Abstract
Isoprenoid biosynthesis in the widespread diatomaceous algae, Rhizosolenia setigera (Brightwell) and Haslea ostrearia (Simonsen), results not only in the production of diterpenoids, triterpenoids, and sterols but, unusually for diatoms, also in the production of sesterterpenoids. By using 13C and 2H isotopic labeling techniques followed by NMR and mass spectrometry, specific inhibition of mevalonate (MVA) and methylerythritol (MEP) pathways, and comparison with the natural 13C/12C isotope ratios of the lipids, the different biosynthetic pathways of the sesterterpenes and other isoprenoids have now been determined. Surprisingly, whereas the sesterterpenes (Δ7(20)-haslenes) in R. setigera were made by the MVA pathway, as were the related triterpenoid rhizenes and desmosterol, in H. ostrearia the structurally similar Δ6(17)-haslenes and the major sterol, 24-ethylcholest-5-en-3β-ol, were instead biosynthesized by the MEP route. Phytol was biosynthesized in both diatoms by the MEP route. Subfractionation of R. setigera cells revealed that although phytol was located in the chloroplasts, the haslenes, rhizenes, and sterols were present in the cytoplasm. The observations described here for R. setigera and H. ostrearia show that terpenoid biosynthesis in diatoms is species-dependent and cannot simply be grouped according to structural type. Triterpenes appear to be made by the MVA route as in higher plants, whereas sesterterpenes and sterols can be made by either the MVA or MEP routes. In neither organism were the isoprenoids biosynthesized by leucine metabolism. Sesterterpene and triterpene biosynthesis in diatoms has not been investigated previously.
Although all isoprenoids are biosynthesized from isopentenyl diphosphate (IDP), IDP itself can be produced by several different routes. In addition to the mevalonate (MVA) route, a MVA-independent pathway has been described, yielding IDP from pyruvate and glyceraldehyde 3-phosphate, with 1-deoxy-d-xylulose 5-phosphate and 2-C-methyl-d-erythritol 4-phosphate (MEP) identified as intermediates (1). In addition, IDP in some parasitic protozoa appears to be biosynthesized both by the MVA route and by direct incorporation of leucine (2). Many authors have studied the distribution of the MVA and MEP pathways within a large number of organisms by incorporation of 13C- or 2H-labeled precursors (for reviews see refs. 1, 3, and 4), by use of highly specific inhibitors of the MVA and MEP pathways (5, 6), or by measuring the distribution of the genes of both pathways (7, 8). Jux and coworkers (9) demonstrated that natural 13C/12C isotope ratios of individual lipids could also be used to distinguish between the pathways. Based on these four approaches, it has been established that archaea, certain bacteria, yeasts, fungi, and some protozoa and animals use only the MVA pathway, whereas many bacteria, green algae, and some protists rely on the MEP pathway (10). Some algae, some streptomycetes, mosses, and liverworts, two marine diatoms, and higher plants appear to use both routes. Recent findings (4, 11, 12) have shown that the MVA pathway is implicated in cytosolic sterol biosynthesis, whereas the MEP pathway appears to be involved in the formation of plastidic isoprenoids such as phytol and the carotenoids. However, compartmental separation of the two biosynthetic pathways is not absolute (4, 12, 13) and the biosynthesis of some isoprenoid classes such as C25 sesterterpenes has received virtually no attention. Some diatoms are important producers of sesterterpenes and other isoprenoids (14) and, because only two species of these important primary producers have been studied (11), it seemed essential to determine the biosynthesis of isoprenoids in further diatom species.
In a recent study, Rowland et al. (15) reported that highly branched isoprenoid (HBI) sesterterpenes (haslenes) synthesized by the diatom Haslea ostrearia exhibited in vitro activity against lung cancer cells. A dramatic effect of the growth temperature on the biosynthesis of haslenes was also reported (15), suggesting that these isoprenoids may have a significant biological function in diatoms. Pozzi et al. suggested that phosphate esters of acyclic isoprenoids, including haslenes, may be membrane lipids (16), and Takajo and coworkers (17) showed that synthetic HBI phosphates formed stable vesicles. As yet, the location and biological function of HBIs in diatoms are not known. The potential medical applications, marine geochemical interests, and possible membrane functions prompted a study herein of two HBI-producing diatoms chosen to represent both planktonic (Rhizosolenia setigera) and benthic (H. ostrearia) species. In a first set of experiments, each species was grown in the presence of a specific inhibitor of each biosynthetic pathway (mevinolin and fosmidomycin for the MVA and MEP pathways, respectively). In a second set of experiments, the same species were grown in the presence of a variety of isotopically labeled precursors (13CO2, [1-13C]acetate, [2-13C]acetate, [2H3]acetate, [1,1,1,4-2H4]deoxyxylulose, l-[2-13C]leucine, and d-[1-13C]glucose), and the extent and nature of incorporation of these compounds into individual isoprenoids was investigated by using MS and NMR techniques. For both diatoms, the results of these experiments were used to interpret the differences in natural 13C/12C isotope ratios of individual compounds obtained from the current and previous studies. R. setigera cells were also fractionated according to established procedures, and the individual terpenoid composition of the cellular fractions was investigated by GC-MS.
Experimental Procedures
Algal Cultures. R. setigera (strains RS 99 and RS 02/A) was isolated in Spring 1999 and 2002 from surface waters at Le Croisic (France), and H. ostrearia was isolated from microphytobenthic communities in oyster ponds from the Bay of Bourgneuf (France) during Spring 1999. Small- and large-scale cultures were grown under standard conditions (Guillard's F/2 medium) as described (18). Cells were harvested by filtration at the end of each exponential growing phase.
Cell Fractionation. R. setigera cells from a 300-liter culture were filtered (30-μm gauze), washed in 0.2 μm filtered seawater, and resuspended in isolation buffer (19). Chloroplast-enriched fractions were obtained by centrifugation in Percoll gradients (19), whereas free lipid-enriched fractions were obtained by differential centrifugation (20).
Inhibition Experiments. Mevinolin/lovastatin is a well known inhibitor of 3-hydroxy-3-methylglutaryl-CoA reductase, a key enzyme in the MVA pathway (5), whereas fosmidomycin has been demonstrated to be a specific inhibitor of 1-deoxy-d-xylulose-5-phosphate reductoisomerase, an enzyme involved in the MEP route (21). R. setigera and H. ostrearia were each grown in the presence of increasing concentrations of fosmidomycin and, in separate experiments, increasing concentrations of mevinolin, to investigate the effect on the nonsaponifiable lipid content of the cells and on the lipid distributions. Each species was grown in 50-ml culturing tubes containing 15 ml of F/2 Guillard's medium under standard controlled conditions. Fosmidomycin (sodium salt) and mevinolin/lovastatin were purchased from Molecular Probes and from Calbiochem-Novabiochem, respectively. Concentrations of the inhibitors were based on those used in previous investigations (21, 22). Cell counts and monitoring of the cultures, together with the extraction, purification and analysis of the HBIs by GC-MS, were performed as described (23).
Isotopic Labeling. At least two alternative biosynthetic pathways exist for the formation of IDP, the common precursor of all isoprenoids. Acetate is directly incorporated into the MVA pathway after activation into acetyl-CoA but can enter into the MEP pathway only by way of the glyoxylate and tricarboxylic acid cycles (24, 25). In contrast, CO2 is efficiently incorporated into the MEP route (11). Glucose, when metabolized, provides carbon to both pathways. Further, when [1-13C]glucose is used, the MVA and MEP pathways yield distinctive labeling patterns in IDP (3, 25). It was therefore decided to grow R. setigera and H. ostrearia in the presence of isotopically enriched [13C]acetate, glucose, and CO2. Deuterium (2H)-enriched acetate and deoxyxylulose (26) were also used for comparison. 13CO2-incorporation experiments were performed according to the method of Cvejić and Rohmer (11). Experiments using sodium [1-13C]acetate (20–50% 13C), sodium [2-13C]acetate (20–50% 13C), [2H3]acetate (20% 2H), l-[2-13C]leucine (50% 13C), and d-[1-13C]glucose (20% 13C) were performed in 250-ml (R. setigera) or 2-liter (H. ostrearia) flasks. Sodium [1-13C]acetate (99% 13C), sodium [2-13C]acetate (99% 13C), sodium [2H3]acetate (99% 2H), l-[2-13C]leucine (99% 13C), d-[1-13C]glucose (99% 13C), and Ba13CO3 (99% 13C) were obtained from Sigma-Aldrich. Lipids were analyzed quantitatively by GC-MS.
To obtain quantities of HBIs sufficient for NMR spectroscopic analysis (>0.1 mg), large-scale cultures (≥50-liters) were needed. Sodium [1-13C]acetate (20% or 100% 13C) and sodium [2-13C]acetate (20% 13C) were used at a concentration of 250 mg·liter–1. To control the availability of carbon from other sources, culture media were bubbled with CO2-free air (15 liter·h–1), and Na2CO3 (150 or 10 mg·liter–1) was added to provide an inorganic carbon source. Total lipids were extracted from filtered cells by methods as described (23). Individual HBI isomers were separated by silver-ion (Ag+) chromatography (Chromspher 5 lipid, 250 × 4.6 mm i.d.) under isocratic conditions (hexane/isopropyl alcohol, 98.75:1.25, vol/vol). A Hewlett–Packard 5010 HPLC system coupled to a Hewlett–Packard diode array detector was used.
Monitoring of Stable Isotope Incorporation by GC-MS: Isotopic Enrichment Factors. For stable isotope enrichment experiments, the extent of 2H and 13C incorporation were determined by MS. In their recent study of the biosynthesis of 2-methyl-3-buten-2-ol from pine needles, Zeidler and Lichtenthaler (27) estimated the degree of isotopic labeling in 2-methyl-3-buten-2-ol after incubation of the needles with labeled precursors by using a simple ratio of mass spectral peak intensities. However, in contrast to 2-methyl-3-buten-2-ol, the increased molecular masses together with the variable structures of the ≥ C20 terpenoids synthesized by the two diatoms studied here meant that such ratios would not provide accurate measures of the degree of labeling. Instead, 13C or 2H isotopic enrichment factors (IEF) were calculated for each compound according to Eq. 1,
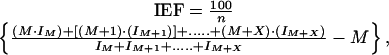 |
[1] |
where M, M + 1, etc. are the values of the molecular ions for various isotopomers, IM is the intensity of the molecular ion, IM + 1 is the intensity of the M + 1 peak, IM+X is the intensity of the highest mass ion (quantifiable), and n is the number of carbon atoms in the molecule. Comparison of isotopic enrichment factors from compounds obtained from cells cultured in the presence of isotopically labeled precursors with those obtained from control cultures thus revealed the extent of isotopic incorporation within individual compounds.
Monitoring of Stable Isotope Incorporation by NMR Spectroscopy. 13C NMR spectra of individual compounds were recorded in CDCl3 by using a Jeol EX 270 spectrometer equipped with a DELTA station. 13C peak assignments of the individual terpenoids were made by comparison with published data (18, 28–31). To measure specific enhancements in 13C, a carbon atom needed to be identified that should show no enrichment from 13C-enriched acetate according to either the MVA pathway or the MEP route by way of the glyoxylate shunt. For example, incorporation of [1-13C]acetate into IDP by the MVA and MEP routes results in labeling at C-1/C-3 and C-4, respectively (32), and so a carbon atom corresponding to C-2 was chosen as a reference. Having normalized the intensities of all remaining 13C resonances to the reference carbon, relative 13C enhancements for individual carbon atoms were calculated by comparison of 13C peak intensities between 13C-labeled and unlabeled material.
Results
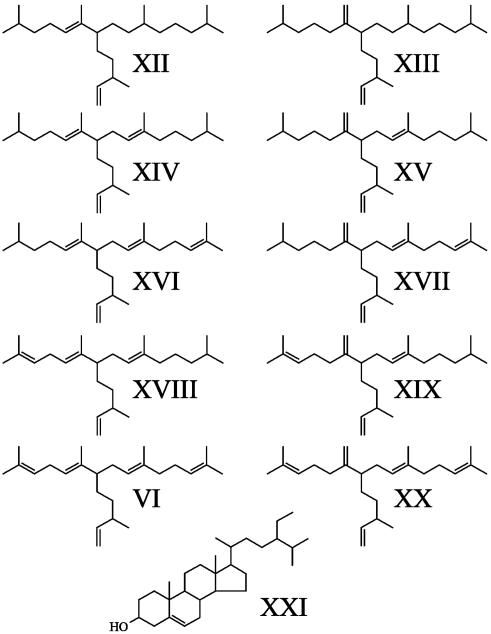
R. setigera. The structures and distributions of the nonsaponifiable lipids synthesized by the diatom R. setigera have been described (30, 33). In brief, GC-MS analysis of the nonsaponifiable lipids of R. setigera (RS 99) showed the presence of n-C21:6 (I), phytol (II), desmosterol (XI, cholesta-5,24-dien-3β-ol), four C30 HBI alkene isomers (VII–X), and up to three C25 HBI alkene isomers (III–V) (Fig. 1), depending on the culture analyzed. For the isoprenoid-localization study, phytol and small amounts of n-C21:6 were observed in the chloroplast-enriched fraction, whereas depleted amounts of the other isoprenoids were detected. In contrast, in the free lipid-enriched fraction, phytol and n-C21:6 were not detected, but large amounts of HBIs and desmosterol were observed (Table 1).
Fig. 1.
Nonsaponifiable lipids obtained from cultures of strains RS 99 and RS 02/A of R. setigera: n-C21:6 (I), phytol (II), C25 HBIs (III–VI), C30 HBIs (VII–X), and desmosterol (XI).
Table 1. Nonsaponifiable lipid (NSL) composition (expressed as percentage of total NSL content) of subcellular fractions of R. setigera.
| NSL extract | Chloroplast | Free lipid | |
|---|---|---|---|
| n-C21:6 (I) | 6.1 | 4.1 | 0.0 |
| Phytol (II) | 38.0 | 81.8 | 0.0 |
| C25:3 (III) | 1.0 | 0.0 | 1.5 |
| C30:5 (VII) | 12.3 | 3.7 | 29.0 |
| C30:5 (VIII) | 11.5 | 3.1 | 25.2 |
| C30:6 (IX) | 7.5 | 1.8 | 14.4 |
| C30:6 (X) | 8.7 | 2.0 | 14.3 |
| Desmosterol (XI) | 14.9 | 3.6 | 14.8 |
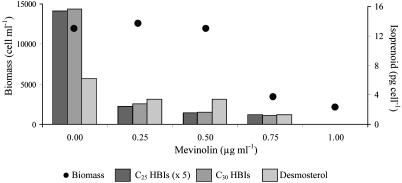
The growth of R. setigera was dramatically inhibited by increasing concentrations of mevinolin (Table 2 and Fig. 2). The concomitant inhibition of C25 and C30 HBI biosynthesis, together with that of desmosterol, indicates that the involvement of the MVA pathway is essential for the biosynthesis of these compounds in R. setigera. In contrast, fosmidomycin did not reduce the C25 or C30 HBI alkene or sterol content of the cells, even though cell growth was slightly inhibited at the highest concentrations used (75–100 μg·ml–1). For both sets of inhibition experiments, the concentrations of phytol were too low (<2% of the nonsaponifiable lipids) to be quantified satisfactorily.
Table 2. Influence of mevinolin (μg·ml–1) on the final cell biomass (cells per ml) and nonsaponifiable lipid concentrations (pg per cell) of R. setigera.
| Mevinolin
|
|||||
|---|---|---|---|---|---|
| 0 | 0.25 | 0.50 | 0.75 | I | |
| Biomass | 11,960 | 12,600 | 11,920 | 3,480 | 2,200 |
| C25:3 (III) | 1.61 | 0.29 | 0.16 | — | — |
| C25:4 (IV) | 1.25 | 0.20 | 0.16 | 0.26 | — |
| C25:5 (V) | 0.21 | — | — | — | — |
| C30:5 (VII) | 4.89 | 1.29 | 0.72 | 0.58 | — |
| C30:5 (VIII) | 5.32 | 1.14 | 0.66 | 0.66 | — |
| C30:6 (IX) | 2.02 | 0.06 | — | — | — |
| C30:6 (X) | 3.38 | 0.34 | 0.29 | — | — |
| Desmosterol (XI) | 6.22 | 3.37 | 3.41 | 1.31 | — |
| Total C25 HBIs | 3.07 | 0.49 | 0.32 | 0.26 | — |
| Total C30 HBIs | 15.61 | 2.83 | 1.67 | 1.24 | — |
—, below limit of detection.
Fig. 2.
Influence of mevinolin (μg·ml–1) on the final cell biomass (cells per ml) and nonsaponifiable lipid concentrations (pg per cell) of R. setigera.
When R. setigera (strain RS 99) was cultured in the presence of 13CO2 (20% 13C) and unlabeled sodium acetate, MS analysis of the nonsaponifiable lipids (Table 3), showed that 13C was incorporated into phytol, all of the C25 and C30 HBI isomers (III–V and VII–X), and desmosterol (XI). In contrast, phytol was not labeled when the cells were cultured in the presence of [1-13C]acetate, but a significant incorporation of 13C was detected for all of the HBIs and desmosterol. When a different strain of R. setigera (RS 02/A) was used, which produced the C25 pentaene VI as the only HBI, incorporation of 13Cfrom[2-13C]acetate was again observed (Table 3). Phytol, but not the HBIs or desmosterol, was efficiently labeled when cells (strains RS 99 and RS 02/A) were grown in the presence of [1,1,1,4-2H4]deoxyxylulose, but none of these lipids were labeled when cells were cultured in the presence of l-[2-13C]leucine or d-[1-13C]glucose.
Table 3. Isotope enrichment factors determined by MS for the nonsaponifiable lipids isolated from R. setigera grown in the presence of isotopically enriched (13C 20%) substrates.
| Control | Acetate | CO2 | Glucose | Leucine | Deoxyxylulose* | |
|---|---|---|---|---|---|---|
| Phytol (II) | 1.0 (0.9)† | 1.2 | 6.1 | 1.4 | — | 14.2 (26.0)† |
| C25:3 (III) | 1.1 | 4.5 | 5.1 | 1.1 | 1.0 | 0.8 |
| C25:4 (IV) | 1.2 | 4.8 | 5.6 | 1.0 | 1.0 | — |
| C25:5 (V) | 1.0 | 4.3 | 5.5 | 0.9 | — | — |
| C30:5 (VII) | 0.9 | 3.9 | 4.9 | 1.0 | 1.0 | 1.0 |
| C30:5 (VIII) | 0.9 | 3.9 | 5.4 | 0.8 | 0.9 | 1.0 |
| C30:6 (IX) | 1.1 | 4.1 | 4.7 | 0.9 | 0.5 | — |
| C30:6 (X) | 0.9 | 3.9 | 5.4 | 1.0 | 0.4 | — |
| Desmosterol (XI) | 1.3 | 4.6 | 5.5 | 1.2 | 1.3 | 0.8 (1.1)† |
| C25:5 (VI) | 0.9† | 4.5† | — | — | — | 0.9† |
Deoxyxylulose was used with 100% 2H enrichment.
Strain RS 02/A, all other entries, strain RS 99.
The incorporation of 13Cfrom[1-13C]acetate into the HBIs and desmosterol was investigated further by isolating these compounds from larger-scale cultures and analyzing them by 13C NMR spectroscopy. The 13C NMR spectra of VII and VIII isolated from cells (strain RS 99) grown in the presence of [1-13C]acetate showed that only 13C signals corresponding to C-2, C-4, C-6, C-8, C-10, C-12, C-14, C-16, C-18, C-25, C-27, and C-29 were enhanced in 13C relative to the other carbon nuclei (Table 4). In both cases, the 13C-enriched carbon atoms corresponded to C-1 and C-3 of the (six) IDP precursors from which these C30 HBIs are biosynthesized (Fig. 3). The 13C NMR spectrum of the most abundant C25 HBI (III) revealed that 13C signals corresponding to C-2, C-4, C-6, C-8, C-10, C-12, C-14, C-20, C-22, and C-24 were enhanced in 13C (Table 5) in excellent agreement with the observations made for the pseudohomologous C30 HBIs. In a further experiment with strain RS 02/A and [2-13C]acetate, the C25 pentaene VI was identified as the only HBI alkene consistent with the small-scale studies (see above). Comparison of the 13C NMR spectrum obtained for this compound with that isolated from a control culture revealed selective 13C incorporation in positions C-1, C-3, C-5, C-7, C-9, C-11, C-13, C-15–C-19, C-21, C-23, and C-25 corresponding to C-2, C-4, and C-5 of IDP (Table 5). The 13C NMR spectrum of desmosterol (RS 99, [1-13C]acetate) showed selective enhancement of signals corresponding to C-2, C-4, C-6, C-8, C-10, C-11, C-12, C-14, C-16, C-20, C-23, and C-25 (Fig. 1 and Table 4) equivalent to C-1 and C-3 of IDP.
Table 4. 13C NMR analysis of two C30 pentaenes (VII and VIII) and desmosterol (XI) isolated from R. setigera grown in the presence of [1-13C] acetate.
| C30:5 Z (VII)
|
C30:5 E (VIII)
|
Desmosterol (XI)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ ppm | Carbon | [13C 20%] | [13C 100%] | δ ppm | Carbon | [13C 20%] | [13C 100%] | δ ppm | Carbon | [13C 20%] |
| 144.7 | 28† | 1.0 | 1.0 | 144.7 | 28† | 1.0 | 1.0 | 140.8 | 5 | 1.0 |
| 143.1 | 7 | 0.8 | 0.6 | 142.7 | 7 | 0.8 | 0.8 | 131.1 | 25 | 1.9 |
| 135.7 | 10 | 1.5 | 5.2 | 135.7 | 10 | 1.6 | 5.5 | 125.3 | 24† | 1.0 |
| 135.1 | 14 | 1.8 | 6.1 | 135 | 14 | 1.9 | 5.3 | 121.8 | 6 | 1.6 |
| 131.3 | 18 | 1.5 | 5.9 | 131.4 | 18 | 1.7 | 5.9 | 71.9 | 3 | 0.7 |
| 124.5 | 13 | 1.0 | 1.0 | 124.5 | 13 | 0.8 | 1.1 | 56.8 | 14 | 1.5 |
| 124.3 | 17 | 0.9 | 0.9 | 124.4 | 17 | 0.9 | 0.9 | 56.1 | 17 | 0.8 |
| 124.1 | 9 | 1.1 | 1.2 | 123.4 | 9 | 0.9 | 0.9 | 50.2 | 9 | 0.6 |
| 122.9 | 25 | 2.0 | 6.3 | 122.8 | 25 | 2.1 | 6.5 | 42.4 | 13 | 1.1 |
| 112.2 | 29 | 2.0 | 7.6 | 112.3 | 29 | 1.9 | 6.0 | 42.3 | 4 | 1.8 |
| 39.8 | 15 | 0.8 | 0.9 | 39.9 | 11 | 1.0 | 0.9 | 39.8 | 12 | 2.1 |
| 39.4 | 3 | 0.9 | 1.0 | 39.8 | 15 | 0.7 | 0.7 | 37.3 | 1 | 0.8 |
| 38.3 | 27 | 2.1 | 7.2 | 39.3 | 3 | 0.8 | 0.8 | 36.6 | 10 | 1.6 |
| 35.4 | 5 | 1.1 | 1.1 | 38.3 | 27 | 1.8 | 6.1 | 36.2 | 22 | 0.8 |
| 34.5 | 26 | 0.9 | 1.0 | 35.4 | 5 | 0.8 | 0.8 | 35.7 | 20 | 1.6 |
| 34.4 | 6 | 2.2 | 7.4 | 34.5 | 26 | 1.0 | n/a | 31.9 | 7, 8 | 1.7 |
| 31.9 | 11 | 1.0 | 1.0 | 34.4 | 6 | 2.2 | 6.2 | 31.7 | 2 | 2.1 |
| 29.1 | 8 | 1.9 | 7.5 | 29.3 | 8 | 2.1 | 7.3 | 28.3 | 16 | 1.7 |
| 28 | 2 | 2.1 | 7.6 | 28 | 2 | 1.8 | 5.6 | 25.9 | 27 | 0.8 |
| 26.8 | 12 | 2.0 | 6.8 | 26.8 | 12 | 2.0 | 6.6 | 24.8 | 23 | 2.2 |
| 26.7 | 16 | 2.1 | 7.7 | 26.7 | 16 | 2.1 | 7.3 | 24.4 | 15 | 0.8 |
| 25.8 | 4, 19 | 1.9 | 5.4 | 25.8 | 4, 19 | 1.6 | 4.7 | 21.2 | 11 | 1.6 |
| 23.6 | 22 | 0.9 | 1.0 | 22.7 | 1, 20 | 0.8 | 0.8 | 19.5 | 19 | 0.8 |
| 22.7 | 1, 20 | 0.9 | 1.2 | 19.7 | 21 | 0.7 | 0.9 | 18.7 | 21 | 0.7 |
| 19.6 | 21, 30 | 0.8 | 1.2 | 19.6 | 30 | 0.8 | 0.9 | 17.8 | 26 | 0.8 |
| 17.8 | 24 | 0.9 | 1.2 | 17.8 | 24 | 0.6 | 0.7 | 11.9 | 18 | 0.8 |
| 16 | 23 | 0.7 | 0.8 | 16, 15.9 | 23, 22 | 0.7 | 0.6 | |||
Entries correspond to relative enhancements in peak intensities compared with the unlabeled compound. Carbon atoms that should be labeled according to the MVA pathway are indicated in bold.
Reference carbon corresponding to C-2 of IDP, unlabeled by either the MVA or MEP pathway.
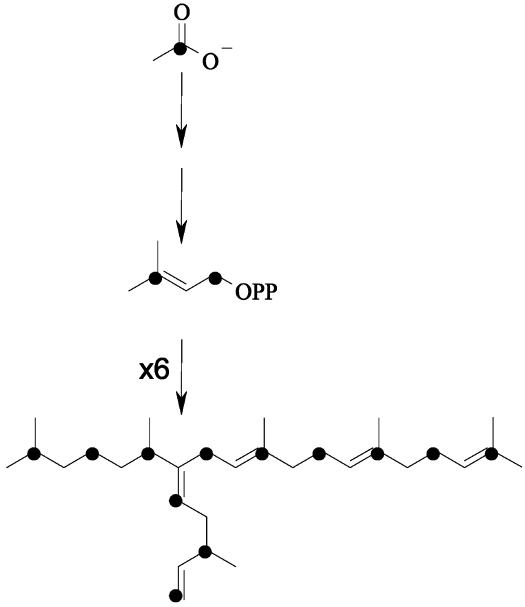
Fig. 3.
Hypothetical scheme showing the formation of a C30 HBI from [1-13C]acetate according to the MVA pathway.
Table 5. 13C NMR analysis of C25 alkenes (III and VI) isolated from R. setigera grown in the presence of 13C-labeled acetate.
| C25 (III)
|
C25 (VI)
|
||||
|---|---|---|---|---|---|
| δ ppm | Carbon | [13C 100%] | δ ppm | Carbon | [13C 20%] |
| 144.7 | 23* | 1.0 | 145 | 23 | 1.6 |
| 142.8 | 7 | — | 136.7 | 6 | 0.9 |
| 136 | 10 | 5.8 | 135 | 10 | 0.9 |
| 123.2 | 9 | 1.4 | 131.3 | 2,14 | 1.0 |
| 122.8 | 20 | 5.2 | 124.6 | 3 | 1.1 |
| 112.2 | 24 | 6.2 | 124.5 | 5 | 1.4 |
| 40.1 | 11 | 0.8 | 123.6 | 9,13 | 1.4 |
| 39.4 | 3 | 0.8 | 112.4 | 24* | 1.0 |
| 38.8 | 13 | 0.7 | 49.2 | 7 | 1.3 |
| 38.3 | 22 | 7.1 | 39.9 | 11 | 1.3 |
| 35.4 | 5 | 0.8 | 37.9 | 22 | 0.9 |
| 34.5 | 21 | 0.7 | 34.5 | 21 | 1.5 |
| 34.4 | 6 | 6.6 | 32.4 | 8 | 0.9 |
| 29.3 | 8 | 5.4 | 30.3 | 20 | 0.9 |
| 28 | 2,14 | 7.5 | 26.9 | 4,12 | 0.9 |
| 25.9 | 12 | 6.3 | 25.8 | 1,15 | 1.6 |
| 25.8 | 4 | 7.5 | 20.6 | 25 | 1.4 |
| 22.7 | 1,15,16,19 | 0.8 | 17.8 | 16,19 | 1.5 |
| 19.6 | 17,25 | 0.9 | 16.3 | 18 | 1.3 |
| 15.8 | 18 | 0.5 | 12 | 17 | 1.5 |
C25:3 (III) was isolated from strain RS 99 ([1-13C]acetate) and C25:5 (VI) was isolated from strain RS 02/A ([2-13C]acetate). Entries correspond to relative enhancements in peak intensities compared with the unlabeled compound. Carbon atoms that should be labeled according to the MVA pathway are indicated in bold.
Reference carbon corresponding to C-2 (III) or C-1 (VI) of IDP, unlabeled by either MVA or MEP pathway
H. ostrearia. A description of the distributions of the major nonsaponifiable lipids synthesized by the diatom H. ostrearia has been published (see, e.g., refs. 18 and 23), but the structures are summarized in Fig. 4. These lipids exhibit differences from those of R. setigera. For example, the dominant sterol in H. ostrearia is 24-ethylcholest-5-en-3β-ol (XXI; ref. 34), whereas, in terms of HBIs, H. ostrearia produces C25 HBI alkenes only, often as mixtures of isomers (XII–XX). These alkenes are regioisomeric with the C25 HBIs found in R. setigera, with the exception of C25:5 (VI), which has been identified in both species (23, 31). In further contrast to HBIs found in R. setigera, the double bonds in HBIs from H. ostrearia are biosynthesized with E stereochemistry only.
Fig. 4.
Representative structures of C25 HBI alkenes (XII–XX) together with the major sterol, 24-ethylcholest-5-en-3β-ol (XXI), produced by various cultures of H. ostrearia.
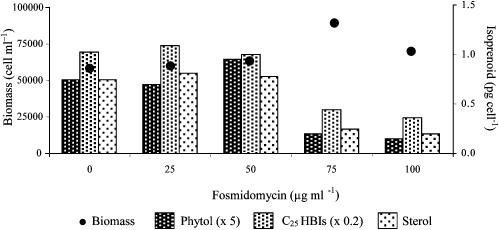
When H. ostrearia was cultured in the presence of mevinolin at concentrations equivalent to those used for R. setigera (namely, ≤1 μg·ml–1), neither the cell growth nor the nonsaponifiable lipid concentrations were affected. At much higher concentrations of inhibitor (10 and 20 μg·ml–1), cell growth was diminished (by 41% and 72%, respectively), but total concentrations of the C25 HBIs and sterols were unchanged. In contrast, fosmidomycin did not inhibit cell growth even at high concentrations of the inhibitor, but concentrations of phytol, 24-ethylcholest-5-en-3β-ol, and the C25 HBIs were dramatically reduced, although their relative distributions were essentially invariant (Table 6 and Fig. 5).
Table 6. Effect of fosmidomycin on the final biomass (cells per ml) and nonsaponifiable lipid concentrations (pg per cell) for cells of H. ostrearia.
| Fosmidomycin
|
|||||
|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | |
| Biomass | 58100 | 60000 | 63600 | 89500 | 70000 |
| Phytol (II) | 0.15 | 0.14 | 0.19 | 0.04 | 0.03 |
| C25:2(XII, XIII) | 1.76 | 2.12 | 2.25 | 0.86 | 0.80 |
| C25:3(XIV, XV) | 2.13 | 2.13 | 1.91 | 0.99 | 0.76 |
| C25:4(XVI-XIX) | 1.10 | 1.06 | 0.74 | 0.35 | 0.26 |
| C25:5(VI, XX) | 0.14 | 0.16 | 0.11 | 0.03 | — |
| Sterol (XXI) | 0.75 | 0.81 | 0.78 | 0.25 | 0.20 |
| Total C25 HBIs | 5.14 | 5.47 | 5.01 | 2.23 | 1.82 |
—, below limit of detection.
Fig. 5.
Effect of fosmidomycin on the final biomass (cells per ml) and nonsaponifiable lipid concentrations (pg per cell) for cells of H. ostrearia.
When H. ostrearia was cultured in the presence of 13CO2 and unlabeled sodium acetate, 13C was efficiently incorporated within phytol, all the C25 HBI isomers, and 24-ethylcholest-5-en-3β-ol (average isotopic enrichment factor, 6.5% ± 0.6%). In contrast, phytol was not labeled when the cells were cultured in the presence of either [1-13C]- or [2-13C]acetate, and a relatively small incorporation of 13C (≈2%) was detected for the C25 HBIs and sterols. When the cells were grown in the presence of [2H3]acetate, no incorporation of 2H was observed in any of the compounds examined. Because [2H3]acetate was not used directly by the cells, the small incorporation of 13C from the labeled acetates was presumed to be due to the partial catabolism of the acetate into 13CO2 before incorporation (11). This presumption was subsequently verified by 13C NMR analysis of the C25 trienes XIV and XV, which showed that the average enhancement [relative to the reference carbon (C-23)] of C-1 and C-3 from IDP was 1.06 ± 0.2, whereas that for C-2, C-4, and C-5 was 1.00 ± 0.12, confirming that the small incorporation determined by MS was nonspecific. [Note that the corresponding values for the C30 HBIs from R. setigera were the following: VII, C-1 and C-3 (1.93 ± 0.22) and C-2, C-4, and C-5 (0.91 ± 0.11); and VIII, C-1 and C-3 (1.93 ± 0.11) and C-2, C-4, and C-5 (0.82 ± 0.12)].
H. ostrearia did not grow in the presence of d-[1-13C]glucose. Cell growth was not perturbed by [1,1,1,4-2H4]deoxyxylulose, but, surprisingly, no incorporation of 2H was detected in any of the HBIs or 24-ethylcholest-5-en-3β-ol. Significantly however, phytol was also not labeled with 2H, indicating that H. ostrearia was not able to metabolize deoxyxylulose at all. No incorporation of 13C was detected in any of the C25 HBIs or the 24-ethylcholest-5-en-3β-ol when the cells were cultured in the presence of l-[2-13C]leucine.
Discussion
From both pathway-specific inhibition and isotopic labeling experiments, it now seems clear that both MEP and MVA pathways are in operation for the biosynthesis of terpenoids in the diatom R. setigera. Although concentrations of phytol were too low for satisfactory quantification for inhibition experiments, both small- and large-scale labeling experiments revealed that this compound was not labeled when [1-13C]acetate was applied to the cells. In contrast, substantial labeling was observed when the cells were cultured in the presence of 13CO2 or [1,1,1,4-2H4]deoxyxylulose, consistent with the biosynthesis of phytol by the MEP pathway. In agreement with the observations made by Cvejić and Rohmer (11) with the diatom Phaeodactylum tricornutum, glucose was not used for the biosynthesis of isoprenoids in R. setigera.
In contrast to phytol, we have found clear evidence for the involvement of the MVA pathway in the biosynthesis of other terpenes in R. setigera, including sesterterpenes. Thus, very low concentrations of mevinolin (<1 μg·ml–1) were required to reduce the cell concentrations of desmosterol, whereas from isotopic labeling experiments, [1-13C]acetate was directly incorporated within this sterol, resulting in a site-specific labeling pattern as determined by 13C NMR spectroscopy. No incorporation of [1,1,1,4-2H4]deoxyxylulose into desmosterol was observed in either of the two R. setigera strains studied. C27 to C29 sterols are common in diatoms, and Cvejić and Rohmer (11) showed that intact acetate was incorporated into C27 and C28 sterols from P. tricornutum and Nitzschia ovalis. Thus, the sterols in P. tricornutum, N. ovalis, and two strains of R. setigera are all synthesized via the MVA pathway. Our inhibition and labeling studies also revealed the formation of C25 and C30 HBIs in both strains of R. setigera by the MVA route. Mevinolin efficiently inhibited the formation of these compounds, and, from labeling experiments, [1-13C]acetate was incorporated within these molecules according to the expected labeling patterns resulting from the MVA route. These isotopic incorporation effects were independent of the specific strain of R. setigera used, despite significant variations in the isomeric forms of the HBIs produced.
Observations from the fractionation of R. setigera cells revealed that phytol was located in the chloroplasts, whereas sterols and HBIs were present in the cytoplasm. Numerous studies performed on the formation of mono-, di-, sester-, tri-, and tetraterpenes in plants have shown that an apparent division exists for the biosynthesis of these compounds in higher plants (4). Thus, the MVA pathway is located in the cytoplasm contributing to the biosynthesis of sesquiterpenes and triterpenes, whereas the MEP pathway is located in the plastids and accounts for the biosynthesis of mono-, di-, sester-, tri- and tetraterpenes. The observations described here for diatomaceous algae are consistent with these previous findings from higher plants and extend our knowledge of the biosynthesis of sester- and triterpenes in microscopic plants. Thus, although phytol in the chloroplast is made exclusively by the MEP pathway, C25 and C30 HBIs, along with desmosterol, are found in the free lipid fraction (cytoplasm) and are made from the MVA pathway. Our data also aid the interpretation of previous studies of natural isotope patterns in diatom isoprenoids. This finding is important because the latter studies involve no perturbation of isoprenoid biosynthesis by addition of labeled substrates. Hence, the results are complementary to the labeling and inhibition studies described above. For instance, we and others (33, 35) have shown that the natural 13C/12C isotopic ratios (so-called δ13C values) of C25 and C30 HBIs and desmosterol in two different strains of R. setigera were 2.5–4.5‰ depleted in 13C compared with phytol. Jux et al. (9) showed that in higher plants such differences are due to the biosynthesis of isoprenoids by the MVA and MEP routes. From the results of the current labeling and inhibition experiments for two strains of R. setigera (Tables 2, 3, 4, 5), it is clear that the differences in δ13C values observed previously do indeed reflect the difference between MVA-derived isoprenoids (C25 and C30 HBIs and desmosterol) and MEP-derived lipids (phytol).
In contrast to the findings for R. setigera, a parallel investigation into the biosynthesis of terpenoids in H. ostrearia supports the hypothesis that the MVA pathway does not contribute significantly to the biosynthesis of many of the nonsaponifiable lipids in this species under the conditions investigated. Instead, these compounds appear to be made predominantly by the MEP route. Similar conclusions were made by Schwender and co-workers (32) for the green alga Scenedesmus obliquus. These workers, among others (1), also provided evidence for the operation of the glyoxylate shunt or the involvement of acetate in the MEP pathway, but no such contribution was found for the diatom H. ostrearia described here.
Although high (10–20 μg·ml–1) mevinolin concentrations did inhibit cell growth, this inhibitor was found to have no effect on the cell isoprenoid concentrations, whereas fosmidomycin dramatically inhibited their synthesis, even in the presence of acetate. From labeling experiments, no 2H was incorporated in these terpenoids when the cells were cultured in the presence of [2H3]acetate and an unselective incorporation of 13C from [1-13C]acetate was established by 13C NMR spectroscopy. This result confirmed that acetate was not incorporated directly into these isoprenoids and that the small 13C enrichment detected in these compounds arose from in situ degradation of the labeled substrates (11). When the cells were cultured in the presence of 13CO2, 13C was efficiently incorporated into all of the isoprenoids investigated.
Part of our reasoning behind the identification of the MEP route as the major biosynthetic route for isoprenoid lipids in H. ostrearia is the absence of any specific incorporation of 13C from [1-13C]acetate or 2H from [2H3]acetate. Arguably, this absence may partly reflect an inability for H. ostrearia to undergo mixotrophic or heterotrophic growth, rather than a complete lack of involvement of the MVA route. Indeed, cell growth was inhibited at high concentrations of mevinolin, so it would appear that the MVA pathway is partly in operation in H. ostrearia, even if its contribution is relatively minor to the compounds studied. It is also plausible that an additional compensatory involvement of the MEP pathway occurs when the MVA route is inhibited (26). However, the natural isotope ratios (δ13C) of the isoprenoid lipids of H. ostrearia were also consistent with a major involvement of the MEP rather than MVA route. Thus, the mean differences between the δ13C values of phytol and the C25 HBIs were much smaller than for R. setigera (see above). For example, for replicate analyses of three separate cultures of H. ostrearia, the mean difference in δ13C values between phytol and the C25 HBIs was –0.5 ± 1.3‰ (n = 13). Because the δ13C values for the C25 HBIs and phytol are within experimental error of each other, these findings are entirely consistent with the proposal that these compounds are biosynthesized by the same MEP route in H. ostrearia.
Research is needed to obtain further natural isotope (δ13C) values of phytol, HBIs, and sterols of H. ostrearia under more perturbed or stressed conditions. Such studies should establish in H. ostrearia to what extent (if any) the MVA route functions and, if so, whether any “cross-talk” occurs between the two mechanisms.
Acknowledgments
We thank Dr Ian Bull (Natural Environment Research Council MS facility, University of Bristol) for δ13C measurements on R. setigera and H. ostrearia isoprenoids.
Abbreviations: MVA, mevalonate; MEP, methylerythritol phosphate; HBI, highly branched isoprenoid; IDP, isopentenyl diphosphate.
References
- 1.Rohmer, M. (1999) Nat. Prod. Rep. 16, 565–574. [DOI] [PubMed] [Google Scholar]
- 2.Ginger, M. L, Chance, M. L., Sadler, I. H. & Goad L. J. (2001) J. Biol. Chem. 276, 11674–11682. [DOI] [PubMed] [Google Scholar]
- 3.Eisenreich, W., Schwarz, M., Cartayrade, A., Arigoni, D., Zenk, M. H. & Bacher, A. (1998) Chem. Biol. 5, 221–233. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenthaler, H. K. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 47–65. [DOI] [PubMed] [Google Scholar]
- 5.Bach, T. J. & Lichtenthaler, H. K. (1982) Z. Naturforsch. (C) 37, 46–50. [DOI] [PubMed] [Google Scholar]
- 6.Zeidler, J., Schwender, J., Muller, C., Wiesner, J., Weidemeyer, C., Beck, E., Jomaa, H. & Lichtenthaler, H. K. (1998) Z. Naturforsch. C 53, 980–986. [Google Scholar]
- 7.Boucher, Y. & Doolittle, W. F. (2000) Mol. Microbiol. 37, 703–716. [DOI] [PubMed] [Google Scholar]
- 8.Lange, B. M., Rujan, T., Martin, W. & Croteau, R. (2000) Proc. Natl. Acad. Sci. USA 97, 13172–13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jux, A., Gleixner, G. & Boland, W. (2001) Angew. Chem. Int. Ed. Engl. 40, 2091–2093. [PubMed] [Google Scholar]
- 10.Wilson, R. J. M. (2002) J. Mol. Biol. 319, 257–274. [DOI] [PubMed] [Google Scholar]
- 11.Cvejić, J. H. & Rohmer, M. (2000) Phytochemistry 53, 21–28. [DOI] [PubMed] [Google Scholar]
- 12.Nabeta, K., Kawae, T., Kikuchi, T., Saitoh, T. & Okuyama, H. (1995) Chem. Commun. 2529–2530.
- 13.Arigoni, D., Eisenreich, W., Latzel, C., Sagner, S., Radykewicz, T., Zenk, M. H. & Bacher, A. (1999) Proc. Natl. Acad. Sci. USA 96, 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volkman, J. K., Barrett, S. M. & Dunstan, G. A. (1994) Org. Geochem. 21, 407–413. [Google Scholar]
- 15.Rowland, S. J., Belt, S. T., Wraige, E. J., Massé, G., Roussakis, C. & Robert, J.-M. (2001) Phytochemistry 56, 597–602. [DOI] [PubMed] [Google Scholar]
- 16.Pozzi, G., Birault, V., Werner, B., Dannenmuller, O., Nakatani, Y., Ourisson, G. & Terakawa, S. (1996) Angew. Chem. Int. Ed. Engl. 35, 177–180. [Google Scholar]
- 17.Takajo, S., Nagano, H., Dannenmuller, O., Ghosh, S., Albrecht, A. M., Nakatani, Y. & Ourisson, G. (2001) New J. Chem. 25, 917–929. [Google Scholar]
- 18.Belt, S. T., Cooke, D. A., Robert, J.-M. & Rowland, S. J. (1996) Tetrahedron Lett. 37, 4755–4758. [Google Scholar]
- 19.Wittpoth, C., Kroth, P. G., Weyrauch, K., Kowallik, K. V. & Strotmann, H. (1998) Planta 206, 79–85. [Google Scholar]
- 20.Sullivan, C. W. (1978) in Handbook of Phycological Methods, eds. Hellebust, J. A. & Craigie, J. S. (Cambridge Univ. Press, New York), pp. 39–55.
- 21.Kuzuyama, T., Shimizu, T., Takahashi, S. & Seto, H. (1998) Tetrahedron Lett. 39, 7913–7916. [Google Scholar]
- 22.Schindler, S., Bach, T. J. & Lichtenthaler, H. K. (1984) Z. Naturforsch. C 40, 208–214. [Google Scholar]
- 23.Wraige, E. J., Belt, S. T., Lewis, C. A., Cooke, D. A., Robert, J.-M., Massé, G. & Rowland, S. J. (1997) Org. Geochem. 27, 497–505. [Google Scholar]
- 24.Flesch, G. & Rohmer, M. (1988) Eur. J. Biochem. 175, 405–411. [DOI] [PubMed] [Google Scholar]
- 25.Rohmer, M., Knani, M., Simonin, P., Sutter, B. & Sahm, H. (1993) Biochem J. 295, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemmerlin, A., Hoeffler, J. F., Meyer, O., Tritsch, D., Kagan, Grosdemange-Billiard, C., Rohmer, M. & Bach, T. J. (2003) J. Biol. Chem. 278, 26666–26676. [DOI] [PubMed] [Google Scholar]
- 27.Zeidler, J. & Lichtenthaler, H. K. (2001) Planta 213, 323–326. [DOI] [PubMed] [Google Scholar]
- 28.Goad, J. L. & Akihisha, T. (1997) Analysis of Sterols (Blackie Academic and Professional, London).
- 29.Arigoni, D., Sagner, S., Latzel, C., Eisenreich, W., Bacher, A. & Zenk, M. H. (1997) Proc. Natl. Acad. Sci. USA 94, 10600–10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belt, S. T., Allard, W. G., Massé, G., Robert, J.-M. & Rowland, S. J. (2001) Tetrahedron Lett. 42, 5583–5585. [Google Scholar]
- 31.Sinninghe Damsté, J. S., Schouten, S., Rijpstra, W. C., Hopmans, E. C., Peletier, H., Gieskes, W. W. C. & Geenevasen, J. J. (1999) Org. Geochem. 30, 1581–1583. [DOI] [PubMed] [Google Scholar]
- 32.Schwender, J., Seemann, M., Lichtenthaler, H. K. & Rohmer, M. (1996) Biochem. J. 316, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowland, S. J., Allard, W. G., Belt, S. T., Massé, G., Robert, J.-M., Blackburn, S., Frampton, D., Revill, A. T. & Volkman, J. K. (2001) Phytochemistry 58, 717–728. [DOI] [PubMed] [Google Scholar]
- 34.Barrett, S. M., Volkman, J. K., Dunstan, G. A. & Le Roi, J.-M. (1995) J. Phycol. 31, 360–369. [Google Scholar]
- 35.Schouten, S., Klein Breteler, W. C. M., Blokker, P., Schogt, N., Rijpstra, W. I. C., Grice, K., Baas, M. & Sinninghe Damsté, J. S. (1998) Geochim. Cosmochim. Acta 62, 1397–1406. [Google Scholar]