Abstract
Chlamydiae are important pathogens and symbionts, with unique cell biology features. They lack the cell-division protein FtsZ, which functions in maintaining cell shape and orchestrating cell division in almost all other bacteria. In addition, the existence of peptidoglycan (PG) in chlamydial cell envelopes has been highly controversial. Using electron cryotomography, mass spectrometry and fluorescent labeling dyes, here we show that some environmental chlamydiae have cell-wall sacculi consisting of an unusual PG type. Treatment with fosfomycin (a PG synthesis inhibitor) leads to lower infection rates and aberrant cell shapes, suggesting that PG synthesis is crucial for the chlamydial life cycle. Our findings demonstrate for the first time the presence of PG in a member of the Chlamydiae. They also present a unique example of a bacterium with a PG sacculus but without FtsZ, challenging the current hypothesis that it is the absence of a cell wall that renders FtsZ non-essential.
Introduction
Chlamydiae are members of the Planctomycetes-Verrucomicrobia-Chlamydiae (PVC) bacterial superphylum1. Like most other bacteria, some PVC bacteria are already known to possess peptidoglycan, i.e. chains of alternating N-acetylglucosamine and N-acetylmuramic acid sugars crosslinked by short peptides. PVC bacteria also display striking eukaryote-like and archaea-like cell biological features, which have suggested intriguing hypotheses about their role in cellular evolution2, 3.
In Verrucomicrobia and almost all other bacteria, septal PG synthesis is orchestrated by the FtsZ cytoskeleton4, 5. In contrast, Planctomycetes lack both PG and FtsZ5. This fits to the notion that PG-loss renders FtsZ dispensable, like in mycoplasmas6 or L-form bacilli7. While from genome sequences it is clear that chlamydiae do not possess FtsZ, the presence or absence of chlamydial PG has been highly controversial8, 9, 10, 11, 12, 13, 14, 15, 16. One early study reported the colorimetric detection of muramic acid in chlamydiae14, but more reliable chromatographic methods subsequently failed to confirm this result11, 15. All attempts to purify chlamydial sacculi have failed10, 12 and no periplasmic density layers have been detected between the inner and outer membranes of chlamydiae by electron microscopy (including for instance17, 18, 19). The apparent absence of PG in chlamydiae is surprising, however, since despite their highly reduced genomes, a nearly complete pathway for the synthesis of PG is present in the genomes of all chlamydiae13. In addition, several of the chlamydial PG biosynthetic enzymes have been characterized and shown to be functional in vitro and in complementation assays20, 21, 22, 23, 24, 25.
Here we look for evidence of peptidoglycan cell walls in two diverse and deeply rooting chlamydiae26, Protochlamydia amoebophila and Simkania negevensis. Through electron cryotomography (ECT), biochemical purification, enzymatic digestion, mass spectrometry, fluorescence microscopy, and antibiotic treatment, we show that P. amoebophila are indeed surrounded by sacculi containing a new type of PG. In contrast, no evidence of PG is found in S. negevensis. These results prove that some chlamydiae do in fact synthesize PG sacculi, explaining the presence of PG-synthetic genes, but raising new questions about the identity and purpose of the modifications and the mechanisms of cell division in the absence of FtsZ.
Results
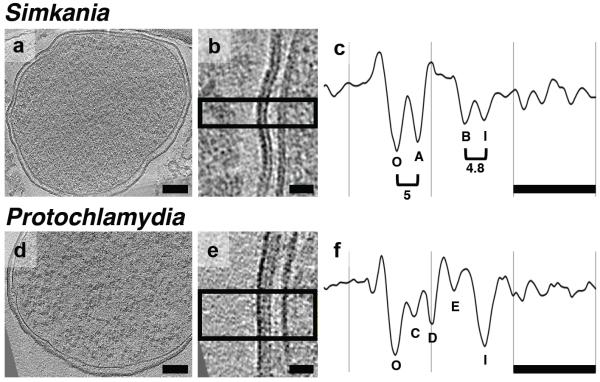
Electron cryotomography of the chlamydial cell envelope
Two diverse and deeply rooting members of the chlamydial phylum, Simkania negevensis and Protochlamydia amoebophila, were imaged by electron cryotomography (ECT) in a near-native state. Bacteria were purified from amoeba cultures, plunge-frozen, and 25 and 20 tomograms were collected of intact cells (Figure 1). Density profiles through the cell envelopes of the two species were quite different. While four layers were resolved in Simkania envelopes (Figure 1 B, C), five layers were resolved in Protochlamydia (Figure 1 E, F). Because the individual leaflets of lipid bilayers can be resolved in some cryotomograms, especially when the images are taken close to focus, in the case of Simkania, it is unclear whether the four layers represent the two leaflets of the outer and inner membranes (“O” and “A” being the two leaflets of the outer membrane, and layers “B” and “I” being the two leaflets of the inner membrane), or whether one or more of these layers are non-membranous. The facts that layers O and A have fairly similar contrast and are consistently spaced even through the undulations are consistent with them being two leaflets of a single (outer) bilayer membrane. Their separation (~5 nm), however, is much larger than typical phospholipid bilayer membranes, whose two density peaks (from the phospholipid head groups) are only 3.7-4 nm apart27. Similarly, Simkania layers B and I may be the two leaflets of a single (inner) membrane, since they have similar contrast and a consistent spacing, but again they appear too far apart. In contrast to the Simkania envelope, the profile of Protochlamydia surprisingly resembled those of other Gram-negative bacteria with two membranes and a peptidoglycan cell wall28, 29, 30. Between the Protochlamydia outer and inner membranes (labeled “O” and “I”, respectively) there appeared to be three additional layers (labeled “C”-“E”). The similar-looking three layers in Treponema pallidum (from the outside in) were identified as proteinaceous (lipoproteins), peptidoglycan, and again proteinaceous29. By analogy this suggests that layer C is composed of lipoproteins (perhaps connecting the outer membrane to the cell wall) and other outer-membrane-associated proteins, layer D is a cell wall, and layer E is composed of lipoproteins and inner-membrane-associated proteins (perhaps including the penicillin binding proteins responsible for cell wall synthesis, the lipoprotein OmcA, and the cysteine-rich protein OmcB). While other interpretations remain possible (cysteine-rich disulphide-cross-linked envelope proteins have been suggested to be the functional equivalent of PG in chlamydiae31), the most important and clear observation was that Protochlamydia exhibit a distinct periplasmic layer (D).
Figure 1. Chlamydial cell envelopes are multi-layered.
Simkania (A-C) and Protochlamydia (D-F) cells were purified from asynchronously infected amoeba cultures, plunge-frozen and imaged by ECT. Shown are tomographic slices through reticulate bodies (A, D and B, E enlarged) and corresponding density profiles (C, F) of the cell envelopes. Profiles are enlarged, aligned and cropped relative to the outer membrane. Distances between peaks (in nm) are indicated. In contrast to the Simkania profile, the Protochlamydia profile resembles those of other bacteria with peptidoglycan cell walls (see text for a full discussion of each profile and layer). Bar, 100 nm in A, D and 20 nm in B, C, E, F.
Purification and imaging of sacculi
In order to explore whether any of the observed periplasmic layers consisted of peptidoglycan (PG), we attempted to purify sacculi by boiling chlamydial cells (obtained from asynchronously infected amoeba cultures) in 4% sodium dodecyl sulfate. Strikingly, in three independent experiments, we observed sacculus-like structures in preparations from Protochlamydia (Figure 2, A-D), but not from Simkania (two experiments). Protochlamydia sacculi diameters (679 nm +/−34 s.d. n=10) and morphologies matched the size and shape of intact cells. Protochlamydia sacculi had one or two 5-7 nm thick layers (arrowheads in Figure 2 E), plus mesh-like (up to 30 nm long) high-density aggregates attached to the outside (arrows in Figure 2 D).
Figure 2. Protochlamydia synthesize purifiable sacculi that contain peptidoglycan.
Cryoprojections (A) and tomographic slices (B-E) through sacculi-like structures purified from Protochlamydia cells. We were unable to obtain similar structures from Simkania. Sacculi had one or two layers (arrowheads) plus short high-density filaments (arrows) on the outside (C enlarged in D, E). Sacculi were digested, reduced and separated by high-pressure liquid chromatography (F). Mass spectrometry analysis of peaks 1-3 (neutral masses indicated in Da) indicated the presence of modified peptidoglycan (see also Table 1 and Supplementary Fig. S1). Bars, 100 nm.
Digestion and biochemical analyses of sacculi
To check for the presence of peptidoglycan in the purified sacculi, we digested the samples with cellosyl, a glycan strand-cleaving peptidoglycan muramidase. Cellosyl released soluble material from insoluble sacculi, which was reduced with sodium borohydride and analyzed by high pressure liquid chromatography (HPLC) using conditions for separating muropeptides32. The chromatogram (Figure 2 F) showed three main peaks in the monomeric region (20-50 min) and many peaks after 60 min that are poorly separated at higher retention time (>75 min) forming a “hump”, which is typical for highly cross-linked and/or incompletely digested PG material33. The retention times and overall pattern of cellosyl digestion products were different, however, from those of muropeptide mixtures obtained from other Gram-positive and Gram-negative bacteria.
To characterize this material, the three main cellosyl products in the monomeric region and one well-separated main product at the beginning of the “hump” region were analyzed by mass spectrometry (MS). The determined neutral masses of the earlier three products were higher than what would be expected for monomeric muropeptides, but the masses of products 1 and 2 and of products 2 and 3 both differed by 71 Da, a typical feature of monomeric muropeptides with a tri-, tetra- and pentapeptide, respectively, due to the presence of none, one or two D-alanine residues (Figure 2 F). In MS/MS analysis the three products fragmented in a similar way, showing that they are related (Supplementary Fig. S1, Table 1). For all three peaks, we observed mass differences to the parent ion corresponding to the loss of GlcNAc, GlcNAcMurNAc(r) (r, indicates reduction to N-acetylmuramitol), GlcNAcMurNAc(r)-l-alanine and GlcNAcMurNAc(r)-l-alanine-d-glutamate, confirming that products 1-3 are all muropeptides. The neutral masses of the Protochlamydia products 1, 2 and 3 were all 314.12 Da larger than the masses of the reduced monomeric muropeptides (with tri-, tetra- or pentapeptide) from Gram-negative bacteria34, however, suggesting the presence of a common modification in the Protochlamydia muropeptides. The neutral mass of product 4 was consistent with a peptide cross-linked dimer of product 2. Additional mass differences that occurred in all fragmentation spectra indicated the presence of the same and as yet unknown modifications with 129 and 203 Da, respectively, explaining the higher mass of Protochlamydia muropeptides 1-3 compared to the monomeric muropeptides of E. coli.
Table 1.
Fragmentation masses from fractions of reduced P. amoebophila muropeptides.
| Fraction number or muro- peptide |
Fragment | Mass of fragments, H+ form (m/z) |
Proposed structure | |
|---|---|---|---|---|
| determined | calculated | |||
| 1 | A | 1167.3447 | 1167.4908 | [M+H]+-H2O |
| B | 1056.3231 | 1056.5014 | [M+H]+-129.2 | |
| C | 982.3848 | 982.4220 | [M+H]+-GlcNAc | |
| D | 853.3392 | 853.4220 | [M+H]+-129.0-GlcNAc | |
| E | 705.3032 | 705.3059 | [M+H]+-GlcNAcMurNAc(r) | |
| F | 634.3148 | 634.2688 | [M+H]+-GlcNAcMurNAc(r)Ala | |
| G | 505.2668 | 505.2262 | [M+H]+-GlcNAcMurNAc(r)AlaGlu | |
|
| ||||
| 2 | A | 1238.3288 | 1238.5255 | [M+H]+-H2O |
| B | 1127.4075 | 1127.5361 | [M+H]+-129.1 | |
| C | 1053.3859 | 1053.4567 | [M+H]+-GlcNAc | |
| D | 924.3934 | 924.4567 | [M+H]+-129.0-GlcNAc | |
| E | 776.3376 | 776.3406 | [M+H]+-GlcNAcMurNAc(r) | |
| F | 705.3202 | 705.3035 | [M+H]+-GlcNAcMurNAc(r)Ala | |
| G | 576.3141 | 576.2609 | [M+H]+-GlcNAcMurNAc(r)AlaGlu | |
|
| ||||
| 3 | A | 1309.4104 | 1309.5684 | [M+H]+-H2O |
| B | 1198.3875 | 1198.5790 | [M+H]+-129.2 | |
| C | 1124.3796 | 1124.4996 | [M+H]+-GlcNAc | |
| D | 995.3871 | 995.4996 | [M+H]+-129.0-GlcNAc | |
| E | 847.3485 | 847.3835 | [M+H]+-GlcNAcMurNAc(r) | |
| F | 776.3356 | 776.3464 | [M+H]+-GlcNAcMurNAc(r)Ala | |
| G | 647.3268 | 647.3038 | [M+H]+-GlcNAcMurNAc(r)AlaGlu | |
|
| ||||
| Tetra from C. jejuni |
A | 946.4128 | 946.3869 | [M+Na]+-H2O |
| B | n.d. | 835.4202 | [M+Na]+-129 | |
| C | 761.3908 | 761.3181 | [M+Na]+-GlcNAc | |
| D | n.d. | 632.3181 | [M+Na]+-129-GlcNAc | |
| E | 484.3317 | 484.2019 | [M+Na]+-GlcNAcMurNAc(r) | |
| F | 413.2619 | 413.1648 | [M+Na]+-GlcNAcMurNAc(r)Ala | |
| G | 284.3200 | 284.1222 | [M+Na]+-GlcNAcMurNAc(r)AlaGlu | |
Fraction numbers correspond to peak numbers in Figure 2 F. Fragments correspond to fragments in Supplementary Fig. S1. The parental ion ([M+H]+ or [M+Na]+) of each muropeptide fragments to ions lacking either water, an unknown modification with ~129 Da, a GlcNAc residue, ~129 Da and GlcNAc, a GlcNAcMurNAc(r) disaccharide (r, indicates reduction to muramitol), GlcNAcMurNAc(r)Ala or GlcNAcMurNAc(r)AlaGlu (see column ‘Proposed structure’). Tetra, reduced disaccharide tetrapeptide muropeptide fraction from Campylobacter jejuni56. The Na+ containing ion with m/z 964.4202 was fragmented. n.d., not detected.
To confirm that PG was a major component of Protochlamydia sacculi and check for the presence of disulfide cross-linked protein components, purified sacculi were subjected to lysozyme and DTT treatment, respectively, and imaged with negative stain electron microscopy. Only the incubation with lysozyme degraded the sacculi (Supplementary Fig. S2).
Fluorescence imaging of D-alanine incorporation in vivo
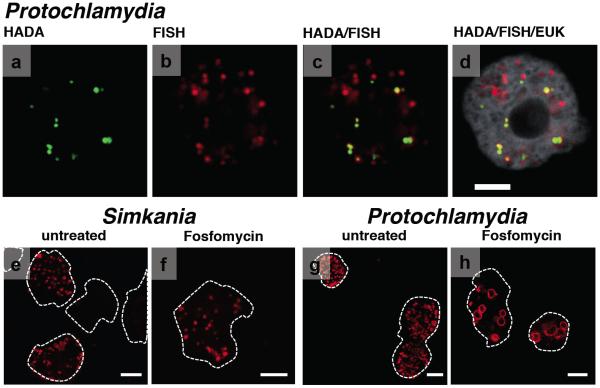
To further confirm the presence of PG in Protochlamydia sacculi, we tested whether fluorescently labeled amino acids (FDAA; labeled D-alanine in our experiments)35 would be incorporated into chlamydial cells in vivo. Incubation of amoeba cultures continuously infected with Protochlamydia (including reticulate bodies, elementary bodies, and transitional stages) with FDAA (HADA and BADA) resulted in multiple strong and chlamydial cell-sized signals inside amoeba cells (Figure 3 A-D, Supplementary Fig. S3). In many cases the FDAA labeling in infected amoebae overlapped with staining of the chlamydial cells by DAPI or chlamydiae-specific fluorescence in situ hybridization (FISH). Not all cells stained by FISH/DAPI showed a corresponding FDAA signal, at least in part because chlamydial cells were in different developmental stages, including non-replicating elementary bodies. No signals were detected when uninfected amoebae were incubated with FDAA (Supplementary Fig. S3 D, E), or when infected amoebae were incubated with DMSO only. Interestingly, purified Protochlamydia elementary bodies (which cannot undergo cell division and are therefore probably not actively synthesizing new PG) also did not show labeling upon incubation with FDAA (Supplementary Fig. S3 B, C), indicating that PG synthesis takes place during Protochlamydia replication inside the host. Amoeba cultures infected with Simkania, on the other hand, showed either no signals (using BADA) or signals similar to the background level (using HADA) upon labeling with FDAAs (Supplementary Fig. S4 A, B), consistent with the absence of purifiable sacculi. Purified Simkania cells were not labeled by either dye (Supplementary Fig. S4 C, D).
Figure 3. Protochlamydia incorporate D-alanine in vivo and are sensitive to fosfomycin.
Protochlamydia-infected amoebae (A-D), but not purified Protochlamydia cells (Supplementary Fig. S2) or uninfected amoeba cells (Supplementary Fig. S2), stained positively for new peptidoglycan synthesis with fluorescently labeled D-alanine dyes HADA (A) and BADA (Supplementary Fig. S2), confirming the synthesis of peptidoglycan in actively-growing Protochlamydia. Specific FISH staining of chlamydial cells (B, overlay with A shown in C) and the eukaryotic host (D, shows overlay with C) are shown. The treatment of Protochlamydia-infected amoeba cultures (amoeba cell outlines in white) with cell wall synthesis-targeting fosfomycin (H, control shown in G) resulted in a dramatic decrease in infection rate and aberrant or larger chlamydial cell shapes (shown are immunofluorescent stainings of chlamydial outer membrane proteins), suggesting a crucial role of PG in the Protochlamydia life cycle. Fosfomycin did not affect Simkania (F, control shown in E). Bars, 5 μm in D, 10 μm in E-H.
Protochlamydia sensitivity to cell-wall-targeting antibiotics
Due to the high conservation of PG throughout the bacterial domain of life, many antibacterial drugs target PG synthesis. The so-called “chlamydial anomaly”16 is that despite the fact that PG has not been detected in pathogenic chlamydiae, these organisms are sensitive to cell wall-targeting β-lactam antibiotics. Penicillin, for instance, leads to the formation of enlarged aberrant cells36, 37 and blocks the conversion between developmental stages38. Environmental chlamydiae, in contrast, are resistant to β-lactams39, 40 - possibly due to putative β-lactamases encoded in their genomes. To explore the role of the PG sacculus in the Protochlamydia life cycle, we used an alternative PG synthesis-targeting antibiotic (fosfomycin) to treat infected amoeba cells. The addition of 500 μg/ml fosfomycin to Protochlamydia-infected amoeba cultures led to a significant decrease in infection rate (20.2% ± 8 infected amoebae for fosfomycin-treated cultures vs. 95.8% ± 2.2 infected amoebae for untreated cultures; p <0.0001, unpaired t-test). Protochlamydia cells within treated cultures were also up to eight-times larger than normal (diameters of up to 6 μm) (Figure 3 G, H). Lower fosfomycin concentrations (25 μg/ml and 100 μg/ml) induced the formation of fewer aberrant forms and did not affect the infection rate (not shown). Fosfomycin-treatment of Simkania-infected amoeba cultures led to only a slight decrease in infection rate (59.9% ± 5.6 infected amoebae for fosfomycin-treated cultures vs. 67% ± 1.7 infected amoebae for untreated cultures) and no differences in cell size were detected (Figure 3 E, F).
Discussion
We conclude that Protochlamydia synthesize sacculi containing peptidoglycan that can be hydrolyzed by cellosyl, contains monomeric and cross-linked muropeptides, and carries yet unknown modifications at virtually every subunit. No evidence of peptidoglycan in Simkania was found. Fluorescence imaging of D-Ala incorporation in vivo and monitoring of cell wall antibiotic sensitivity further suggested that the Protochlamydia PG sacculus plays an important role in cell cycle and shape. This challenges previous speculations that chlamydiae synthesize a small ring of PG only during cell division41. Because this might still be true for Simkania and pathogenic chlamydiae, however, our data prompts a reconsideration of whether these organisms lack PG entirely (and the effects of β-lactams are pleiotropic) or if they synthesize novel PG structures that are not purified by standard sacculus preparation protocols.
The presence of sacculi in Protochlamydia but not in Simkania matches the less complete set of synthetic genes in the latter: Simkania, as well as pathogenic chlamydiae, lack an undecaprenyl-diphosphate phosphatase (UppP) and alanine/glutamine racemases (Alr, MurI) (Supplementary Table S1)13, 42, 43. Interestingly, transglycosylases have not been found in any chlamydial genomes (Supplementary Table S1)13 or in the genomes of a few other PG-possessing bacteria44, 45, so some other enzyme(s) must be capable of synthesizing glycan strands.
The presence of PG sacculi in Protochlamydia and in the Chlamydiae’s sister phylum Verrucomicrobia46, together with the fact that the more basal chlamydial lineages have more complete PG synthesis pathways make it likely that the last common chlamydial ancestor synthesized a PG sacculus. The detection of a PG-containing sacculus in Protochlamydia challenges the view that FtsZ is essential in PG-possessing bacteria4, 6, 7, however, because to our knowledge, Protochlamydia is the first example of a bacterium with a PG cell wall, but without FtsZ. Studying cell division and septal development in this organism could help clarify the role of FtsZ and the evolutionary transition to PG- and FtsZ-independency.
Methods
Cultivation of organisms
Acanthamoeba castellanii Neff infected with Protochlamydia amoebophila UWE25, or A. castellanii UWC1 infected with Simkania negevensis, were cultivated in TSY medium (30 g/L trypticase soy broth, 10 g/L yeast extract, pH 7.3) at 20°C. Amoebal growth was monitored by light microscopy and medium was exchanged every 3-6 days. The presence and identity of the chlamydial symbionts was verified by isolation of DNA from cultures followed by amplification and sequencing of the 16S rRNA genes. In addition, fluorescence in situ hybridization (FISH) using specific probes combined with 4′,6-diamidino-2-phenylindole (DAPI) staining of infected cultures was performed using specific probes for the respective symbiont47. Amoebae infected with chlamydiae were allowed to attach on slides and were fixed with 4% formaldehyde at 20°C. Cells were hybridized for 1.5 hours at 46°C at a formamide concentration of 25% with the Protochlamydia-specific probe E25-454 (5′ -GGATGT TAG CCA GCT CAT-3′ ) and the probe EUB33848. Subsequently, cells were stained with DAPI (0.5 μg/ml in PBS) for 5 minutes, and slides were analyzed using an epifluorescence microscope.
Purification of chlamydiae
Infected A. castellanii cultures were harvested by centrifugation (7,197 × g, 10 min), washed in Page’s Amoebic Saline (PAS)49, centrifuged and resuspended in PAS. Amoeba cells were disrupted by vortexing with an equal volume of glass beads for 3 minutes. Glass beads and cell debris were removed by centrifugation (5 min, 300 × g). The supernatant was filtered through a 1.2 μm filter and centrifuged at maximum speed for 10 min. The obtained pellet was resuspended in PAS.
Plunge-freezing
For plunge-freezing, copper/rhodium electron microscopy (EM) grids (R2/2 or R2/1, Quantifoil) were glow-discharged for 1 min. A 20×-concentrated bovine serum albumin-treated solution of 10 nm colloidal gold (Sigma) was added to purified chlamydiae or sacculi (1:4 v/v) immediately before plunge freezing. A 4-μl droplet of the mixture was applied to the EM grid, then automatically blotted and plunge-frozen into a liquid ethane-propane mixture50 using a Vitrobot (FEI Company)51.
Electron cryotomography
Images were collected using a Polara 300 kV FEG transmission electron microscope (FEI Company) equipped with an energy filter (slit width 20 eV; Gatan) on a lens-coupled 4 k×4 k UltraCam CCD (Gatan). Pixels on the CCD represented 0.95 nm (22,500×) or 0.63 nm (34,000×) at the specimen level. Typically, tilt series were recorded from −60° to +60° with an increment of 1° at 10 μm under-focus. The cumulative dose of a tilt-series was 180-220 e−/Å2. UCSF Tomo52 was used for automatic acquisition of tilt-series and 2D projection images. Three-dimensional reconstructions were calculated using the IMOD software package53 or Raptor54. Tomograms were visualized using 3dMOD53. Density profiles were generated using ImageJ.
Fluorescent labeling of peptidoglycan
Newly synthesized peptidoglycan was labeled using fluorescent D-amino acids35. A. castellanii cells continuously infected with Protochlamydia or Simkania were harvested and resuspended in a mixture of TSY and PAS (1:1). Cells were incubated with 1.5 mM HADA (hydroxy coumarin-carbonyl-amino-D-alanine)35 or BADA (4,4-Difluoro-5,7-Dimethyl-4-Bora-3a,4a-Diaza-s-Indacene-3-Propionic Acid-3-amino-D-alanine) for 6 h with gentle shaking. Cells were pelleted, washed three times and fixed with 4% formaldehyde followed by FISH using the chlamydia-specific probe Chls-0523 or DAPI-staining. As a control, uninfected amoebae and purified chlamydiae were treated in the same way.
Antibiotic treatment of infected amoebae cultures
A. castellanii were seeded into the wells of a multi-well dish and infected with purified Simkania and Protochlamydia elementary bodies55. After centrifugation at 600 × g for 10 min, the medium was exchanged for TSY supplemented with fosfomycin (0, 25, 100 or 500 μg/ml, respectively). Medium was exchanged once at 48 hours post infection. Cells were fixed with methanol at 96 hours post infection followed by immunofluorescence analysis using either anti-PomS antibodies55 or anti-Simkania antibodies raised against purified chlamydiae. The number of infected amoebae was counted for each treatment.
Preparation and composition analysis of sacculi
Chlamydial cells were purified from 1.8 L (Protochlamydia) and 3 L (Simkania) of infected amoeba culture and, depending on the amount of harvested cells, resuspended in 2.6-5.2 ml 4% SDS (w/v). After shipping (overnight, room temperature), the suspensions were dripped into 4% SDS (preheated to 90°C, 6 ml final volume) and stirred for 2.5 h at 90°C. Sacculi were pelleted (30 min, 135,000 × g) in a TLA-100.3 rotor (Beckman Coulter), washed 4× in 3 ml water, resuspended in 150 μl water and supplemented with 0.02% (w/v) sodium azide. Muropeptides were released from the PG by an overnight incubation with the muramidase Cellosyl (Hoechst, Frankfurt am Main, Germany) on a thermal shaker at 37°C and 800 rpm. The sample was boiled for 10 min and centrifuged for 10 min at 13,500 × g. The muropeptides present in the supernatant were reduced with sodium borohydride and separated on a 250×4.6 mm 3-μm Prontosil 120-3-C18 AQ reversed phase column (Bischoff, Germany) at 55°C using a 135 min gradient from 50 mM sodium phosphate pH 4.31 to 75 mM sodium phosphate pH 4.95, 15% methanol and a flow rate of 0.5 ml/min32. Muropeptides were detected at 205 nm. Fractions were collected, concentrated in a SpeedVac, acidified by 1% trifluoroacetic acid, and analyzed by offline electrospray mass spectrometry on a Finnigan LTQ-FT mass spectrometer (ThermoElectron, Bremen, Germany) in positive ion mode using mass scans over the mass range from m/z = 300 to m/z = 1900 at a typical spray voltage of 1.1-1.5 kV34. Parent ion scans were acquired with an FT MS resolution setting of 100,000 (at m/z = 400) with a typical mass accuracy of 3 ppm. MS/MS scans were performed in the ion trap, which has a typical mass accuracy for the fragment ion of ± 0.3 Da. MS spectra were deconvoluted to generate uncharged masses using the QualBrowser program (ThermoElectron, Bremen, Germany)34.
Negative-stain electron microscopy of treated sacculi preparations
Purified sacculi were incubated at 37°C for 12 h in 20 μl total volume with either lysozyme (10 mg/ml), dithiothreitol (5 mM) or phosphate buffered saline. Samples were applied to a Formvar-coated, carbon-coated, glow-discharged copper EM grid (Electron Microscopy Sciences). Samples were aspirated, stained with 1.5% uranylacetate and imaged on an FEI Tecnai T12 electron microscope.
Supplementary Material
Acknowledgments
This work was funded by the Austrian Science Fund FWF (Y277-B03 to MH), the European Research Council (ERC StG “EvoChlamy” to MH), NIH grant GM094800B (to GJJ), the Caltech Center for Environmental Microbiology Interactions (to GJJ, MP), a gift from the Gordon and Betty Moore Foundation to Caltech, the BBSRC (BB/I020012/1 to WV), NIH grant AI059327 (to MSV) and NIH grant GM051986 (to YVB). We thank Elitza Tocheva for discussions on the preparation of sacculi.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Wagner M, Horn M. The Planctomycetes, Verrucomicrobia, Chlamydiae and sister phyla comprise a superphylum with biotechnological and medical relevance. Curr Opin Biotechnol. 2006;17:241–249. doi: 10.1016/j.copbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Devos DP, Reynaud EG. Evolution. Intermediate steps. Science. 2010;330:1187–1188. doi: 10.1126/science.1196720. [DOI] [PubMed] [Google Scholar]
- 3.Reynaud EG, Devos DP. Transitional forms between the three domains of life and evolutionary implications. Proc Biol Sci. 2011;278:3321–3328. doi: 10.1098/rspb.2011.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson HP, Anderson DE, Osawa M. FtsZ in Bacterial Cytokinesis: Cytoskeleton and Force Generator All in One. Microbiol Mol Biol Rev. 2010;74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pilhofer M, et al. Characterization and Evolution of Cell Division and Cell Wall Synthesis Genes in the Bacterial Phyla Verrucomicrobia, Lentisphaerae, Chlamydiae, and Planctomycetes and Phylogenetic Comparison with rRNA Genes. J Bacteriol. 2008;190:3192–3202. doi: 10.1128/JB.01797-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lluch-Senar M, Querol E, Pinol J. Cell division in a minimal bacterium in the absence of ftsZ. Mol Microbiol. 2010;78:278–289. doi: 10.1111/j.1365-2958.2010.07306.x. [DOI] [PubMed] [Google Scholar]
- 7.Leaver M, DomA-nguez-Cuevas P, Coxhead JM, Daniel RA, Errington J. Life without a wall or division machine in Bacillus subtilis. Nature. 2009;457:849–853. doi: 10.1038/nature07742. [DOI] [PubMed] [Google Scholar]
- 8.Manire GP, Tamura A. Preparation and Chemical Composition of the Cell Walls of Mature Infectious Dense Forms of Meningopneumonitis Organisms. J Bacteriol. 1967;94:1178–1183. doi: 10.1128/jb.94.4.1178-1183.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura A, Manire GP. Preparation and Chemical Composition of the Cell Membranes of Developmental Reticulate Forms of Meningopneumonitis Organisms. J Bacteriol. 1967;94:1184–1188. doi: 10.1128/jb.94.4.1184-1188.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox A, et al. Muramic acid is not detectable in Chlamydia psittaci or Chlamydia trachomatis by gas chromatography-mass spectrometry. Infect Immun. 1990;58:835–837. doi: 10.1128/iai.58.3.835-837.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbour AG, Amano K, Hackstadt T, Perry L, Caldwell HD. Chlamydia trachomatis has penicillin-binding proteins but not detectable muramic acid. J Bacteriol. 1982;151:420–428. doi: 10.1128/jb.151.1.420-428.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCoy AJ, Maurelli AT. Building the invisible wall: updating the chlamydial peptidoglycan anomaly. Trends Microbiol. 2006;14:70–77. doi: 10.1016/j.tim.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Perkins HR, Allison AC. Cell-wall Constituents of Rickettsiae and Psittacosis-Lymphogranuloma Organisms. J Gen Microbiol. 1963;30:469–480. doi: 10.1099/00221287-30-3-469. [DOI] [PubMed] [Google Scholar]
- 15.Garrett AJ, Harrison MJ, Manire GP. A Search for the Bacterial Mucopeptide Component, Muramic Acid, in Chlamydia. J Gen Microbiol. 1974;80:315–318. doi: 10.1099/00221287-80-1-315. [DOI] [PubMed] [Google Scholar]
- 16.Moulder JW. Why is Chlamydia sensitive to penicillin in the absence of peptidoglycan? Infect Agents Dis. 1993;2:87–99. [PubMed] [Google Scholar]
- 17.Matsumoto A. Recent progress of electron microscopy in microbiology and its development in future: from a study of the obligate intracellular parasites, chlamydia organisms. J Electron Microsc. 1979;28:57–64. [Google Scholar]
- 18.Tamura A, Matsumoto A, Manire GP, Higashi N. Electron Microscopic Observations on the Structure of the Envelopes of Mature Elementary Bodies and Developmental Reticulate Forms of Chlamydia psittaci. J Bacteriol. 1971;105:355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Z, Chen M, Li K, Dong X, Han J, Zhang Q. Cryo-electron tomography of Chlamydia trachomatis gives a clue to the mechanism of outer membrane changes. J Electron Microsc. 2010;59:237–241. doi: 10.1093/jmicro/dfp057. [DOI] [PubMed] [Google Scholar]
- 20.McCoy AJ, Maurelli AT. Characterization of Chlamydia MurC-Ddl, a fusion protein exhibiting D-alanyl-D-alanine ligase activity involved in peptidoglycan synthesis and D-cycloserine sensitivity. Mol Microbiol. 2005;57:41–52. doi: 10.1111/j.1365-2958.2005.04661.x. [DOI] [PubMed] [Google Scholar]
- 21.McCoy AJ, Sandlin RC, Maurelli AT. In vitro and in vivo functional activity of Chlamydia MurA, a UDP-N-acetylglucosamine enolpyruvyl transferase involved in peptidoglycan synthesis and fosfomycin resistance. J Bacteriol. 2003;185:1218–1228. doi: 10.1128/JB.185.4.1218-1228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hesse L, Bostock J, Dementin S, Blanot D, Mengin-Lecreulx D, Chopra I. Functional and Biochemical Analysis of Chlamydia trachomatis MurC, an Enzyme Displaying UDP-N-Acetylmuramate:Amino Acid Ligase Activity. J Bacteriol. 2003;185:6507–6512. doi: 10.1128/JB.185.22.6507-6512.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patin D, Bostock J, Blanot D, Mengin-Lecreulx D, Chopra I. Functional and biochemical analysis of the Chlamydia trachomatis ligase MurE. J Bacteriol. 2009;191:7430–7435. doi: 10.1128/JB.01029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patin D, Bostock J, Chopra I, Mengin-Lecreulx D, Blanot D. Biochemical characterisation of the chlamydial MurF ligase, and possible sequence of the chlamydial peptidoglycan pentapeptide stem. Arch Microbiol. 2012;194:505–512. doi: 10.1007/s00203-011-0784-8. [DOI] [PubMed] [Google Scholar]
- 25.Henrichfreise B, et al. Functional conservation of the lipid II biosynthesis pathway in the cell wall-less bacteria Chlamydia and Wolbachia: why is lipid II needed? Mol Microbiol. 2009;73:913–923. doi: 10.1111/j.1365-2958.2009.06815.x. [DOI] [PubMed] [Google Scholar]
- 26.Horn M. Chlamydiae as symbionts in eukaryotes. Annu Rev Microbiol. 2008;62:113–131. doi: 10.1146/annurev.micro.62.081307.162818. [DOI] [PubMed] [Google Scholar]
- 27.Leforestier A, Lemercier N, Livolant F. Contribution of cryoelectron microscopy of vitreous sections to the understanding of biological membrane structure. Proc Natl Acad Sci U S A. 2012;109:8959–8964. doi: 10.1073/pnas.1200881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tocheva EI, Li Z, Jensen GJ. Electron cryotomography. Cold Spring Harb Perspect Biol. 2010;2:a003442. doi: 10.1101/cshperspect.a003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Howell JK, Bradley SD, Zheng Y, Zhou ZH, Norris SJ. Cellular architecture of Treponema pallidum: novel flagellum, periplasmic cone, and cell envelope as revealed by cryo electron tomography. J Mol Biol. 2010;403:546–561. doi: 10.1016/j.jmb.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tocheva EI, Matson EG, Morris DM, Moussavi F, Leadbetter JR, Jensen GJ. Peptidoglycan remodeling and conversion of an inner membrane into an outer membrane during sporulation. Cell. 2011;146:799–812. doi: 10.1016/j.cell.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatch T. Disulfide cross-linked envelope proteins: the functional equivalent of peptidoglycan in chlamydiae? J Bacteriol. 1996;178:1–5. doi: 10.1128/jb.178.1.1-5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glauner B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988;172:451–464. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- 33.de Jonge BL, Tomasz A. Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2A in cell wall synthesis. Antimicrob Agents Chemother. 1993;37:342–346. doi: 10.1128/aac.37.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui NK, Gray J, Schwarz H, Schumann P, Blanot D, Vollmer W. The peptidoglycan sacculus of Myxococcus xanthus has unusual structural features and is degraded during glycerol-induced myxospore development. J Bacteriol. 2009;191:494–505. doi: 10.1128/JB.00608-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuru E, et al. In Situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent D-amino acids. Angewandte Chem. 2012;51:12519–12523. doi: 10.1002/anie.201206749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto A, Manire GP. Electron Microscopic Observations on the Effects of Penicillin on the Morphology of Chlamydia psittaci. J Bacteriol. 1970;101:278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skilton RJ, et al. Penicillin Induced Persistence in Chlamydia trachomatis: High Quality Time Lapse Video Analysis of the Developmental Cycle. PLoS ONE. 2009;4:e7723. doi: 10.1371/journal.pone.0007723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beatty WL, Morrison RP, Byrne GI. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maurin M, Bryskier A, Raoult D. Antibiotic susceptibilities of Parachlamydia acanthamoeba in amoebae. Antimicrob Agents Chemother. 2002;46:3065–3067. doi: 10.1128/AAC.46.9.3065-3067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahane S, Gonen R, Sayada C, Elion J, Friedman MG. Description and partial characterization of a new chlamydia-like microorganism. FEMS Microbiol Lett. 1993;109:329–334. doi: 10.1016/0378-1097(93)90041-y. [DOI] [PubMed] [Google Scholar]
- 41.Brown WJ, Rockey DD. Identification of an antigen localized to an apparent septum within dividing chlamydiae. Infect Immun. 2000;68:708–715. doi: 10.1128/iai.68.2.708-715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horn M, et al. Illuminating the evolutionary history of chlamydiae. Science. 2004;304:728–730. doi: 10.1126/science.1096330. [DOI] [PubMed] [Google Scholar]
- 43.Collingro A, et al. Unity in Variety—The Pan-Genome of the Chlamydiae. Mol Biol Evol. 2011;28:3253–3270. doi: 10.1093/molbev/msr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McPherson DC, Popham DL. Peptidoglycan Synthesis in the Absence of Class A Penicillin-Binding Proteins in Bacillus subtilis. J Bacteriol. 2003;185:1423–1431. doi: 10.1128/JB.185.4.1423-1431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arbeloa A, et al. Role of Class A Penicillin-Binding Proteins in PBP5-Mediated β-Lactam Resistance in Enterococcus faecalis. J Bacteriol. 2004;186:1221–1228. doi: 10.1128/JB.186.5.1221-1228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hedlund BP, Gosink JJ, Staley JT. Phylogeny of Prosthecobacter, the fusiform caulobacters: Members of a recently discovered division of the Bacteria. Int J Syst Bacteriol. 1996;46:960–966. doi: 10.1099/00207713-46-4-960. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz-Esser S, et al. Diversity of Bacterial Endosymbionts of Environmental Acanthamoeba Isolates. Appl Environ Microbiol. 2008;74:5822–5831. doi: 10.1128/AEM.01093-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Page FC. An illustrated key to freshwater and soil amoebae. Freshwater Biological Association; 1976. [Google Scholar]
- 50.Tivol WF, Briegel A, Jensen GJ. An improved cryogen for plunge freezing. Microsc Microanal. 2008;14:375–379. doi: 10.1017/S1431927608080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iancu CV, et al. Electron cryotomography sample preparation using the Vitrobot. Nat Protocols. 2006;1:2813–2819. doi: 10.1038/nprot.2006.432. [DOI] [PubMed] [Google Scholar]
- 52.Zheng SQ, et al. UCSF tomography: an integrated software suite for real-time electron microscopic tomographic data collection, alignment, and reconstruction. J Struct Biol. 2007;157:138–147. doi: 10.1016/j.jsb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;1996/01/01:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 54.Amat F, Moussavi F, Comolli LR, Elidan G, Downing KH, Horowitz M. Markov random field based automatic image alignment for electron tomography. J Struct Biol. 2008;161:260–275. doi: 10.1016/j.jsb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Aistleitner K, et al. Identification and Characterization of a Novel Porin Family Highlights a Major Difference in the Outer Membrane of Chlamydial Symbionts and Pathogens. PLoS ONE. 2013;8:e55010. doi: 10.1371/journal.pone.0055010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frirdich E, et al. Peptidoglycan-modifying enzyme Pgp1 is required for helical cell shape and pathogenicity traits in Campylobacter jejuni. PLoS Pathog. 2012;8:e1002602. doi: 10.1371/journal.ppat.1002602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCoy AJ, Maurelli AT. Building the invisible wall: updating the chlamydial peptidoglycan anomaly. Trends Microbiol. 2006;14:70–77. doi: 10.1016/j.tim.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Lovering AL, Safadi SS, Strynadka NCJ. Structural Perspective of Peptidoglycan Biosynthesis and Assembly. Ann Rev Biochem. 2012;81:451–478. doi: 10.1146/annurev-biochem-061809-112742. [DOI] [PubMed] [Google Scholar]
- 59.Chopra I, Storey C, Falla TJ, Pearce JH. Antibiotics, peptidoglycan synthesis and genomics: the chlamydial anomaly revisited. Microbiology. 1998;144(Pt 10):2673–2678. doi: 10.1099/00221287-144-10-2673. [DOI] [PubMed] [Google Scholar]
- 60.McCoy AJ, Adams NE, Hudson AO, Gilvarg C, Leustek T, Maurelli AT. l,l-diaminopimelate aminotransferase, a trans-kingdom enzyme shared by Chlamydia and plants for synthesis of diaminopimelate/lysine. Proc Natl Acad Sci U S A. 2006;103:17909–17914. doi: 10.1073/pnas.0608643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.