Abstract
A 43-year-old woman presented with a few weeks’ history of discomfort and swelling in her left breast. She had undergone bilateral breast augmentation 8 years previously. There were no risk factors for breast cancer. Clinical examination, mammography and breast ultrasound revealed a large left breast mass adjacent to the breast implant with enlarged axillary lymph nodes. Owing to diagnostic uncertainty, core biopsies were sent to a specialist unit which confirmed breast implant-associated anaplastic large cell lymphoma with involved lymph nodes. Staging investigations confirmed no distant disease. The lymphoma multidisciplinary team recommended cyclophosphamide, doxorubicin, vincristine and prednisone chemotherapy, followed by implant removal and local radiotherapy. However, the patient's disease progressed on first-line, and then second-line chemotherapy. She therefore had a mastectomy and axillary node clearance followed by radiotherapy, with a planned delayed left breast reconstruction and removal of the right breast implant.
Background
Breast augmentation is the commonest cosmetic operation in the UK. Data from the British Association of Aesthetic Plastic Surgeons reveals that almost 10 000 women in the UK had breast augmentation in 2012. The complications associated with augmentation include implant rotation, capsular contraction, implant rupture and potential difficulties in interpreting mammograms.
Recent publications have reported an increased incidence of anaplastic large cell lymphoma (ALCL) of the breast in women with breast implants. This is a type of non-Hodgkin's lymphoma involving T cells. The recent Poly Implant Prothese (PIP) scandal caused great anxiety among women with PIP breast implants. Some PIP implants contained non-medical grade silicone, with a rupture rate twice that of other implants. But thorough testing of PIP gel material revealed no carcinogenic potential.
We report a case of breast implant-associated anaplastic lymphoma kinase (ALK) negative ALCL that was chemoresistant and therefore needed a mastectomy and axillary clearance followed by radiotherapy. Because of its rarity and resistance to both first-line and second-line chemotherapy, this case posed an initial diagnostic dilemma and then further therapeutic challenges.
Worldwide, less than 50 cases of breast implant-associated ALCL (BIA-ALCL) have been reported. Most of these live in the USA and the authors believe this is the first reported case of BIA-ALCL from the UK.
Case presentation
A 43-year-old woman presented to the breast clinic with a few weeks’ history of discomfort and hardening of the left breast. She denied nipple discharge and change in breast shape. Her appetite had been poor for 3 weeks and she felt lethargic. There were no risk factors for breast cancer. She denied weight loss, fever and night sweats. There was no family history of breast or ovarian cancer. She had bilateral breast augmentation with silicone breast implants 8 years previously. Interestingly, she had presented to the breast clinic with vague left breast symptoms 3 years previously and had seroma fluid aspirated from the left breast.
Investigations
Clinical examination revealed a vague mass measuring about 5 cm in the upper outer quadrant of the left breast together with palpable axillary lymph nodes. There was bilateral capsular contracture, left worse than the right.
A mammogram showed bilateral breast implants and a diffuse density in the upper outer quadrant of the left breast around the implant, measuring 4 cm, with enlarged axillary lymph nodes (figure 1). An ultrasound revealed a large, irregular, hypoechoic mass in the left breast, adjacent to the implant, measuring over 4 cm (figure 2). The mass was relatively vascular. Multiple enlarged axillary lymph nodes suggestive of metastasis were also seen. Triple assessment was completed with US-guided core biopsy of both the breast mass and axillary lymph nodes.
Figure 1.
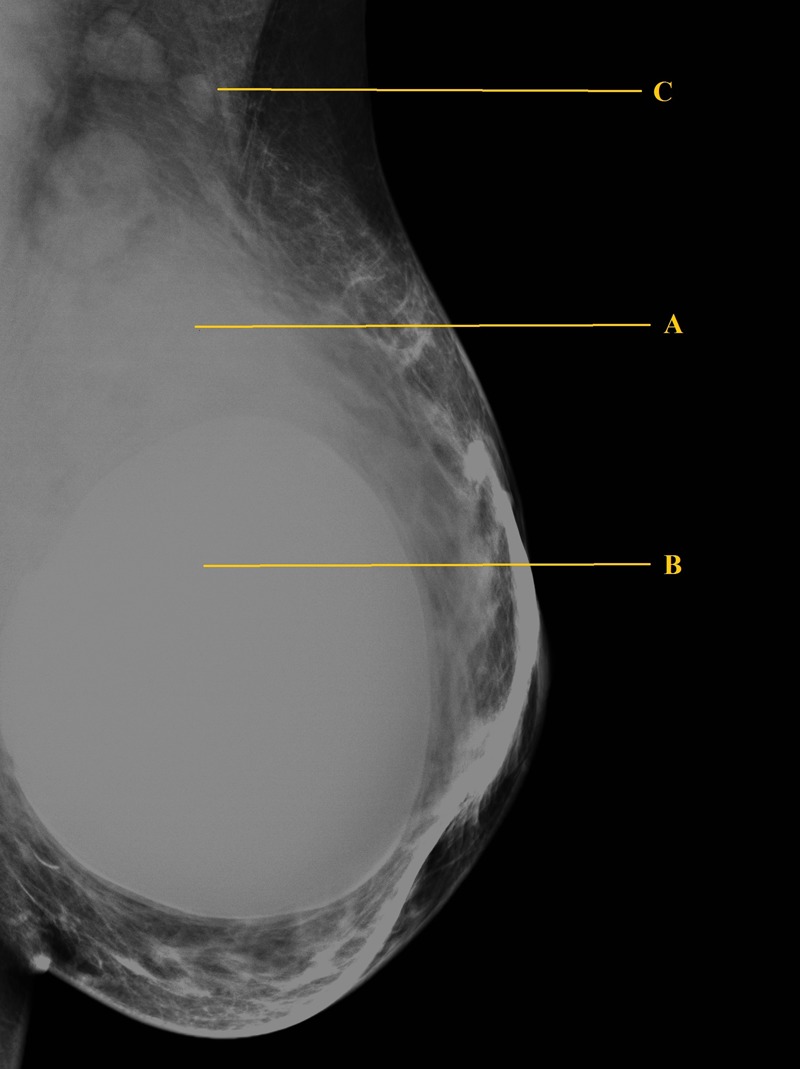
Left breast mammogram showing a diffuse density in the upper outer quadrant of the left breast (A) around the implant (B) and enlarged axillary lymph nodes (C).
Figure 2.
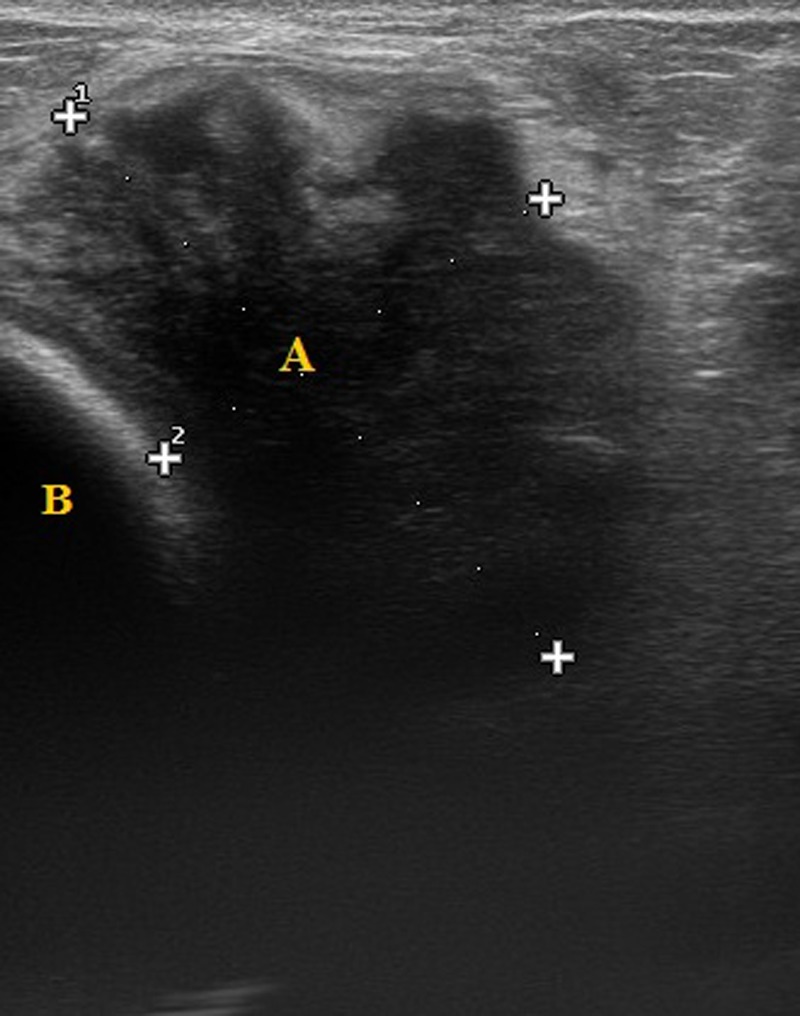
Left breast ultrasound scan showing a large, irregular, hypoechoic mass (A), adjacent to the implant (B).
Differential diagnosis
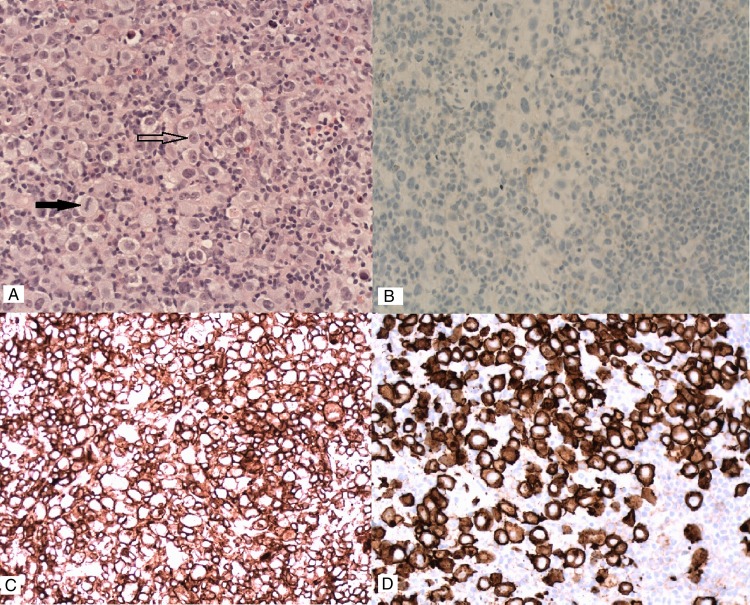
The core biopsies showed numerous, large, mitotically active pleomorphic cells with abundant cytoplasm. The majority were mononuclear, with occasional bi-nucleate and multinucleate cells. Silicone material was not seen. The immunohistochemistry excluded breast carcinoma (negative for cytokeratins CAM 5.2, AE1/3 and CK7) and malignant melanoma (negative for melanocyte markers S100, HMB45 and melan A). The cells were negative for leukocyte common antigen (LCA) and CD45 and did not express CD20. There was strong staining for CD30 and CD4 and focal staining for CD15 but they did not express CD3. They were negative for both epithelial membrane antigen (EMA) and ALK (figure 3).
Figure 3.
(A) H&E staining showing numerous large pleomorphic cells (arrow outline) and mitosis (solid arrow). Immunohistochemistry showing (B) anaplastic lymphoma kinase negative (B), CD4 positive (C) and CD30 positive (D) status.
The differentials was between Hodgkin's lymphoma (though exceptionally rare in the breast) and ALCL. Hence given the ambiguity, a second opinion from St Thomas’ Hospital, London was sought. This confirmed ALK protein negative—ALCL of the breast.
A staging CT scan of the chest, abdomen and pelvis confirmed an 8×6×9 cm mass behind the left breast implant, multiple enlarged left axillary lymph nodes and a small low attenuation lesion in the liver (figure 4). A bone marrow aspirate showed no evidence of marrow infiltration. A positron emission tomography (PET)-CT scan confirmed a macroscopic, metabolically active, fluorodeoxyglucose avid 100×61 mm mass seen within the left breast and left axillary lymph nodal disease but no malignant liver lesions (figure 5).
Figure 4.
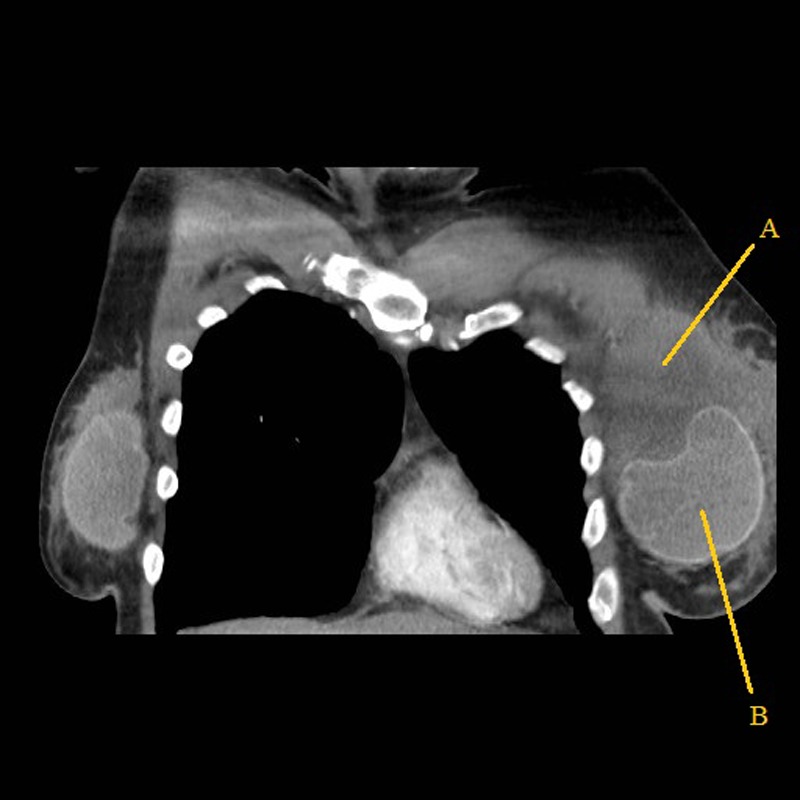
CT scan showing the left breast mass (A) and the breast implant (B).
Figure 5.
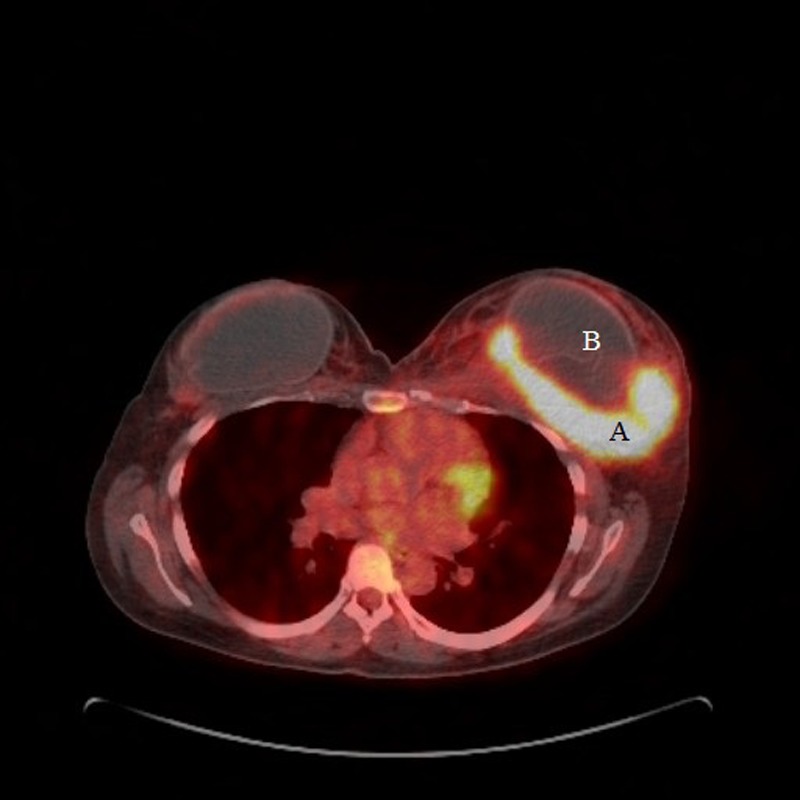
Positron emission tomography-CT scan showing a metabolically active left breast mass (A) and the breast implant (B).
Treatment
Given the nature of the disease, a multidisciplinary team (MDT) comprising oncologists, lymphoma specialists, radiologists, haematologists and breast surgeons were involved. The patient was given two cycles of first-line chemotherapy using cyclophosphamide, doxorubicin, vincristine and prednisone with a view to re-assess the response. Clinical examination and a repeat PET-CT scan showed a persistent, 100×60 mm mass within the left breast, unchanged from the previous PET-CT (figure 6).
Figure 6.
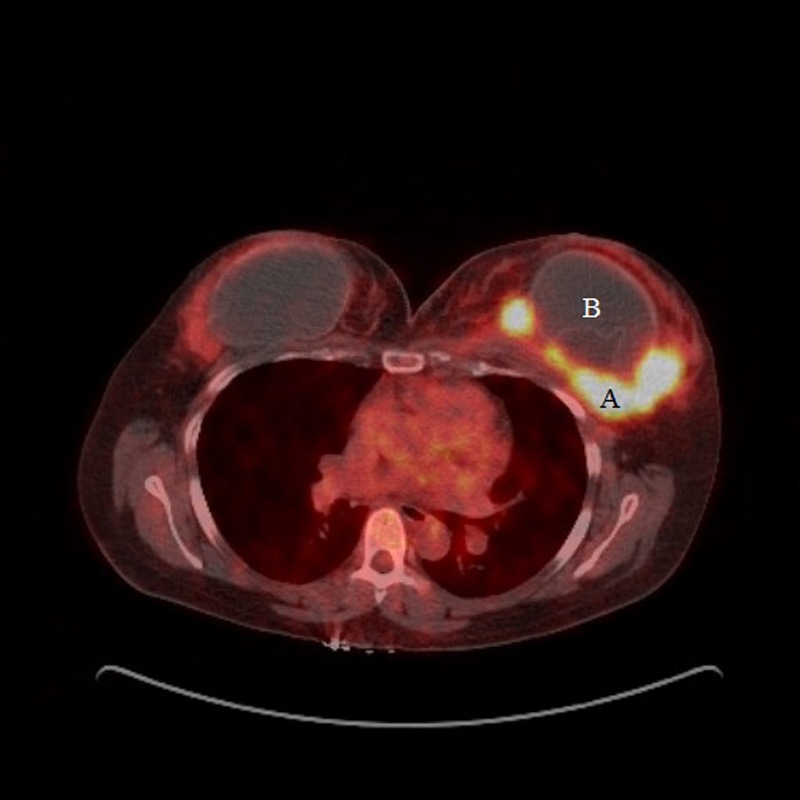
Positron emission tomography-CT after two cycles of first-line chemotherapy showing persisting left breast mass (A) and the breast implant (B).
The patient was therefore started on second-line chemotherapy (cisplatin and gemcitabine). A repeat PET-CT scan confirmed axillary lymph node resolution but progressive disease in the left breast measuring 120×80 mm (figure 7) which correlated with the clinical findings (figure 8). A repeat core biopsy of the breast mass confirmed persistent ALK negative ALCL.
Figure 7.
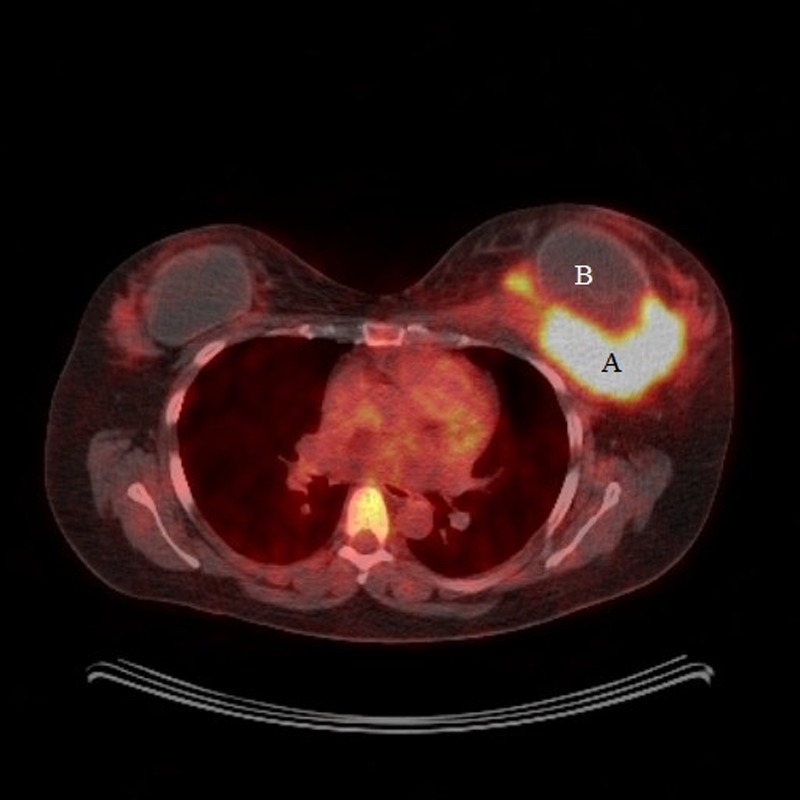
Positron emission tomography-CT after two cycles of second-line chemotherapy showing progressive disease in the left breast (A) and the breast implant (B).
Figure 8.
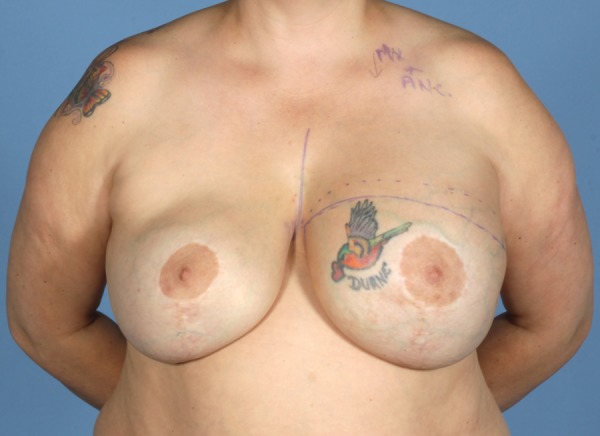
Preoperative photograph.
After MDT discussion, the patient had a left mastectomy (including pectoralis major muscle fibres) and removal of the breast implant and an axillary clearance (figure 9). The patient made an uneventful postoperative recovery.
Figure 9.
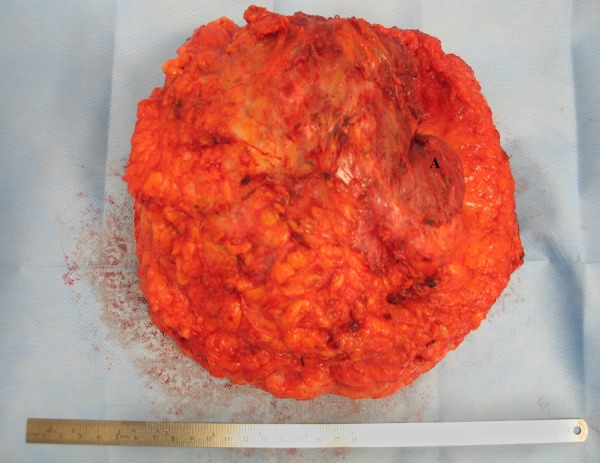
Left mastectomy specimen showing pectoralis major muscle fibres (A).
The mastectomy specimen contained residual ALCL with areas of necrosis in keeping with a partial response to chemotherapy. The tumour was separated from the silicone implant by a band of fibrous tissue. The skeletal muscle was infiltrated by the lymphoma but the margins were clear. The axillary lymph nodes were negative for lymphoma (complete response to chemotherapy).
Her adjuvant treatment was completed with radiotherapy (RT) to the chest wall, 40 Gy in 15 fractions over 3 weeks (higher end of normal dose for lymphoma), to optimise her care.
Outcome and follow-up
The patient has recovered following her RT with no signs of local recurrence (figure 10). She is 3 months postsurgery and will have regular surveillance CT scans and a planned delayed left breast reconstruction and removal of the right breast implant.
Figure 10.
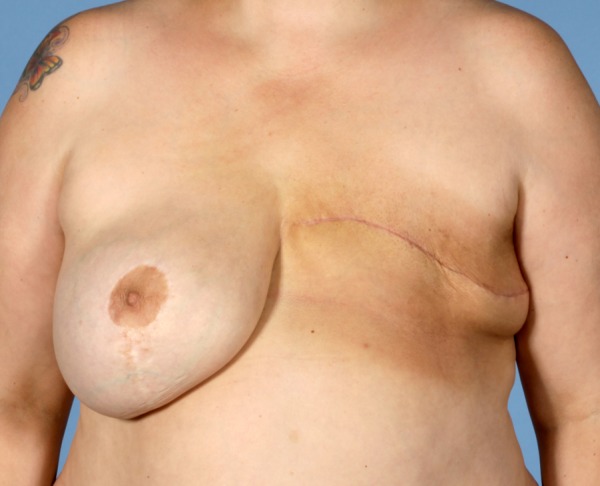
Postoperative photograph taken 3 months after surgery and completion of radiotherapy.
Discussion
Breast lymphoma is very rare. A diagnosis of primary breast lymphoma (PBL) is made when the breast is the first or major manifestation site of lymphoma, with or without ipsilateral axillary lymph node involvement but without the involvement of lymphoma in any other organ.1
PBL accounts for less than 1% of all breast malignancies, and the majority are B-cell lymphomas. Recently, ALCL, a type of non-Hodgkin's lymphoma involving the T cells has been reported in association with breast implants. Although the estimated number of breast implant procedures performed worldwide is between 5 and 10 million,2 only 50 cases of BIA-ALCL have been reported worldwide. The US Food and Drug Administration (FDA) noted a possible association between breast implants and ALCL and believed that women with breast implants had a small but increased risk of developing ALCL adjacent to the implant.2 Given the number of reported cases and the total number of breast implants performed worldwide, the calculated risk of developing ALCL of the breast is 1:100 000 to 1:200 000 implants3 which is extremely low, even after accounting for some under-reporting.
ALCL develops in women who have had breast implants for both cosmetic and reconstructive reasons. Both saline-filled and silicone implants have been associated with ALCL. There are suggestions that texturing (a process to make the outer surface of the implant rough to reduce implant contracture) might play a role in the development of ALCL secondary to chronic inflammation and increased antigenic stimulation.4 A mini-meta analysis of 49 patients with BIA-ALCL found the median age at diagnosis was 54 years, and the median time for development of ALCL was 9 years from implant surgery.3
Clinical presentation of BIA-ALCL can be vague and therefore easily missed. Patients present in two different ways with completely different management options and outcomes. Those presenting with an effusion in the absence of a mass lesion have an excellent long-term survival after surgical removal of the implant and usually do not need any further treatment. On the contrary, patients presenting with a mass lesion tend to have a less favourable prognosis, often requiring aggressive treatment in the form of combination chemotherapy +/− involved field RT. In our case, the BIA-ALCL was chemoresistant, hence necessitating a mastectomy to remove the mass together with the breast implant and axillary clearance, followed by RT.
IHC differentiates ALCL into ALK-positive and ALK-negative types depending on the expression of ALK protein. Patients with ALK-negative ALCL tend to be older (median age 54–61 years), with a poor 5-year overall survival (OS) of 30–49% whereas patients with ALK-positive ALCL tend to be younger, with a much better 5-year OS of 70–86%.5 Cases associated with an effusion within the implant capsule are typically ALK-positive while those presenting with a solid mass, like our patient, are typically ALK negative.
Though the literature reports an association between breast implant and ALCL, large clinical studies based on over 200 000 person-years of follow-up do not show an increased risk of lymphoma in women with breast implant.6 Patients with breast implants should not be unduly concerned about the risk of developing lymphoma, but doctors should be wary of patients with breast implants presenting with vague symptomatology, especially a persisting seroma or a mass lesion.
Learning points.
Anaplastic large cell lymphoma is associated with breast implants.
Patients presenting with an effusion tend to be anaplastic lymphoma kinase (ALK) positive with a good prognosis whereas patients presenting with a mass lesion tend to be ALK negative with a worse prognosis.
A proactive, multidisciplinary team approach is needed to treat such rare conditions.
Patients with breast implants presenting with vague symptoms (swelling, deformity, masses) should be referred to a breast clinic for a triple assessment.
Acknowledgments
The authors would like to acknowledge Mr Garg, Dr Whitear and Dr Sherwin for their contribution.
Footnotes
Contributors: MP, the corresponding author for this article, certify that this manuscript has not been submitted elsewhere. My co-authors did contribute to the writing up of the article and agree to allow me to correspond with the editorial office.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wiseman C, Liao KY. Primary lymphoma of the breast. Cancer 1972;2013:1705–12 [DOI] [PubMed] [Google Scholar]
- 2.Center for Devices and Radiological Health, US Food and Drug Administration Anaplastic large cell lymphoma (ALCL) in women with breast implants: preliminary FDA findings and analyses. FDA/CDRH; 2011. http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM240003.pdf (accessed 4 Sep 2013).
- 3.Thompson PA, Prince HM. Breast implant-associated anaplastic large cell lymphoma: a systematic review of the literature and mini-meta analysis. Curr Hematol Malig Rep 2013;2013:196–210 [DOI] [PubMed] [Google Scholar]
- 4.Lechner MG, Megiel C, Church CH, et al. Survival signals and targets for therapy in breast implant-associated ALK-anaplastic large cell lymphoma. Clin Cancer Res 2012;2013:4549–59 [DOI] [PubMed] [Google Scholar]
- 5.Ferreri AJ, Govi S, Pileri SA, et al. Anaplastic large cell lymphoma, ALK negative. Crit Rev Oncol Hematol 2012;2013:206–15 [DOI] [PubMed] [Google Scholar]
- 6.Largent J, Oefelein M, Kaplan HM, et al. Risk of lymphoma in women with breast implants: analysis of clinical studies. Eur J Cancer Prev 2012;2013:274–80 [DOI] [PubMed] [Google Scholar]