepsin IV have been localized to the food vacuole and shown to hydrolyze hemoglobin (15), and these proteases likely participate with falcipain-2 and falcipain-3 in hemoglobin degradation. However, specific functions have not yet been definitively delineated for any plasmodial proteases.
To begin to characterize specific functions of the falcipains, we disrupted the falcipain-2 gene and evaluated the impact of this genetic manipulation. Recombinant parasites demonstrated altered hemoglobin hydrolysis and increased sensitivity to protease inhibitors. However, they recovered from the transient block in hemoglobin hydrolysis, presumably because of expression of falcipain-2′ and falcipain-3. These results confirm a key role for falcipain-2 in hemoglobin hydrolysis by trophozoites and highlight the cooperative roles of multiple proteases in this process.
Materials and Methods
Materials. P. falciparum strain D10 and the plasmid pHRP/GFPM2 were obtained from the Malaria Research and Reference Reagent Resource Center (MR4, managed by the American Type Culture Collection), contributed by Yemin Wu and Kasturi Haldar (25), respectively. The transfection plasmid pDC, the experimental drug WR99210, and anti-plasmepsin antibodies were kind gifts from David Fidock (26), David Jacobus (Jacobus Pharmaceutical, Princeton), and Daniel Goldberg (Washington University School of Medicine, St. Louis), respectively. Benzyloxycarbonyl-Leu-Arg-7-amino-4-methylcoumarin (Z-Leu-Arg-AMC) was from Peptides International. All other biochemical reagents were from Sigma or Fisher.
Parasite Culture, Collection, and Processing. P. falciparum strain D10 was cultured in human erythrocytes at 2% hematocrit in RPMI medium 1640 supplemented with 10% human serum (27). Synchronization was maintained by serial treatment with 5% d-sorbitol (28). Parasites were harvested at 6–10% parasitemia as described earlier (19), and parasite pellets were suspended in 300 μl of PBS [with 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM EDTA, 2 mM benzamidine·HCl, 10 μM pepstatin, and 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF)] for immunoblot and activity analysis or TRIzol (Invitrogen) for RNA isolation. For processing, half of each parasite suspension was solubilized in reducing SDS/PAGE buffer (total lysate) and the other half was subjected to fractionation as described earlier (19) to prepare total parasite extracts or vacuolar fractions. Genomic DNA (gDNA) was isolated from schizont-rich parasites by using the Puregene DNA isolation kit (Gentra Systems). Total parasite RNA was isolated by using TRIzol (Invitrogen).
Construction of Transfection Plasmids. Primers and probes are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. DNA encoding the proregion of falcipain-2 (TFP2-F/TFP2-R) and the green fluorescent protein (GFP) (GFP-F/3R) were PCR amplified from the pTOP-FP2 (18) and pHRP/GFPM2 plasmids (25), respectively, by using Vent DNA polymerase (New England Biolabs). XhoI- and NotI-digested PCR products were cloned into XhoI-digested and dephosphorylated pDC plasmid (29) to obtain transfection plasmids with a human dihydrofolate reductase selectable marker for WR99210 and the PFP2/GFP construct within a cam 5′/hsp86–3′ expression cassette in sense (pDC-PFP2/GFPS) or antisense (pDC-PFP2/GFPA) orientation.
Generation of Falcipain-2 Knockout (FP2KO) Parasites. Ring-stage parasites (100-μl packed cell volume, 4–6% parasitemia) were transfected with 100 μg of pDC-PFP2/GFP plasmids [prepared with the Mobius 1000 Plasmid kit (Novagen)] in a 0.2-cm cuvette by using a Gene Pulser II (Bio-Rad) at 0.31 kV, 950 μF, and maximum resistance (29). WR99210 (2.5 nM) was added 48 h after the electroporation and was maintained thereafter. One week after emergence of the resistant parasites (3–4 weeks after transfection), WR99210 was increased to 5.0 nM. gDNA was extracted from parasites and analyzed for chromosomal integration of the plasmid by PCR using integration (1F/3R) and WT specific (1F/2R) primers. To eliminate parasites containing episomal plasmids, cultures were subjected to two cycles of incubation with and without WR99210 (5 nM), each for 3 weeks. Recombinant parasites were then cloned by limiting dilution.
Southern Hybridization. For Southern hybridization, gDNA was digested with restriction endonucleases, separated on agarose gels, transferred to Hybond-N membranes (Amersham Pharmacia Biosciences), and probed with the [α-32P]dATP-labeled prodomain of falcipain-2, (Megaprime DNA labeling system; Amersham Pharmacia Biosciences) as described earlier (19).
Immunoblotting. Proteins were resolved by SDS/PAGE (30) and transferred onto poly(vinylidene difluoride) (PVDF) membranes (Bio-Rad). Membranes were incubated with blocking buffer (PBS/0.2% casein/0.1% Tween 20) containing appropriate dilutions of antibodies to falcipains (19), GFP (Sigma, 1/2,000), and plasmepsins (15) followed by alkaline phosphatase-conjugated IgG in blocking buffer (goat anti-rat IgG for falcipains, goat anti-mouse IgG for plasmepsin I and histoaspartic protease, and goat anti-rabbit IgG for plasmepsin II and IV, all 1/10,000 dilutions), and reactions were developed by using a Western-Star chemiluminescence kit (Tropix).
Analysis of Parasites by Microscopy. For bright-field microscopy, Giemsa-stained parasite smears were analyzed. Transmission electron microscopy was performed as described in Supporting Materials and Methods. Immunofluorescence microscopy was carried out as described for a prior study (25).
Assay of Protease Activity. Parasite extracts were assessed by measuring hydrolysis of the fluorogenic peptide substrate Z-Leu-Arg-AMC and native hemoglobin, both as described (19).
Analysis of Falcipain Transcription. cDNA was produced from 5–10 μg of DNA-free RNA by using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). For real-time PCR, specific primers (RTP1F/RTP1R, falcipain-1; RTP2F/1R, falcipain-2; RTP2′F/RTP2′R, falcipain-2′; RTP3F/RTP3R, falcipain-3; and 18SF/18SR, 18S rRNA) and probes were designed based on 3′ regions of falcipains and 18S rRNA by using primer3 software (www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). 5′ FAM/3′-TAMRA-labeled probes were obtained from Biosearch Technologies, and reactions were carried out as described in Supporting Materials and Methods. To analyze transcription loci, PCR was performed with 50 ng of gDNA and 250 ng of pooled multistage cDNA of WT and FP2KO parasites by using Taq DNA polymerase (Invitrogen) and primers specific for full-length WT falcipain-2 (1F/1R), recombined falcipain-2 (2F/1R), falcipain-3 (FP3UP/RTP3R), and falcipain-2′ (2′UP/RTP2′R).
Measurement of Parasite Growth Rates and Inhibitor Sensitivity. Synchronized parasites were cultured in complete or minimal medium (amino acid-free RPMI medium 1640/10% dialyzed human serum, containing 41.1 mg/liter hypoxanthine, 300 mg/liter glutamine, 20 mg/liter glutamic acid, 50 mg/liter isoleucine, 15 mg/liter methionine, and 50 mg/liter cysteine), as previously described (31). At each time point, parasites were fixed with 1% formaldehyde in PBS, pH 7.4, for 48 h at room temperature and then labeled with YOYO-1 (1 nM; Molecular Probes) in 0.1% Triton X-100 in PBS (32). Parasitemias were determined from dot plots (forward scatter vs. fluorescence) acquired on a FACSort flow cytometer using cellquest software (Becton Dickinson).
To evaluate the effects of protease inhibitors and antimalarial drugs, parasites were cultured for 48 h with various concentrations of compounds added from ×1,000 stocks, parasitemias were determined as described above, and IC50 values were calculated as previously described (33). In each case, goodness of curve fit was documented by R2 values of >0.95.
Results
Disruption of the Falcipain-2 Locus. To disrupt the falcipain-2 gene, erythrocytes infected with ring-stage P. falciparum D10 parasites were electroporated with pDC-PFP2/GFPA and pDC-PFP2/GFPS plasmids, selected with WR99210, and periodically analyzed for chromosomal integration of plasmid DNA by PCR (Fig. 1). WR99210-resistant parasites appeared after 3–4 weeks in pDC-PFP2/GFPA-transfected cultures only. About 12 weeks after electroporation, the mixed population of recombinant and WT parasites was subjected to two alternating cycles of growth with and without WR99210 to eliminate parasites containing episomal plasmids. Cloning by limiting dilution then yielded 11 clones with insertion of the plasmid at the proregion of the falcipain-2 locus and absence of intact WT falcipain-2, as determined by PCR (Fig. 2A).
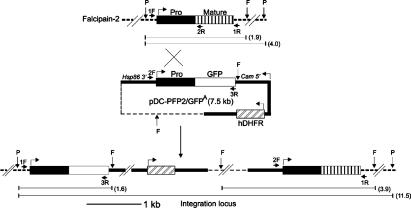
Fig. 1.
Schematic of falcipain-2 gene disruption. The WT falcipain-2 locus, transfection plasmid (pDC-PFP2/GFPA), and integration locus after a single crossover are shown. Thick lines represent regulatory regions, thin dotted lines indicate the plasmid backbone, and thick dotted lines represent the chromosomal DNA. Bent arrows indicate orientations, horizontal arrows represent the locations of primers, and vertical arrows indicate the locations of restriction endonuclease sites. The sizes of restriction fragments are shown in kilobases in parentheses.
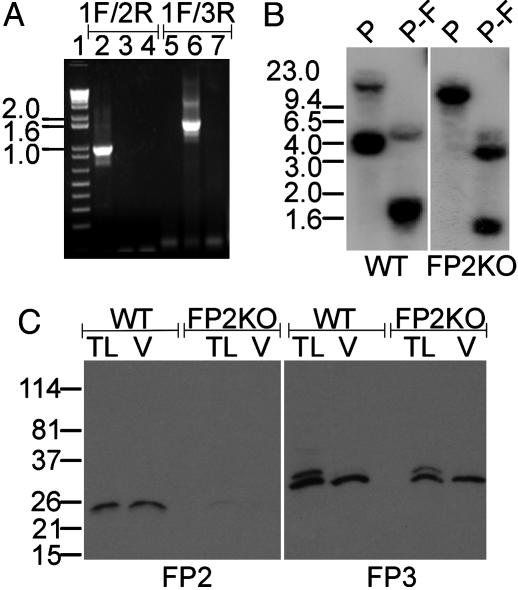
Fig. 2.
Verification of falcipain-2 gene disruption. (A) Ethidium bromide-stained agarose gel showing PCR products from WT (lanes 2 and 5) and FP2KO (lanes 3 and 6) gDNAs and pDC-PFP2/GFPA plasmid DNA (lanes 4 and 7) when primers specific for WT falcipain-2 (1F/2R) and the integration locus (1F/3R) are used. The sizes of DNA markers (lane 1) are indicated (kb). (B) Southern blot of PacI (P) and PacI–FokI (P-F) gDNA digests of WT and FP2KO parasites probed with the falcipain-2 proregion. Fragments of 11.5 kb (P) and 1.6 and 3.9 kb (P-F) for FP2KO gDNA and of 4 kb (P) and 1.9 kb (P-F) for WT gDNA indicate integration of a single copy of pDC-PFP2/GFPA into the proregion of falcipain-2 by single-crossover homologous recombination. The weaker signals of 15 kb (P) and 4 kb (P-F) correspond to falcipain-2′.(C) Immunoblots of early (24–28 h) trophozoite lysates (TL) and vacuolar fractions (V) of WT and FP2KO parasites (2 × 107 per lane) probed with antibodies to falcipain-2 (FP2) and falcipain-3 (FP3). The positions of molecular mass standards are indicated (kDa).
Integration of the plasmid within the proregion of falcipain-2 by a single-site crossover was predicted to generate FP2KO parasites, with an upstream proregion-GFP fusion under the control of the falcipain-2 promoter, but lacking a 3′ untranslated region, and a downstream, recombined falcipain-2 ORF lacking a native 5′ untranslated region (Fig. 1). Hybridization of gDNA digests of FP2KO and WT parasites with a proregion probe showed patterns consistent with this prediction (Fig. 2B). To confirm the disruption of falcipain-2 expression, WT and recombinant early trophozoites were evaluated by immunoblotting. Multiple clones of recombinant trophozoites showed a dramatic diminution in falcipain-2 expression (although a faint band was seen), but expressed falcipain-3 equivalently to WT parasites (Fig. 2C). A single recombinant clone was studied in all subsequent experiments. Expression of GFP was not seen by either immunofluorescence or immunoblotting, perhaps because the proregion-GFP fusion did not have an intact 3′ untranslated region.
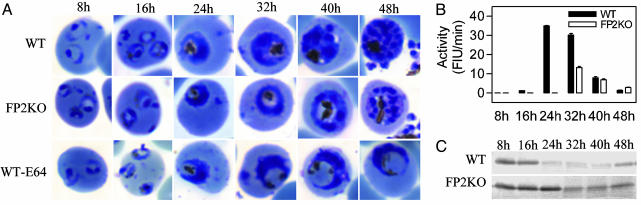
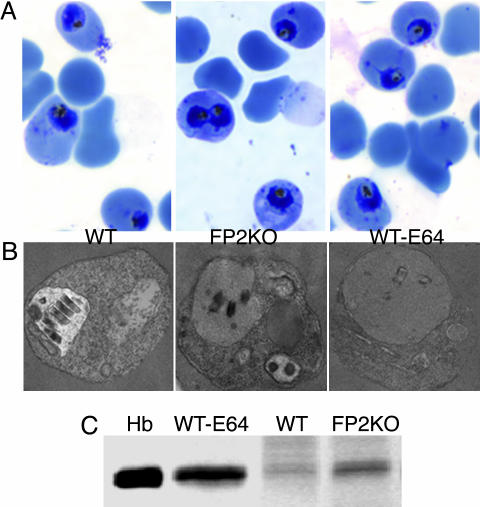
Effect of Falcipain-2 Gene Disruption on Parasite Development and Cysteine Protease Activity. FP2KO parasites developed a phenotype similar to that seen in cysteine protease inhibitor-treated parasites, whereby the food vacuole of early trophozoites was swollen and dark-staining, although the abnormality was not as prominent as in WT parasites treated with E-64 (Fig. 3A). However, this phenotype was transient, as more mature FP2KO parasites were not distinguishable from WT parasites, and they developed normally into schizonts (Fig. 3A). Compared to WT, the FP2KO parasites had markedly reduced activity against Z-Leu-Arg-AMC and hemoglobin in early-mid trophozoites, but in more mature parasites activities were similar (Fig. 3 B and C). To further characterize the phenotype of FP2KO early trophozoites, these parasites were examined by electron microscopy, and intracellular hemoglobin was quantified. Compared with WT parasites, both FP2KO and E-64-treated WT parasites showed accumulation of undigested hemoglobin (Fig. 4), although inhibitor-treated parasites demonstrated a more marked abnormality. Taken together, these data indicate that falcipain-2 plays a major role in the hydrolysis of hemoglobin by P. falciparum trophozoites.
Fig. 3.
Effect of falcipain-2 gene disruption on parasite development. Synchronized WT, FP2KO, and E-64-treated (10 μM, beginning at 0 h) (WT-E64) parasites were collected at different stages, stained with Giemsa stain, and evaluated by light microscopy (A). Extracts were assessed for the hydrolysis of Z-Leu-Arg-AMC [4.8 × 106 parasites per reaction, measured as fluorescent intensity units (FIU)/min, B] and native hemoglobin (1.2 × 107 parasites and 2 μg of hemoglobin per reaction for2hat37°C) visualized by Coomassie staining after SDS/PAGE (C), each in 100 mM sodium acetate, pH 5.5, with 10 mM DTT (B) or 2.5 mM reduced glutathione (C). Error bars represent the standard deviations of means from three replicate assays.
Fig. 4.
Evaluation of trophozoite hemoglobin hydrolysis. Early (24-h) trophozoites of FP2KO, WT, and E-64-treated (10 μM, beginning at 0 h) (WT-E64) parasites were evaluated by Giemsa staining (A) and electron microscopy (B). Erythrocytes infected with equal numbers of these trophozoites were lysed with saponin, washed to remove erythrocyte hemoglobin, and solubilized in reducing sample buffer, and proteins were resolved by SDS/PAGE and stained with Coomassie blue (C). Hb represents a hemoglobin control.
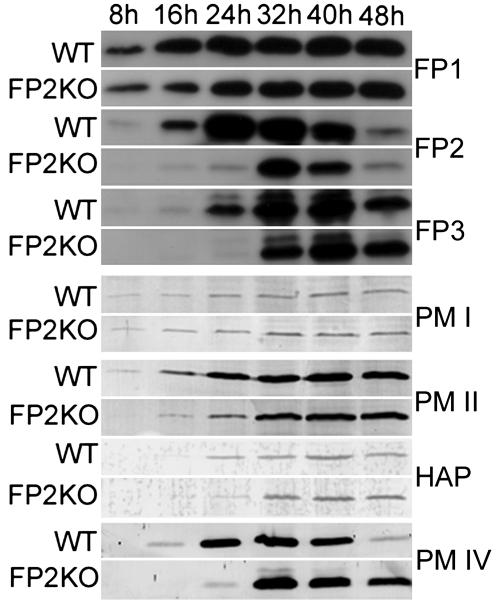
Expression of Proteases in FP2KO Parasites. After a transient abnormal phenotype, FP2KO parasites developed normally, suggesting the recovery of essential protease activity in mature parasites. We examined the expression profiles of falcipains and plasmepsins across the life cycle by immunoblotting with specific antibodies (Fig. 5). For falcipain-2, which was nearly absent from early trophozoites, recovery of expression, albeit at below normal levels, was seen in late trophozoites and early schizonts. However, immunoblotting could not distinguish falcipain-2 and the nearly identical falcipain-2′. Expression of the other well characterized hemoglobinase, falcipain-3, was somewhat delayed in FP2KO compared with WT parasites, although expression levels did not change markedly. Expression of falcipain-1 was not appreciably changed between FP2KO and WT parasites. Expression of food vacuole plasmepsins was generally similar between WT and FP2KO parasites, although as with falcipain-3, expression was somewhat delayed for some of these enzymes, in particular plasmepsin IV.
Fig. 5.
Expression of proteases in FP2KO parasites. Synchronized FP2KO and WT parasites were collected at the indicated time points, and immunoblots (2 × 107 parasites per lane) were probed with monospecific antibodies to falcipain-1 (FP1), falcipain-2 (FP2), falcipain-3 (FP3), plasmepsin I (PM I), plasmepsin II (PM II), histoaspartic protease (HAP), and plasmepsin IV (PM IV).
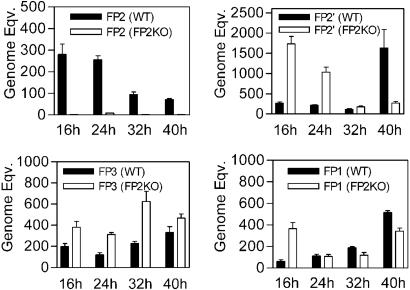
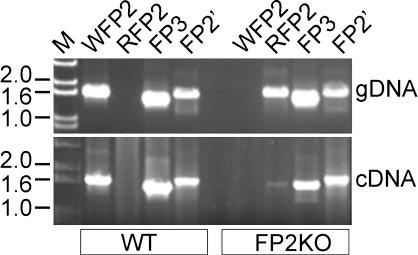
To compare transcription of falcipain genes and discriminate expression of falcipain-2 and falcipain-2′, we determined transcript levels of each falcipain by using real-time PCR (Fig. 6). Compared with WT, FP2KO parasites showed negligible levels of FP2 mRNA throughout the life cycle. In contrast, FP2KO parasites maintained transcription of falcipain-2′ mRNA, with increased transcription in early-stage parasites. mRNA levels of falcipain-3 and falcipain-1 showed relatively modest changes between FP2KO and WT parasites. We additionally performed PCR with genomic DNA and pooled multistage cDNA by using primers designed to discriminate WT and recombined falcipain-2 coding sequences. WT falcipain-2 specific primers amplified a product of expected size from WT, but not FP2KO, cDNA and gDNA (Fig. 7). Primers specific for recombined falcipain-2 amplified the coding sequence in FP2KO gDNA, as expected, and also identified very low level transcription of the promoterless recombined falcipain-2 gene. Transcription of falcipain-2′ and falcipain-3 was the same in WT and FP2KO parasites. These data confirm that FP2KO parasites contain a disrupted falcipain-2 locus, but suggest that limited transcription of recombined falcipain-2 is present, possibly driven by an upstream expression cassette promoter. In addition, our PCR data suggest that the protein reacting with anti-falcipain-2 antisera in FP2KO parasites is principally falcipain-2′.
Fig. 6.
Quantitation of falcipain transcript levels. cDNA from synchronized FP2KO and WT parasites collected at the indicated time points was evaluated for transcript levels of an 18S rRNA control, falcipain-2 (FP2), falcipain-2′ (FP2′), falcipain-3 (FP3), and falcipain-1 (FP1) by real-time PCR, and normalized genome equivalents (transformed by 104) were determined. Error bars represent standard deviations of means from three replicate experiments.
Fig. 7.
PCR analysis of transcription loci. The ethidium bromide-stained agarose gel shows amplification from gDNA and pooled multistage cDNA of WT and FP2KO parasites by using primers specific for full-length WT falcipain-2 (WFP2), recombined falcipain-2 (RFP2), falcipain-3 (FP3), and falcipain-2′ (FP2′). The positions of DNA size markers (M) are indicated (kb).
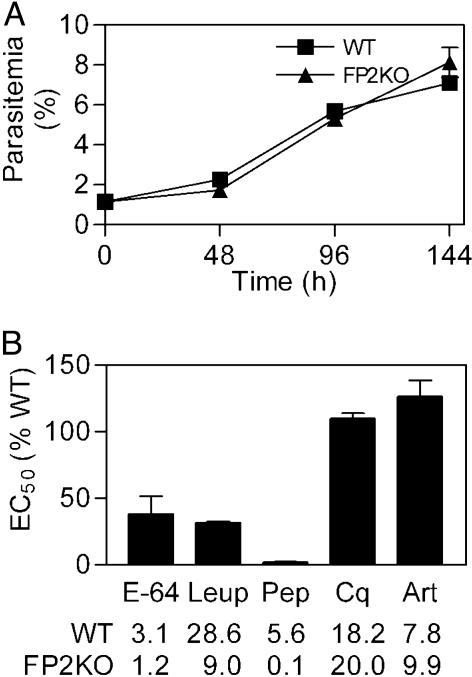
Growth of FP2KO Parasites and Susceptibility to Protease Inhibitors. Under standard culture conditions, WT and FP2KO parasites showed very similar multiplication rates over three cycles of growth (Fig. 8A). We suspected that the growth of the FP2KO parasites might be altered in cultures depleted of essential amino acids. However, with depletion of all but five essential amino acids (31), the multiplication rates of WT and FP2KO parasites were the same (approximately half that of parasites in complete medium; data not shown).
Fig. 8.
Multiplication rates and inhibitor sensitivity. (A) WT and FP2KO parasites were cultured in complete medium for three life cycles, and parasitemias were assessed at the end of each cycle. (B) Parasites were incubated with various concentrations of chloroquine (Cq), artemisinin (Art), E-64, leupeptin (Leup), and pepstatin (Pep) for one full life cycle, beginning at the ring stage, parasitemias were then determined, and IC50 values were calculated. Error bars represent the standard deviations of means from three (two for Cq and Art) independent experiments, each performed in duplicate. IC50 values for WT and FP2KO parasites are shown (μM, except nM for Cq and Art).
We also suspected that FP2KO parasites might be more sensitive than WT to the antimalarial effects of protease inhibitors. Indeed, FP2KO parasites were much more sensitive to cysteine protease inhibitors (Fig. 8B), with about 3 times higher sensitivity to E-64 and leupeptin. Remarkably, differences in sensitivity were even more pronounced with the aspartic protease inhibitor pepstatin, with FP2KO parasites about 50 times more sensitive to this compound than were the WT parasites. FP2KO and WT parasites were equally sensitive to standard antimalarial compounds. These data support a cooperative role for cysteine and aspartic proteases in hemoglobin hydrolysis.
Discussion
Biochemical studies indicate that plasmodial proteases play key roles in hemoglobin hydrolysis, but it has been difficult to gain an understanding of the roles of specific enzymes in this process. To begin to characterize the specific roles of plasmodial proteases, we disrupted the falcipain-2 gene. This alteration yielded FP2KO trophozoites with markedly reduced cysteine protease activity, the accumulation of undegraded hemoglobin in the food vacuole, and increased sensitivity to cysteine and aspartic protease inhibitors. These observations confirmed a specific role for falcipain-2 in trophozoite hemoglobin hydrolysis. However, more mature FP2KO parasites recovered from the alterations seen in trophozoites, suggesting rescue by other enzymes and highlighting the cooperative functions of multiple plasmodial proteases.
Interestingly, the food vacuole phenotype that is indicative of a block in hemoglobin hydrolysis was present only during the trophozoite stage of FP2KO parasites, and subsequent development was normal. In contrast, parasites treated with cysteine protease inhibitors show more pronounced morphological abnormalities, and do not develop beyond the trophozoite stage (23, 34). How can the recovery of cysteine protease activity be explained? Three explanations can be considered, each consistent with a transient loss of function in early trophozoites, followed by recovery in more mature parasites. First, our knockout of the falcipain-2 gene may not have been complete, and, despite separation of the coding sequence by ≈10 kb from its promoter, it may have been expressed, albeit with an altered stage specificity. Second, a copy of the falcipain-2 gene, located downstream of falcipain-2 and falcipain-3 on chromosome 11 but not previously shown to be transcribed, may have been expressed in these parasites to allow delayed replacement of falcipain-2 function. Third, falcipain-3, a similar enzyme known to be expressed later in the life cycle (19), or, less likely, other proteases, may have replaced falcipain-2 function. To test these possibilities, we compared expression of falcipains by immunoblotting and real-time PCR.
Immunoblotting identified expression of falcipain-2 in FP2KO parasites, but this expression was delayed until the midtrophozoite stage. This technique could not distinguish between falcipain-2 and falcipain-2′. Real-time PCR showed that, in FP2KO parasites, transcription of falcipain-2 was negligible throughout the life cycle. In contrast, transcription of falcipain-2′ was increased over that of WT parasites in rings and trophozoites. These results suggest that the protein reacting with antifalcipain-2 antiserum in immunoblots of FP2KO parasites was falcipain-2′, and that this enzyme may have partially complemented falcipain-2 function. Immunoblots further showed that the expression of other falcipains and food vacuole plasmepsins was similar in WT and knockout parasites. Modest delays in expression of some other proteases may have been due to minor differences in the life cycle stages assessed for WT and knockout parasites. However, immunoblot signals for falcipain-1 and plasmepsin I between WT and FP2KO parasites were nearly identical. These results indicate that the marked difference in expression of falcipain-2 between WT and FP2KO trophozoites, and possibly smaller differences for other proteases, were biologically relevant, and not simply due to differences in the timing of cultures. Real-time PCR studies showed only modest differences in the transcription of falcipain-1 and falcipain-3. Taken together, the results suggest that the normalization of morphology and development in FP2KO parasites was brought about by the production of falcipain-2′ and falcipain-3 in more mature parasites. Thus, we hypothesize that falcipain-2 and falcipain-3 have temporally distinct but overlapping roles in hemoglobin hydrolysis, and that the inhibition of both of these enzymes will be required for a potent antimalarial effect. This hypothesis will be testable with the generation of specific inhibitors and the knockout of multiple falcipains.
Differences in transcription of messages other than falcipain-2 between WT and FP2KO parasites were modest but intriguing. Recent preliminary studies have suggested that P. falciparum shows remarkably little transcriptional response to specific insults (35). However, with the knockout of falcipain-2, transcription of falcipain-1 and falcipain-2′ increased in ring-stage parasites and that of falcipain-3 increased in all stages, but particularly in trophozoites. The temporal relationship between transcription and protein expression was not straightforward, and it is not clear if the alterations in transcription afforded parasite rescue. Rather, the scheduled production of falcipain-3 in late trophozoites may alone have provided the necessary hemoglobinolytic activity for mature parasites.
Increased sensitivity of FP2KO parasites to cysteine protease inhibitors was not surprising. Presumably, a transient profound loss of cysteine protease activity left these parasites more susceptible to irreversible killing by the inhibitors. Our more surprising result was a marked increase in sensitivity to the aspartic protease inhibitor pepstatin. It has been suggested that cysteine and aspartic proteases act cooperatively to degrade hemoglobin, and this model is supported by synergistic activities of cysteine and aspartic protease inhibitors against cultured and murine malaria parasites (21, 36). The present results add support for this model. It is not clear why the knockout of falcipain-2 led to such a marked increase in sensitivity to pepstatin, however. One possibility is that falcipain-2 is required for the processing or activation of plasmepsins, although prior evidence argues against this possibility (37). Alternatively, the loss of falcipain-2 may render parasites more dependent on plasmepsins for hemoglobin hydrolysis and development. In any event, our findings support roles for the plasmepsins, in addition to falcipain-2, in hemoglobin hydrolysis and/or other essential parasite processes. They also support the consideration of combining cysteine and aspartic protease inhibitors for optimal antimalarial activity.
Our results identify a specific function for a plasmodial protease. Falcipain-2 plays a key role in the hydrolysis of hemoglobin by P. falciparum trophozoites, most notably at the early trophozoite stage. The results further highlight the complexity of parasite biochemistry, and in particular the difficulty of dissecting specific functions of multiple proteases with similar functions. This discrimination of functions is essential if we are to optimally design chemotherapy against protease targets. Additional strategies to disrupt plasmodial protease genes and evaluate the phenotypes engendered by these alterations will thus be of great value.
Supplementary Material
Acknowledgments
We thank Dr. David Fidock for useful advice and Belinda Lee, Jiri Gut, and Julie Lehman for technical assistance. This work was supported by grants from the Medicines for Malaria Venture and the National Institutes of Health (AI35800 and RR01081).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Z-Leu-Arg-AMC, benzyloxycarbonyl-Leu-Arg-7-amino-4-methylcoumarin; AEBSF, 4-(2-aminoethyl)benzenesulfonyl fluoride; E-64, N-(trans-epoxysuccinyl)-l-leucine-4-guanidinobutylamide; gDNA, genomic DNA; FP2KO, falcipain-2 knockout.
References
- 1.Breman, J. G. (2001) Am. J. Trop. Med. Hyg. 64, 1–11. [DOI] [PubMed] [Google Scholar]
- 2.Olliaro, P. L. & Bloland, P. B. (2001) in Antimalarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery, ed. Rosenthal, P. J. (Humana, Totowa, NJ), pp. 65–83.
- 3.Rosenthal, P. J. & Miller, L. H. (2001) in Antimalarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery, ed. Rosenthal, P. J. (Humana, Totowa, NJ), pp. 3–13.
- 4.Rosenthal, P. J. (1999) Adv. Parasitol. 43, 105–159. [DOI] [PubMed] [Google Scholar]
- 5.Blackman, M. J. (2000) Curr. Drug. Targets 1, 59–83. [DOI] [PubMed] [Google Scholar]
- 6.Klemba, M. & Goldberg, D. E. (2002) Annu. Rev. Biochem. 71, 275–305. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal, P. J., Sijwali, P. S., Singh, A. & Shenai, B. R. (2002) Curr. Pharm. Des. 8, 1659–1672. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal, P. J. & Meshnick, S. R. (1996) Mol. Biochem. Parasitol. 83, 131–139. [DOI] [PubMed] [Google Scholar]
- 9.Francis, S. E., Sullivan, D. J. & Goldberg, D. E. (1997) Annu. Rev. Microbiol. 51, 97–123. [DOI] [PubMed] [Google Scholar]
- 10.Lew, V. L., Tiffert, T. & Ginsburg, H. (2003) Blood 101, 4189–4194. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal, P. J., McKerrow, J. H., Aikawa, M., Nagasawa, H. & Leech, J. H. (1988) J. Clin. Invest. 82, 1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis, S. E., Gluzman, I. Y., Oksman, A., Knickerbocker, A., Mueller, R., Bryant, M. L., Sherman, D. R., Russell, D. G. & Goldberg, D. E. (1994) EMBO J. 13, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill, J., Tyas, L., Phylip, L. H., Kay, J., Dunn, B. M. & Berry, C. (1994) FEBS Lett. 352, 155–158. [DOI] [PubMed] [Google Scholar]
- 14.Eggleson, K. K., Duffin, K. L. & Goldberg, D. E. (1999) J. Biol. Chem. 274, 32411–32417. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee, R., Liu, J., Beatty, W., Pelosof, L., Klemba, M. & Goldberg, D. E. (2002) Proc. Natl. Acad. Sci. USA 99, 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal, P. J. & Nelson, R. G. (1992) Mol. Biochem. Parasitol. 51, 143–152. [DOI] [PubMed] [Google Scholar]
- 17.Greenbaum, D. C., Baruch, A., Grainger, M., Bozdech, Z., Medzihradszky, K. F., Engel, J., DeRisi, J., Holder, A. A. & Bogyo, M. (2002) Science 298, 2002–2006. [DOI] [PubMed] [Google Scholar]
- 18.Shenai, B. R., Sijwali, P. S., Singh, A. & Rosenthal, P. J. (2000) J. Biol. Chem. 275, 29000–29010. [DOI] [PubMed] [Google Scholar]
- 19.Sijwali, P. S., Shenai, B. R., Gut, J., Singh, A. & Rosenthal, P. J. (2001) Biochem. J. 360, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dluzewski, A. R., Rangachari, K., Wilson, R. J. & Gratzer, W. B. (1986) Exp. Parasitol. 62, 416–422. [DOI] [PubMed] [Google Scholar]
- 21.Bailly, E., Jambou, R., Savel, J. & Jaureguiberry, G. (1992) J. Protozool. 39, 593–599. [DOI] [PubMed] [Google Scholar]
- 22.Gamboa de Dominguez, N. D. & Rosenthal, P. J. (1996) Blood 87, 4448–4454. [PubMed] [Google Scholar]
- 23.Rosenthal, P. J., Wollish, W. S., Palmer, J. T. & Rasnick, D. (1991) J. Clin. Invest. 88, 1467–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal, P. J., Lee, G. K. & Smith, R. E. (1993) J. Clin. Invest. 91, 1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanWye, J. D. & Haldar, K. (1997) Mol. Biochem. Parasitol. 87, 225–229. [DOI] [PubMed] [Google Scholar]
- 26.Fidock, D. A., Nomura, T., Talley, A. K., Cooper, R. A., Dzekunov, S. M., Ferdig, M. T., Ursos, L. M., Sidhu, A. B., Naude, B., Deitsch, K. W., et al. (2000) Mol. Cell 6, 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trager, W. & Jensen, J. B. (1976) Science 193, 673–675. [DOI] [PubMed] [Google Scholar]
- 28.Lambros, C. & Vanderberg, J. P. (1979) J. Parasitol. 65, 418–420. [PubMed] [Google Scholar]
- 29.Fidock, D. A. & Wellems, T. E. (1997) Proc. Natl. Acad. Sci. USA 94, 10931–10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. (1970) Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 31.Divo, A. A., Geary, T. G., Davis, N. L. & Jensen, J. B. (1985) J. Protozool. 32, 59–64. [DOI] [PubMed] [Google Scholar]
- 32.Barkan, D., Ginsburg, H. & Golenser, J. (2000) Int. J. Parasitol. 30, 649–653. [DOI] [PubMed] [Google Scholar]
- 33.Singh, A. & Rosenthal, P. J. (2001) Antimicrob. Agents Chemother. 45, 949–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shenai, B. R., Semenov, A. V. & Rosenthal, P. J. (2002) Biol. Chem. 383, 843–847. [DOI] [PubMed] [Google Scholar]
- 35.Nirmalan, N., Wang, P., Sims, P. F. & Hyde, J. E. (2002) Mol. Microbiol. 46, 179–190. [DOI] [PubMed] [Google Scholar]
- 36.Semenov, A., Olson, J. E. & Rosenthal, P. J. (1998) Antimicrob. Agents Chemother. 42, 2254–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee, R., Francis, S. E. & Goldberg, D. E. (2003) Mol. Biochem. Parasitol. 129, 157–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.