Abstract
GLD-2 is a cytoplasmic poly(A) polymerase present in the Caenorhabditis elegans germ line and embryo. It is a divergent member of the DNA polymerase β nucleotidyl transferase superfamily, which includes CCA-adding enzymes, DNA polymerases and eukaryotic nuclear poly(A) polymerases. The polyadenylation activity of GLD-2 is stimulated by physical interaction with an RNA binding protein, GLD-3. To test whether GLD-3 might stimulate GLD-2 by recruiting it to RNA, we tethered C. elegans GLD-2 to mRNAs in Xenopus oocytes by using MS2 coat protein. Tethered GLD-2 adds poly(A) and stimulates translation of the mRNA, demonstrating that recruitment is sufficient to stimulate polyadenylation activity. We use the same tethered assay to identify human and mouse poly(A) polymerases related to GLD-2. This may provide entrees to previously uncharacterized modes of polyadenylation in mammalian cells.
Messenger RNAs are exquisitely controlled in eukaryotic cells. Regulation of mRNA stability, translation, and localization are essential for early development, cell growth, homeostasis, and neuronal plasticity (1–7). A tract of adenosine residues added posttranscriptionally to the 3′ end of the mRNA, poly(A), is a hallmark of mRNAs, and a plexus of control (1, 8–10). In the nucleus, poly(A) addition is linked to cleavage of the pre-mRNA. The machinery involved communicates with splicing and transcription factors (11–16) and is regulated by DNA damage, mitosis, and differentiation (13, 17–19). Shortening of the poly(A) tail in the cytoplasm can trigger translational repression and mRNA decay, whereas lengthening can cause translational activation and mRNA stabilization (1, 5, 9).
mRNAs emerge from the nucleus with long poly(A) tails. In the default state, these tails are shortened. However, poly(A) can be added to specific mRNAs, leading to a net increase in their poly(A) length (1, 5). Cytoplasmic polyadenylation events have been extensively characterized in oocytes and embryos, where they are critical for a diversity of developmental decisions (1, 5). Similarly, in neuronal cells, regulated cytoplasmic polyadenylation at synapses controls local translation (3–5). The enzymes responsible for cytoplasmic polyadenylation in somatic cells have not been identified.
The enzyme that adds poly(A) in the nucleus, a “canonical” eukaryotic poly(A) polymerase (PAP), is highly conserved, and adds poly(A) one nucleotide at a time (20–24). This PAP is a poor RNA-binding protein and relatively inactive on its own (19, 25). Although purified PAP acts as a monomer, in vivo it assembles into a large multiprotein complex that recognizes specific sequences in the pre-mRNA. This complex cleaves the pre-mRNA to generate a 3′ hydroxyl group, to which the PAP then adds poly(A).
A different family of PAPs, termed regulatory cytoplasmic PAPs, recently was identified in Caenorhabditis elegans and Schizosaccharomyces pombe (26–29). The C. elegans gld-2 gene was identified initially through its specific effects on germ-line development (30). The GLD-2 protein is cytoplasmic, and localized to P-granules in the C. elegans embryo (26). GLD-2 is a member of the nucleotidyl transferase superfamily, which also includes canonical nuclear PAPs (31); however, GLD-2 diverges substantially from them throughout its length (26). It appears to lack the C-terminal RNA-binding motifs required for nuclear PAP activity (23, 24, 31–34). A distinct RNA-binding protein, GLD-3 (35), binds to GLD-2 and stimulates its polyadenylation activity in vitro (26).
Based on these findings, we proposed that GLD-2 is the catalytic subunit of a heterodimeric PAP involved in cytoplasmic polyadenylation (26). In this model, RNA-binding proteins recruit a subunit containing the PAP active site (e.g., GLD-2) to specific mRNAs. The RNA-binding proteins provide versatility in control. The enzyme, relatively inactive on its own, acquires activity by recruitment to its substrate.
Here, we test this model by tethering GLD-2 to an mRNA in vivo by using a foreign RNA-binding protein, MS2 coat protein. Tethered GLD-2 adds poly(A) efficiently and selectively, and thereby stimulates translation of the mRNA to which it is attached. We use this tethering assay to identify human and mouse proteins that possess polyadenylation activity. These proteins are putative members of a previously undescribed family of regulatory PAPs.
Methods
DNA Constructs. p3HA-MSP. Three hemagglutinin (HA) tags were inserted into pET15b-CPEB (cytoplasmic polyadenylation element-binding protein), replacing the His-tag (p3HA-CPEB). The MS2 fragment of pET-MS2 (36) was ligated into p3HA-CPEB from NdeI–-NheI. This vector contains the T7 promoter, followed by three HA tags, and one copy of the MS2 coat protein (p3HA-MSP).
MS2 fusions. pMS2-U1A and pMS2-bPAP (bovine nuclear PAP) were described (37). GLD-2 and GLD-2 truncation mutants were PCR-amplified from pLW48 (L.W. and M.W., unpublished data). Hs1 (hGLD-2) and Hs2 were PCR-amplified from cDNA from a human breast cancer cell (MDA231). Hs3, Hs4, and Hs5 were similarly obtained from Jurkat cells, Mm1 was obtained from a mouse macrophage cell (RAW264.7), and Ce2 was obtained from mixed stage C. elegans. At1 was PCR-amplified from H2D8 plasmid (Arabidopsis Biological Resource Center, Columbus, OH).
p3HA-MSP was cut either with NheI and XhoI or with NheI and ClaI to remove CPEB. All of the PCR products except Ce2 were ligated into p3HA-MSP from NheI–XhoI. The Ce2 PCR product was ligated into p3HA-MPS from NheI–ClaI.
GLD-2(mut), which possesses a single amino acid change (D608A) that abolishes PAP activity, was created from p3HA-GLD-2 using the QuikChange mutagenesis system according to the manufacturer's instructions (Stratagene).
Each plasmid carrying an MS2 fusion contains the T7 promoter, followed by three HA tags, one copy of MS2 coat protein, and the protein to be tested. All plasmids were linearized with HindIII, with the exception of Mm1, Hs1, and Ce2 (which were cleaved with ClaI) and At1 (cleaved with SspI).
mRNA reporter plasmids. pLG-MS2 (luciferase) and pJK350 (β-galactosidase) plasmids have been described (37). To make pLGMS2-LucHS, pLG-MS2 was cleaved with HindIII and SpeI to excise the luciferase gene fragment; ends were filled-in and religated. pLG-MS2 and pLGMS2-LucHS were linearized with BglII and transcribed with T7 RNA polymerase. pJK350 was linearized with HindIII and transcribed with SP6 RNA polymerase.
In Vitro Transcription. Plasmids were cleaved with restriction enzymes as indicated above, and transcribed with T7 or SP6 RNA polymerase. Transcriptions were performed in the presence of 6 mM m7GpppG. In some cases, [α-32P]UTP was incorporated during in vitro transcription as described (37).
Western Blotting. Oocytes were injected with an mRNA encoding a fusion protein and collected after 6 h. Oocytes were homogenized in PBS containing a protease inhibitor mixture (Roche Diagnostics), by using 5 μl of buffer per oocyte. Homogenates were centrifuged at room temperature for 10 min, and supernatants were collected. Lysate from three oocytes was loaded onto each lane of an SDS/PAGE gel. Proteins were analyzed by Western blotting using either anti-MS2 coat protein antibody (3H4 antibody, gift of M. Kiledjian, Rutgers University, Camden, NJ) or anti-HA-tag antibody (HA11, from Covance, Princeton).
RNA Analysis. RNA extraction and poly(A) selection. Oocyte RNA was prepared by using TRI reagent, following the manufacturer's instructions (Sigma). Polyadenylated and nonpolyadenylated RNAs were separated by using the PolyATrack mRNA isolation system, following the manufacturer's instructions (Promega).
RNaseH analysis. Oligo(dT)/RNaseH treatment was performed in 200 mM KCl/1 mM EDTA/20 mM Tris·HCl, pH 8.0/30 mM MgCl2/20 units of RNasin. A total of 0.5 μg of oligo(dT)12–18 was annealed to total RNA equivalent to two oocytes (≈10 μg). After addition of 3 units of RNaseH, reactions were incubated at 37°C for 90 min.
Electrophoresis. After ethanol precipitation, RNAs were separated on either a denaturing formaldehyde-agarose gel (full-length mRNAs) or a 6% polyacrylamide gel (short RNAs) and analyzed by autoradiography.
Tethered Function Assays. Oocyte injections and enzyme assays were performed as described (36, 37).
Identification of GLD-2 Relatives and Sequence Alignments. With C. elegans GLD-2 protein sequence as the query sequence (26), we ran blastp using the nonredundant (nr) peptide sequence database at the NCBI blast server (www.ncbi.nlm.nih.gov/blast). We aligned sequences related to GLD-2 using the superfamily program (http://supfam.org/SUPERFAMILY). Based on the multiple sequence alignment, an unrooted tree was created with a phylogenetic tree program (www.ebi.ac.uk/clustalw) by using neighbor joining method (38), setting on Kimura correction of distances (39).
Results
Tethered GLD-2 Is an Efficient Poly(A) Polymerase. GLD-2 polyadenylation activity is stimulated by its interaction with a putative RNA-binding protein, GLD-3 (26). We proposed that GLD-3 stimulates GLD-2 by recruiting it to the RNA (Fig. 1A). If so, then bringing GLD-2 to the RNA by other means also should stimulate its activity. To test this idea, we tethered GLD-2 to a reporter mRNA by using MS2 coat protein (Fig. 1 A, Test; ref. 40). We used Xenopus oocytes for this purpose because they provide a robust assay for cytoplasmic polyadenylation and its effects in vivo (36, 37).
Fig. 1.
Strategy. (A)(Upper Right) PAP heterodimer in which GLD-2 contains the active site and GLD-3 provides RNA-binding specificity. (Lower Right) MS2 coat protein was joined to GLD-2 to recruit it to an RNA. (B) Protocol. MS2/GLD2 protein is expressed in Xenopus oocytes; then two reporter mRNAs are injected. Luciferase mRNA contains MS2 binding sites; β-galactosidase mRNA does not.
Xenopus oocytes were first injected with an mRNA encoding a fusion of MS2 coat protein and GLD-2 (MS2/GLD-2) or with mRNAs encoding control proteins (Fig. 1B). After allowing 6 h for synthesis of the fusion protein, two reporter mRNAs were coinjected: a luciferase mRNA bearing MS2 binding sites in its 3′ UTR and a β-galactosidase mRNA lacking MS2 sites. Luciferase and β-galactosidase activities were measured 16 h later.
MS2/GLD-2 stimulates expression of the luciferase mRNA ≈15-fold (Fig. 2A Upper). Stimulation is specific, because it requires the MS2 RNA binding sites: the activity of the β-galactosidase reporter, which lacks binding sites, is not enhanced (Fig. 2 A Lower). Furthermore, an MS2/GLD-2 fusion in which the catalytic center was abolished by a point mutation (31, 34) lacked stimulatory activity [Fig. 2 A, GLD-2(mut)]. MS2/GLD-2 is comparable in activity to MS2/bPAP, a fusion between MS2 and bovine nuclear PAP (Fig. 2 A, bPAP; ref. 37). As expected, MS2/U1A did not stimulate translation regardless of whether sites were present. The level of each MS2 fusion protein in the oocytes was similar, as assessed by Western blotting (Fig. 2B). We conclude that GLD-2 specifically stimulates translation when bound to an mRNA in oocytes.
Fig. 2.
MS2/GLD-2 stimulates translation and polyadenylation of a reporter RNA. (A) Translational stimulation. (Upper) Translational activity of luciferase mRNA in response to each protein is quantified as the ratio of luciferase activity to β-galactosidase activity. Activity is normalized to that of U1A. (Lower) Translational activity of β-galactosidase mRNA, quantified as the ratio of β-galactosidase activity with the test protein relative to that observed with MS2/U1A. Tethered U1A does not stimulate translation in oocytes (36). Averages and error bars derived from three experiments. (B) Protein levels. Lysate equivalent to three oocytes was loaded onto each lane, and analyzed by Western blotting using an anti-MS2 antibody, 3H4 (gift of M. Kiledjian). Arrowheads, positions of full-length fusion proteins. Shown on the left are positions of protein markers. (C) Poly(A) status of reporter mRNAs. 32P-labeled luciferase mRNA was injected into oocytes expressing the fusion proteins indicated above each pair of lanes. Oocytes were collected after 16 h. RNA was extracted and fractionated by using biotinylated oligo(dT). –, RNAs that did not bind the resin; +, RNAs that bound the resin. (D) Polyadenylation of short RNA substrates. A 32P-labeled RNA containing three MS2 sites was injected into oocytes expressing the indicated fusion proteins. Oocytes were collected after 16 h, and the RNA was extracted and analyzed by electrophoresis. The positions of RNAs carrying various lengths of poly(A) (determined by using markers) are shown to the left. Input RNA, RNA before injection. (E) Poly(A) status of short RNA substrates. RNAs were injected and prepared as in D. RNAs were fractionated by using biotinylated oligo(dT). –, RNAs that did not bind the resin; +, RNAs that bound the resin. The positions of RNAs carrying various lengths of poly(A) (determined by using markers) are shown to the left. Input RNA, RNA before injection.
MS2/GLD-2 protein catalyzed polyadenylation of the reporter to which it was tethered (Fig. 2C). Luciferase mRNAs from oocytes containing MS2/GLD-2 bound to biotinylated oligo(dT), whereas mRNAs from oocytes containing the active site mutant or MS2/U1A did not. The apparent polyadenylation activity of MS2/GLD-2 was comparable to that of MS2/bPAP.
To examine polyadenylation in greater detail, we used short (80-nt) radiolabeled RNA substrates containing three MS2 sites. These RNAs were lengthened substantially (>300 nt) by MS2/GLD-2 and MS2/bPAP, but not by MS2/U1A or the inactive MS2/GLD-2(mut) (Fig. 2D). The lengthening was caused by polyadenylation, as judged by binding to biotinylated oligo(dT) (Fig. 2E).
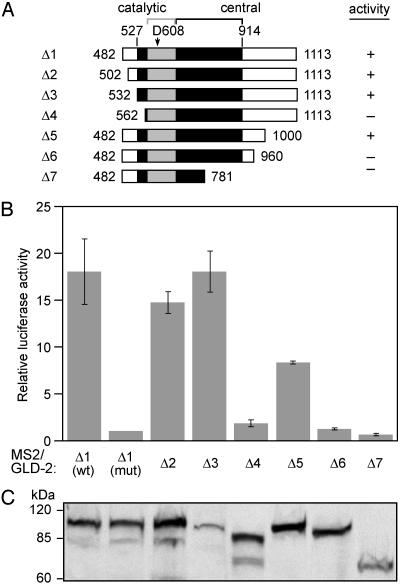
The Minimal Region Required for Activity. GLD-2 possesses hallmark features of the DNA polymerase β (polβ)-nucleotidyl transferase family (31). These include three aspartic acid residues and several amino acids that bind the nucleotide contained in the catalytic and central domains (Fig. 3A). To identify the minimum contiguous portion of GLD-2 capable of supporting polyadenylation, we prepared N- and C-terminal deletions of GLD-2, each fused to MS2 coat protein. A total of 532 amino acids could be pared from the N terminus of GLD-2, and 113 from the C terminus, without significantly reducing activity (Fig. 3 A and B). Further encroachments of only 30 aa at the N terminus, or 40 aa at the C terminus, caused substantial decreases in activity. Mutant proteins, including those without activity, were present at comparable levels (Fig. 3C). We conclude that the minimum contiguous portion of GLD-2 needed for catalysis corresponds roughly to the catalytic and central domains and contains the key residues conserved among polβ-nucleotidyl transferases.
Fig. 3.
The minimal region required for activity corresponds to the catalytic and central domains. (A) Truncated GLD-2 proteins tested. Gray boxes, catalytic domains; black boxes, central domains. “D608” corresponds to one of the three aspartate residues conserved in the polβ-nucleotidyl transferase family, mutated to alanine in GLD-2(mut). (B) Translational activity. Assays were performed as in Fig. 2 A. Relative luciferase activity (ratio of luciferase to β-galactosidase activity) was normalized to that of GLD-2(mut). Averages and error bars are derived from four experiments (except for Δ4, derived from two experiments). (C) Protein levels. Cell lysate equivalent to three oocytes was loaded onto each lane, and analyzed by Western blotting using anti-HA-tag antibody.
Previously Undescribed Mouse and Human PAPs. The members of the polβ-nucleotidyl transferase family include several different biochemical activities, including DNA polymerases, CCA-adding enzymes, and 2′–5′ oligo(A) synthetases, as well as canonical eukaryotic PAPs (Fig. 4A and ref. 31). Sequences more closely related to GLD-2 than to canonical PAPs are detected throughout eukaryotes. A subset of the most closely related sequences are depicted in a dendrogram in Fig. 4B.
Fig. 4.
GLD-2-related sequences and candidate PAPs. (A) polβ-nucleotidyl transferase family. The family includes not only GLD-2 (and the DNA polymerases, Trf4p and Trf5p, of Saccharomyces cerevisiae), but also CCA-adding enzymes (CCA), 2′-5′oligo(A) synthetases (2′-5′A), terminal transferase (TdT), the polX polymerases, and enzymes that transfer nucleotidyl groups to other nonnucleic acid compounds. The image is based on Aravind and Koonin (31). (B) Cladogram of a subset of sequences most similar to GLD-2, emphasizing those relevant for this work. Sequences tested for PAP activity are in boxes. Black boxes, proteins that possessed PAP activity as tethered proteins; white boxes with an “X,” proteins without activity as tethered proteins (although the proteins were expressed); gray boxes, sequences shown in other work to be cytoplasmic PAPs (27, 28). Hs2 and Hs4 proteins were expressed at too low levels to be assessed confidently. “bPAP” indicates the canonical nuclear PAP derived from Bos taurus: the other canonical PAPs are very closely related. (C) Structure of the GLD-2 relatives tested. Gray boxes, catalytic domains; black boxes, central domains; RRM, the RRM-like region present in canonical nuclear PAPs; white boxes, other regions. Hs2 also has a retrovirus zinc-finger-like domain.
The tethered function assay provides a powerful means to identify which of the GLD-2 relatives depicted in Fig. 4 are poly(A) polymerases, because neither the protein partner (the functional equivalent of GLD-3) nor the natural mRNA substrate need to be known. Thus we determined whether any of the GLD-2 relatives in the human, mouse, C. elegans, and Arabidopsis genomes were PAPs. Each of the proteins tested possesses catalytic and central domains more closely related to GLD-2 than to canonical PAPs.
We tethered all five human candidate proteins depicted in Fig. 4B by expressing them as MS2 fusion proteins in oocytes. Of these, only Hs1 was active. This protein stimulated translation (Fig. 5A) and added poly(A) to the reporter mRNA with MS2 sites (Fig. 5C). Its translational activity was ≈40% that of C. elegans GLD-2 (Fig. 5A). Furthermore, it added poly(A) to the short RNA substrate (Fig. 5D), as confirmed by binding to biotinylated oligo(dT) (Fig. 5E) and treatment with RNaseH and oligo(dT) (Fig. 5F). Similarly, one mouse protein, Mm1, that is 93% identical and 95% similar to Hs1, stimulated translation and added poly(A) (Fig. 5). These two proteins, Hs1 and Mm1, are the most closely related to GLD-2 among the human and mouse candidate genes, and so will be referred to as hGLD-2 and mGLD-2, respectively.
Fig. 5.
Identification of poly(A) polymerases. (A) Translational activity. Assays were performed as in Fig. 2 A. Relative luciferase activity (ratio of luciferase to β-galactosidase activity) was normalized to that of GLD-2(mut). (B) Protein levels. Cell lysate equivalent to three oocytes was loaded onto each lane and analyzed by Western blotting using anti-HA antibody. Shown on the left are positions of protein markers used to calibrate molecular mass. (C) Poly(A) status of reporter mRNAs. 32P-labeled luciferase mRNA was injected into oocytes expressing the indicated fusion proteins. RNA was extracted from oocytes after 16 h and fractionated by using biotinylated oligo(dT). –, RNAs that did not bind the resin; +, RNAs that bound the resin. (D) Polyadenylation of short RNA substrates. A 32P-labeled RNA containing three MS2 sites was injected into oocytes expressing the MS2 fusion proteins indicated above each lane. RNA was extracted after 16 h and analyzed by electrophoresis. Shown on the left are positions of RNAs with various lengths of poly(A) (determined by comparison to markers). (E) Oligo(dT) retention. RNAs containing three MS2 sites were injected into oocytes expressing the indicated fusion proteins. Extracted RNAs were fractionated by using biotinylated oligo(dT). –, RNAs that did not bind the resin; +, RNAs that bound the resin. Left, positions of RNAs with various lengths of poly(A) (determined by comparison to markers). Input, RNA before injection. (F) Oligo(dT)/RNaseH. RNAs containing three MS2 sites were injected into oocytes expressing the indicated fusion proteins. Extracted RNAs were incubated with oligo(dT) and RNaseH. –, no RNaseH; +, RNaseH added. Arrowheads indicate positions of the injected RNA and of the same RNA with a 300-nt tail. Input, RNA before injection.
Several proteins tested did not stimulate translation in the tethered function assay. These include all four other candidates from the human genome (Hs2 through Hs5). Of these, Hs3 and Hs5 were produced at levels sufficiently high to conclude they were genuinely inactive (Fig. 5B); full-length Hs2 and Hs4 proteins were expressed inefficiently (not shown). The closest relatives of GLD-2 in the Arabidopsis (At1) and C. elegans (Ce2) genomes also were inactive (Fig. 5A). These proteins were produced in the oocyte at levels comparable to GLD-2 (Fig. 5B). The simplest explanation of these data are that only some, and not all, GLD-2 relatives are poly(A) polymerases.
Discussion
Our findings demonstrate that GLD-2 gains activity by recruitment to an mRNA substrate. Tethering GLD-2 to an mRNA results in polyadenylation and stimulation of translation. The stimulation of translation is due to poly(A) addition, because a point mutation that eliminates GLD-2's polyadenylation activity also eliminates the enhancement. These data support the model that RNA binding partners of GLD-2 bring the protein to the 3′ end of mRNAs, providing sequence specificity (26).
Many enzymes that polymerize nucleotides into nucleic acid gain sequence specificity by interaction with distinct protein partners. Most replication and transcription enzymes follow this principle. In nuclear polyadenylation, a PAP is brought to the pre-mRNA through interactions with a multisubunit RNA-binding protein, called cleavage and polyadenylation specificity factor in mammalian cells (CPSF; refs. 17–19). Cytoplasmic polyadenylation in Xenopus oocytes requires a distinct cytoplasmic form of CPSF (37, 41, 42). An additional protein, CPEB, binds both the RNA and CPSF (37, 41, 42), and hence helps to recruit the PAP. C. elegans GLD-3 binds to GLD-2 and stimulates its polyadenylation activity in vitro (26, 35). RNA-binding proteins, perhaps including CPEB, cytoplasmic CPSF, and GLD-3-related proteins, may enhance the activity of GLD-2 family members.
GLD-2 and its human and mouse homologues, hGLD-2 and mGLD-2, share sequence similarities that distinguish them from other eukaryotic PAPs. All PAPs, including GLD-2, possess a collection of catalytic and nucleotide-binding residues. GLD-2 family members are very closely related to one another, as are the nuclear PAPs to one another. GLD-2 family members possess insertions and deletions relative to the nuclear PAP family, and these might underlie differences in their properties. For example, canonical mammalian PAPs possess two bipartite nuclear localization signals on either side of a S/T-rich domain at their C termini (33); by sequence inspection, GLD-2, mGLD-2, and hGLD-2 do not possess a region related to this C-terminal segment. S. pombe Cid1p and Cid13p, like C. elegans GLD-2, are cytoplasmic poly(A) polymerases (27, 28), yet are more distantly related to the GLD-2 homologues than are Ce2 and Hs3, both of which are inactive in the tethered function assay (Fig. 4 and 5). Thus, further tests are needed to determine which features are critical for PAP activity and biological functions in vivo.
Three other PAP genes, in addition to the one encoding the canonical nuclear PAP, have been identified in mammalian cells. The TPAP gene is expressed in the mouse testes. Its protein product is cytoplasmic and adds poly(A) to a subset of mRNAs in that tissue (43, 44). A second gene, neoPAP, is expressed at elevated levels in human neoplasms (45). A third gene encodes PAPγ, a protein very similar in enzymatic activity to the canonical PAP. Indeed, PAPγ polyadenylates RNAs in an AAUAAA-dependent manner in the presence of CPSF (46). TPAP, neoPAP, and PAPγ are all much more closely related in sequence to the canonical nuclear PAP than are hGLD-2 and mGLD-2. For example, the human canonical PAP (PAPII) is 71% similar in sequence overall to neoPAP (45), but only 23% similar to hGLD-2. Furthermore, hGLD-2 possesses an N-terminal segment lacking in canonical PAPs, and lacks similarity to the C-terminal region of other PAPs.
The diversity of poly(A) polymerases in mammalian cells is striking. Five genes encode identified PAP proteins: the canonical PAPs, neo-PAP (45), TPAP (43, 44, 47), PAPγ (46), and the GLD-2 homologues reported here. Alternative processing of transcripts of the canonical PAP generates additional diversity (48), as does their regulated phosphorylation (49–51). The diversity of poly(A)-adding enzymes suggests an even greater degree of control over poly(A) length than already has been revealed.
The discovery of mouse and human GLD-2 homologues may provide an entree into previously undescribed biological roles of polyadenylation in mammalian cells. In C. elegans, gld-2 regulates stem cell fates and is required for stem cells to complete meiosis (30). Furthermore, gld-2 is required redundantly with gld-1 for stem cell differentiation: gld-1 gld-2 double mutants do not differentiate into gametes, but instead form germ line tumors (30). In S. pombe, CID1 and CID13 monitor DNA damage and nucleotide levels, thereby controlling the cell cycle (27, 28). Perhaps, as with GLD-2, Cid1p, and Cid13p, the mammalian proteins will participate in mitotic controls in certain cell types. Transcripts of mGLD-2 and hGLD-2 appear to be present in a broad range of tissues and cell types (e.g., http://source.stanford.edu/cgi-bin/source/sourceSearch). Thus, the roles of these enzymes, and perhaps of regulated changes in poly(A) length, may now become accessible in mammalian somatic cells.
Acknowledgments
We thank Dr. Mike Kiledjian for his generous gift of anti-MS2 coat protein antibodies. We thank K. Dickson for plasmids, S. Kim and W. Pike for mouse cDNA, and members of the Wickens and Kimble laboratories for advice and discussion of results. We appreciate the help of the Biochemistry Media Lab and of Carol Pfeffer in preparation of the figures and text. This work was supported by National Institutes of Health Grant GM31892 (to M.W.) and a Biochemistry Steenbock Predoctoral Fellowship (to J.E.K.). J.K. is an Investigator with the Howard Hughes Medical Institute.
Abbreviations: PAP, poly(A) polymerase; HA, hemagglutinin; CPSF, cleavage and polyadenylation factor; CPEB, cytoplasmic polyadenylation element-binding protein; bPAP, bovine nuclear PAP; polβ, DNA polymerase β.
References
- 1.Wickens, M., Goodwin, E. B., Kimble, J., Strickland, S. & Hentze, M. (2000) in Translational Control of Gene Expression, eds. Hershey, J. W. B., Mathews, M. B. & Sonenberg, N. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 295–370.
- 2.Dever, T. E. (2002) Cell 108, 545–556. [DOI] [PubMed] [Google Scholar]
- 3.Job, C. & Eberwine, J. (2001) Nat. Rev. Neurosci. 2, 889–898. [DOI] [PubMed] [Google Scholar]
- 4.Martin, K. C., Barad, M. & Kandel, E. R. (2000) Curr. Opin. Neurobiol. 10, 587–592. [DOI] [PubMed] [Google Scholar]
- 5.Richter, J. D. (2000) in Translational Control of Gene Expression, eds. Hershey, J. W. B., Mathews, M. B. & Sonenberg, N. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 785–805.
- 6.Bushell, M. & Sarnow, P. (2002) J. Cell Biol. 158, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil, C. & Walter, P. (2001) Curr. Opin. Cell Biol. 13, 349–355. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson, A. (1996) in Translational Control, eds. Hershey, J. W., Mathews, M. B. & Sonenberg, N. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 451–480.
- 9.Jacobson, A. & Peltz, S. W. (1996) Annu. Rev. Biochem. 65, 693–739. [DOI] [PubMed] [Google Scholar]
- 10.Sachs, A. B., Sarnow, P. & Hentze, M. W. (1997) Cell 89, 831–838. [DOI] [PubMed] [Google Scholar]
- 11.Proudfoot, N. J., Furger, A. & Dye, M. J. (2002) Cell 108, 501–512. [DOI] [PubMed] [Google Scholar]
- 12.Zhao, J., Hyman, L. & Moore, C. (1999) Microbiol. Mol. Biol. Rev. 63, 405–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirose, Y. & Manley, J. L. (2000) Genes Dev. 14, 1415–1429. [PubMed] [Google Scholar]
- 14.McCracken, S., Fong, N., Yankulov, K., Ballantyne, S., Pan, G., Greenblatt, J., Patterson, S. D., Wickens, M. & Bentley, D. L. (1997) Nature 385, 357–361. [DOI] [PubMed] [Google Scholar]
- 15.Shatkin, A. J. & Manley, J. L. (2000) Nat. Struct. Biol. 7, 838–842. [DOI] [PubMed] [Google Scholar]
- 16.Dower, K. & Rosbash, M. (2002) RNA 8, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minvielle-Sebastia, L. & Keller, W. (1999) Curr. Biol. 11, 352–357. [DOI] [PubMed] [Google Scholar]
- 18.Colgan, D. F. & Manley, J. L. (1997) Genes Dev. 11, 2755–2766. [DOI] [PubMed] [Google Scholar]
- 19.Wahle, E. & Ruegsegger, U. (1999) FEMS Microbiol. Rev. 23, 277–295. [DOI] [PubMed] [Google Scholar]
- 20.Raabe, T., Bollum, F. J. & Manley, J. L. (1991) Nature 353, 229–234. [DOI] [PubMed] [Google Scholar]
- 21.Wahle, E., Martin, G., Schiltz, E. & Keller, W. (1991) EMBO J. 10, 4251–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardwell, V. J., Zarkower, D., Edmonds, M. & Wickens, M. (1990) Mol. Cell. Biol. 10, 846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doublie, S. (2000) EMBO J. 19, 4193–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bard, J., Zhelkofsky, A., Helmling, S., Earnest, T., Moore, C. & Bohm, A. (2000) Science 289, 1346–1349. [DOI] [PubMed] [Google Scholar]
- 25.Bienroth, S., Keller, W. & Wahle, E. (1993) EMBO J. 12, 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, L., Eckmann, C., Kadyk, L., Wickens, M. & Kimble, J. (2002) Nature 419, 312–316. [DOI] [PubMed] [Google Scholar]
- 27.Saitoh, S., Chabes, A., McDonald, W., Thelander, L., Yates, J. & Russell, P. (2002) Cell 109, 563–573. [DOI] [PubMed] [Google Scholar]
- 28.Read, R. L., Martinho, R. g., Wang, S.-W., Carr, A. M. & Norbury, C. J. (2002) Proc. Natl. Acad. Sci. USA 99, 12079–12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller, W. & Martin, G. (1996) Nature 419, 267–268. [DOI] [PubMed] [Google Scholar]
- 30.Kadyk, L. C. & Kimble, J. (1998) Development (Cambridge, U.K.) 125, 1803–1813. [DOI] [PubMed] [Google Scholar]
- 31.Aravind, L. & Koonin, E. V. (1999) Nucleic Acids Res. 27, 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhelkovsky, A., Helmling, S. & Moore, C. (1998) Mol. Cell. Biol. 18, 5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raabe, T., Murthy, K. G. K. & Manley, J. L. (1994) Mol. Cell. Biol. 14, 2946–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin, G. & Keller, W. (1996) EMBO J. 15, 2593–2603. [PMC free article] [PubMed] [Google Scholar]
- 35.Eckmann, C. R., Kraemer, B., Wickens, M. & Kimble, J. (2002) Dev. Cell 3, 697–710. [DOI] [PubMed] [Google Scholar]
- 36.Gray, N. K., Coller, J. M., Dickson, K. S. & Wickens, M. (2000) EMBO J. 19, 4723–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickson, K. S., Thompson, S. R. & Wickens, M. (2001) J. Biol. Chem. 276, 41810–41816. [DOI] [PubMed] [Google Scholar]
- 38.Saitou, N. & Nei, M. (1987) Mol. Biol. Evol. 4, 406–425. [DOI] [PubMed] [Google Scholar]
- 39.Kimura, M. (1983) The Neutral Theory of Molecular Evolution (Cambridge Univ. Press, Cambridge, U.K.).
- 40.Coller, J. & Wickens, M. (2002) Methods 26, 142–150. [DOI] [PubMed] [Google Scholar]
- 41.Dickson, K. S., Bilger, A., Ballantyne, S. & Wickens, M. P. (1999) Mol. Cell. Biol. 19, 5707–5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendez, R., Kannenganti, G. K., Murthy, K. R., Manley, J. L. & Richter, J. D. (2000) Mol. Cell 6, 1253–1259. [DOI] [PubMed] [Google Scholar]
- 43.Kashiwabara, S., Zhuang, T., Yamagata, K., Noguchi, J., Fukamizu, A. & Baba, T. (2000) Dev. Biol. 228, 106–115. [DOI] [PubMed] [Google Scholar]
- 44.Kashiwabara, S., Noguchi, J., Ohmura, K., Honda, A., Suguira, S., Miyamoto, K., Takahashi, S., Inoue, K., Ogura, A. & Baba, T. (2002) Science 298, 1999–2002. [DOI] [PubMed] [Google Scholar]
- 45.Topalian, S. J., Kaneko, S., Gonzales, M., Bond, G., Ward, Y. & Manley, J. L. (2001) Mol. Cell. Biol. 21, 5614–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyriakopoulou, C., Nordvarg, H. & Virtanen, A. (2001) J. Biol. Chem. 276, 33504–33511. [DOI] [PubMed] [Google Scholar]
- 47.Kabnick, K. S. & Housman, D. E. (1988) Mol. Cell. Biol. 8, 3244–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao, W. & Manley, J. L. (1996) Mol. Cell. Biol. 16, 2378–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ballantyne, S., Bilger, A., Astrom, J., Virtanen, A. & Wickens, M. (1995) RNA 1, 64–78. [PMC free article] [PubMed] [Google Scholar]
- 50.Thuresson, A.-C., Åström, J., Åström, A., Grönvik, K.-O. & Virtanen, A. (1994) Proc. Natl. Acad. Sci. USA 91, 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colgan, D. F., Murthy, K. G. K., Prives, C. & Manley, J. L. (1996) Nature 384, 282–285. [DOI] [PubMed] [Google Scholar]