Abstract
Wee1, the Cdc2 inhibitory kinase, needs to be down-regulated at the onset of mitosis to ensure rapid activation of Cdc2. Previously, we have shown that human somatic Wee1 (Wee1A) is down-regulated both by protein phosphorylation and degradation, but the underlying mechanisms had not been elucidated. In the present study, we have identified the β-transducin repeat-containing protein 1/2 (β-TrCP1/2) F-box protein-containing SKP1/Cul1/F-box protein (SCF) complex (SCFβ-TrCP1/2) as an E3 ubiquitin ligase for Wee1A ubiquitination. Although Wee1A lacks a consensus DS(p)GXXS(p) phospho-dependent binding motif for β-TrCP, recognition of Wee1A by β-TrCP depended on phosphorylation, and two serine residues in Wee1A, S53 and S123, were found to be the most important phosphorylation sites for β-TrCP recognition. We have found also that the major M-phase kinases polo-like kinase 1 (Plk1) and Cdc2 are responsible for the phosphorylation of S53 and S123, respectively, and that in each case phosphorylation generates an unconventional phospho-degron (signal for degradation) that can be recognized by β-TrCP. Phosphorylation of Wee1A by these kinases cooperatively stimulated the recognition and ubiquitination of Wee1A by SCFβ-TrCP1/2 in vitro. Mutation of these residues or depletion of β-TrCP by small-interfering RNA treatment increased the stability of Wee1A in HeLa cells. Moreover, our analysis indicates that β-TrCP-dependent degradation of Wee1A is important for the normal onset of M-phase in vivo. These results also establish the existence of a feedback loop between Cdc2 and Wee1A in somatic cells that depends on ubiquitination and protein degradation and ensures the rapid activation of Cdc2 when cells are ready to divide.
In higher eukaryotes, Cdc2 (a protein kinase essential for cell division) is inactivated during interphase by negative phosphorylation by two protein kinases, Wee1 and Myt1 (1–3). When cells are ready for mitosis, the Cdc25 phosphatase activates Cdc2 by removing inhibitory phosphates at Thr-14 and Tyr-15. Activated Cdc2 phosphorylates Cdc25 in turn, increasing its activity. This positive feedback loop ensures rapid activation of Cdc2 at the onset of mitosis (4). At the same time, Myt1 is inactivated by phosphorylation (3), whereas Wee1 is inactivated both by phosphorylation and degradation in somatic cells (1). However, the existence of a feedback loop between Cdc2 and Wee1/Myt1 in somatic cells has not been established because phosphorylation of Wee1 and Myt1 by Cdc2 does not affect their catalytic activity (1, 3).
The activity of Wee1 is also regulated at the protein level in the budding yeast Saccharomyces cerevisiae (5), whereas it is down-regulated mainly by protein phosphorylation in the fission yeast, Schizosaccharomyces pombe (6–9). Interestingly, Mik1, another functional homologue of Wee1 in fission yeast, is also regulated at the protein level (10, 11). In budding yeast, Met30p, an F-box protein that is responsible for the substrate recognition in the SKP1/Cul1/F-box protein (SCF) E3 ubiquitin ligase complex, was reported to be responsible for the recognition and ubiquitination-dependent degradation of Swe1p (the budding yeast Wee1 homologue) because Swe1p turnover was inhibited by Met30p deficiency (12). However, this was shown recently (13) to be an indirect effect of Met30p deficiency because Swe1p stability in Δmet30, Δmet4 double-mutant cells was similar to that in wild type cells. SCF complex-dependent Wee1 degradation at the onset of M-phase was reported to occur also in Xenopus laevis egg extracts (14). More recently, a putative F-box protein, Tome-1 was identified, and an SCF complex containing Tome-1 (SCFTome-1) was reported to be responsible for Wee1 (not only embryonic Wee1 but also human Wee1A) ubiquitination and degradation (15), although frog embryonic Wee1 has been reported to be stable at M-phase by other groups (16, 17).
F-box proteins frequently recognize their substrates not only by means of primary amino acid sequence motifs but also as a result of phosphorylation. One of the most extensively studied examples is the interaction between IκBα (an inhibitory subunit of NFκB) and the F-box protein β-transducin repeat-containing protein (β-TrCP) (FBW1A, B, E3RSIKB, HOS, FWD1, and FBXW1) (see ref. 18 for review). β-TrCP recognizes a DSGXXS motif in the N-terminal region of IκBα, only when these serines are phosphorylated by the IκB kinase, IKK. β-TrCP also recognizes other substrates, including β-catenin, HIV Vpu protein and others, in a phosphorylation-dependent manner (19, 20). Most of the known substrates for β-TrCP have the common phospho-binding motif, DS(p)GX2–3S(p). The recently determined crystal structure of β-TrCP complexed with a β-catenin phosphopeptide shows that specific hydrogen bonds and electrostatic interactions are formed between residues in the substrate phospho-binding motif and residues in the WD40 domains of β-TrCP (21).
In the present study, we show that phosphorylation of human somatic Wee1 (Wee1A, or Wee1Hu) by Cdc2, and a second M-phase kinase polo-like kinase 1 (Plk1) creates phosphodegrons (signals for degradation) for the β-TrCP F-box protein-containing SCF E3 ubiquitin ligase (SCFβ-TrCP), thereby inducing proteasome-dependent degradation that is required for the initiation of mitosis. These results also provide evidence for the existence of a feedback loop between Cdc2 and Wee1A in somatic cells that depends on ubiquitination and protein degradation.
Materials and Methods
Plasmids and Baculovirus Constructions. Mammalian expression vectors for Wee1A, Plk1, Tome-1, and components of SCF complexes, including several F-box proteins, were obtained or constructed as described in Supporting Methods and Table 1, which are published as supporting information on the PNAS web site. Details about baculoviruses encoding GST-Wee1 (K328R), GST-cyclin B1, Cdc2 and GST-Plk1 are also described in the Supporting Methods.
Cell Culture and Immunological Techniques. HeLa cells, HtTA1 cells (22), and 293T cells were cultured in DMEM supplemented with 10% FBS in a humidified incubator at 37°C in 10% CO2. Plasmids were transfected into cells by using a standard calcium phosphate-precipitation method. Association between various Wee1A derivatives and F-box proteins in HtTA1 cells was performed as described in Supporting Methods. Endogenous β-TrCP was inactivated by small-interfering RNA (siRNA), as described in Supporting Methods. Levels of endogenous β-catenin were measured by immunoblotting using anti-β-catenin Abs (SC1496; Santa Cruz Biotechnology). Effects of transfected Wee1A and its mutants on cell-cycle progression were analyzed by fluorescence-activated cell sorting, as described (23).
In Vitro Association of Wee1A and β-TrCP. GST-Wee1A (K328R) expressed and purified from insect cells was phosphorylated by cyclin B1/Cdc2 and/or Plk1 in a reaction containing 50 mM Tris·Cl (pH 8.0), 10 mM MgCl2, 1 mM DTT, and 2 mM ATP at 30°C for 1 h. For treatment with bacterial alkaline phosphatase, GST-Wee1A was incubated in the absence of ATP. GST-Wee1A samples were then mixed with 293T cell lysates expressing β-TrCP1 for 30 min on ice and glutathione beads were added, followed by mixing for 30 min at 4°C and washing with cell-lysis buffer. Bound GST-Wee1A and β-TrCP1 were analyzed by immunoblotting.
In Vitro Ubiquitination Assay. Components of SCF complex [SKP1, Cul1, Rbx1, and hemagglutinin (HA)-tagged F-box proteins] were expressed in 293T cells and immunopurified on protein G beads by using 12CA5 anti-HA mAb. GST-Wee1A (K328R) expressed and purified from insect cells was phosphorylated by Cdc2 and/or Plk1. Ubiquitination reactions were carried out essentially as described (24) except GST-UbcH5a (Boston Biochem, Cambridge, MA) was used as a source for E2 enzyme.
Phosphorylation of Synthetic Peptides. Chemically synthesized peptides were phosphorylated by protein kinases, as detailed in Supporting Methods.
Results and Discussion
F-Box Proteins β-TrCP1 and β-TrCP2 Recognize Human Wee1A. As shown (1), Wee1A protein levels rapidly decrease in somatic cells at mitosis, and this decrease is in part responsible for Wee1A inactivation at mitosis when Cdc2 is active. We speculated that Wee1A was degraded by the ubiquitin/proteasome machinery because budding yeast Wee1 (Swe1p) was reported to be degraded by ubiquitination by SCF ubiquitin ligase, as described above (12). Our preliminary results indicated that Wee1A, but not Wee1B (25), was labile in budding yeast and that Wee1A was partially stabilized at the restrictive temperature in strains with thermosensitive SCF components [Skp1p, Cdc53p (CUL-1), and WD40 repeat F-box proteins (Met30p and Cdc4p)] or E2 ubiquitin-conjugating enzyme Cdc34p that works in concert with SCF. Thus, we predicted that Wee1A is recognized and ubiquitinated by an SCF complex with an F-box protein containing WD40 repeats in human cells.
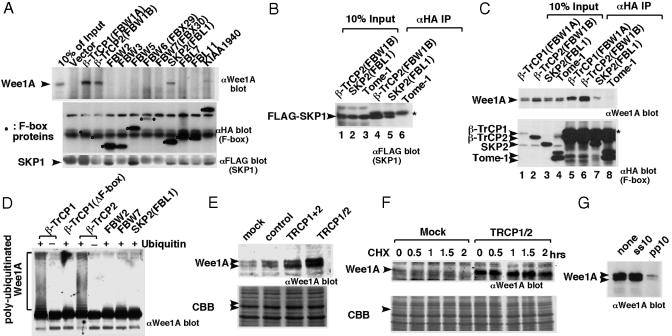
To identify a human F-box protein(s) that recognize(s) Wee1A, we expressed all seven known human WD40 repeat F-box proteins (26) and five other F-box proteins, including Tome-1 [a recently identified F-box protein that recognizes and ubiquitinates both frog embryonic Wee1 and human Wee1A (15)] in HeLa-derived HtTA-1 cells, together with Wee1A and SKP1, and we examined their association. SKP1 was associated with all F-box proteins, excluding Tome-1, although the levels varied, being roughly dependent on the expression level of each F-box protein (Fig. 1 AMiddle and Bottom and B). In contrast, Wee1A coprecipitated only with three F-box proteins, β-TrCP1, β-TrCP2, and SKP2 (FBL1) (Fig. 1 A Top and C). Based on expression levels, β-TrCP1 and β-TrCP2 bound Wee1A much more efficiently than SKP2. Thus, β-TrCP1 and 2 are good candidates for F-box proteins that recognize Wee1A in human cells. We do not know why Tome-1 failed to associate with SKP1 as reported (15), but the lack of Wee1A binding by Tome-1 suggests that Tome-1 may not function as part of a Wee1A SCF E3 ligase in this system.
Fig. 1.
Interaction between human Wee1A and SCF components. (A) Wee1A and FLAG-epitope tagged SKP1 were coexpressed with various HA epitope-tagged F-box proteins in HeLa-derived HtTA-1 cells. Anti-HA Ab immunoprecipitates from the transfected cell lysates were examined by immunoblotting with anti-Wee1A Ab (Top), anti-HA Ab (Middle); and anti-FLAG Ab (Bottom), respectively. Circles indicate F-box proteins. (B) Wee1A and FLAG epitope-tagged SKP1 were coexpressed with various HA epitope-tagged F-box proteins (β-TrCP2, SKP2, and Tome-1) in HeLa-derived HtTA-1 cells. Cell lysates (lanes 1–3) and anti-HA Ab immunoprecipitates (lanes 4–6) were examined by immunoblotting with anti-SKP1 Ab (Santa Cruz Biotechnology; SC-7163). *, signal derived from IgG light chain. (C) The nocodazole-treated transfected cell lysates as in B (lanes 1–4) or anti-HA Ab immunoprecipitates (lanes 5–8) from them were examined by immunoblotting with anti-Wee1A Abs (upper blot) and anti-HA mAb (lower blot), respectively. *, signal derived from IgG heavy chain. (D) GST-Wee1A (K328R) expressed, purified from insect cells and phosphorylated with cyclin B1/Cdc2 was ubiquitinated in vitro by using SCF complexes with various F-box proteins in the presence (+) or absence (–) of ubiquitin and analyzed by immunoblotting by using anti-Wee1A Ab. (E) HeLa cells were transfected with control unrelated siRNA, mixture of siRNAs that are specific for β-TrCP1 or β-TrCP2 siRNA (TRCP1+2) or siRNA that targets both of β-TrCP1 and β-TrCP2 (TRCP1/2). Wee1A levels were analyzed by immunoblotting at 2 days after transfection. Coomassie blue staining (CBB) of the filter is shown also (arrowheads indicate the position of Wee1A). (F) HeLa cells (105 cells per 6-cm dish) were transfected with siRNA (TRCP1/2). Two days later, cells were treated with nocodazole (0.3 μg/μl) for 21 h. CHX (25 μg/ml) was then added, and the stability of Wee1A was compared with that in mock-transfected cells at the times indicated. Wee1A in nocodazole-treated cells slowly migrated as a single band and was not detected in mock-transfected cells later than 0.5 h after the CHX addition. (G) Association of Wee1A to β-TrCP was abolished by the diphosphorylated IκBα peptide pp10 but not by its nonphosphorylated version, ss10 (29). Peptides were added at 100 μM before the addition of the anti-HA mAb.
SCFβ-TrCP1/2 Ubiquitinates Wee1A in Vitro. SCF complexes containing either β-TrCP1 or β-TrCP2 were obtained by expression in 293T cells, followed by immunopurification, and their capacity to ubiquitinate Wee1A was examined. These complexes efficiently ubiquitinated Wee1A in vitro only when ubiquitin was present, whereas SCF complexes with β-TrCP1 lacking the F-box domain or with other F-box proteins, including SKP2, did not ubiquitinate Wee1A (Fig. 1D).
Stabilization of Wee1A by β-TrCP1/2 siRNA in Vivo. We used siRNAs to reduce expression of β-TrCP1 and/or β-TrCP2 in HeLa cells. Transfection of HeLa cells with siRNA corresponding to β-TrCP1 or β-TrCP2 caused increased accumulation of Wee1A compared with mock-transfected cells. Increased levels of β-catenin, a known β-TrCP target, were observed also (data not shown). A further increase was observed on cotransfection of β-TrCP1 and β-TrCP2 siRNAs (TRCP1 and TRCP2) or transfection of siRNA that targets both β-TrCP1 and β-TrCP2 (TRCP1/2) (27) (Fig. 1E, and data not shown). Protein-stability analysis using cycloheximide (CHX) showed that Wee1A was stabilized by TRCP1/2 siRNA in cells enriched at M-phase by nocodazole treatment (Fig. 1F). From these results, we concluded that SCFβ-TrCP1/2 are the major human SCF complexes responsible for ubiquitination and degradation of Wee1A. Because we did not find any significant differences between β-TrCP1 and β-TrCP2, which are very similar (28), in these experiments, we will hereafter refer to these F-box proteins as β-TrCP.
Identification of β-TrCP Recognition Sites in Wee1A. β-TrCP recognizes several phosphoproteins, including the NF-κB inhibitor, IκB, β-catenin, and the HIV-1 accessory protein, Vpu (see ref. 18 for review). Most of the phosphoproteins recognized by β-TrCP contain a common sequence motif (DSG X2–3S, where X is any amino acid), and β-TrCP binds this motif only when both serines are phosphorylated. Peptides (29) comprising the phospho-degrons of IκBα promoted dissociation of Wee1A and β-TrCP only when the two serines were phosphorylated (Fig. 1G), indicating that Wee1A is recognized in a manner similar to IκBα. Unexpectedly, however, Wee1A lacks this motif, and we next determined the region in Wee1A that is required for recognition by β-TrCP.
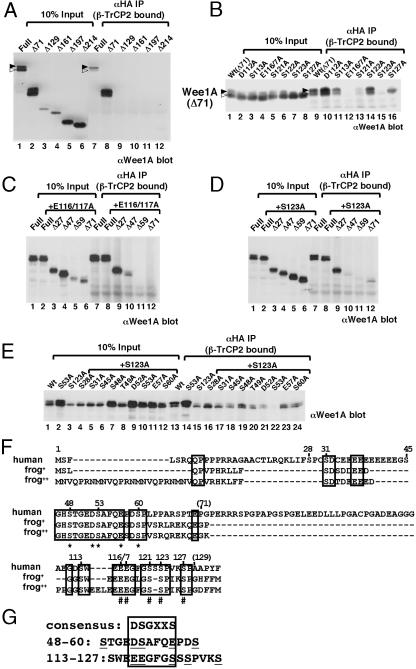
When N-terminal-deletion mutants of Wee1A were tested for binding to β-TrCP by immunoprecipitation and immunoblotting, Wee1A lacking the N-terminal 71 aa (Δ71) bound β-TrCP, but Δ129 did not, indicating that the region between amino acids 71 and 129 is important for recognition (Fig. 2A). The hyperphosphorylated form of Wee1A (Fig. 2 A, upper band and filled arrowheads) bound more efficiently to β-TrCP (Fig. 2 A, compare lanes 1 and 7, and B, compare lanes 1 and 9), suggesting that binding is phosphorylation-dependent. Therefore, the five serines that are conserved between human and frog somatic Wee1 (1, 17, 30) (Fig. 2F) in this region were replaced singly by alanine in Δ71 Wee1A, and the binding capability of the mutants was examined (Fig. 2B). The binding of Δ71 Wee1A to β-TrCP was decreased most dramatically when serine 123 was changed to alanine (S123A), and it was also significantly decreased in the S121A mutant. Interestingly, when glutamates 116 and 117 in Δ71 Wee1A were changed to alanines (E116/117A), binding was abolished. Although the relationship of these important residues (Fig. 2F, #) to the common β-TrCP binding motif DS(p)GX2–3S(p) is not obvious, we suggest that 116EEGFGS(p)121 may mimic the common binding motif when S123 is phosphorylated (Fig. 2G).
Fig. 2.
Amino acid residues in Wee1A important for its recognition by β-TrCP. (A–E) Wee1A (Full) and its N-terminal-deletion mutants (Δ71, Δ129, Δ161, Δ197 and Δ214) (A); Wee1A lacking 71 aa from the N terminus [Wt(Δ71)] and various point mutants as indicated (B); Wee1A (Full), E116/117A mutant (+E116/117A, Full), and its N-terminal-deletion mutants (+E116/117A, Δ27, Δ47, Δ59, and Δ71) (C); Wee1A (Full), S123A mutant (+S123A, Full), and its N-terminal-deletion mutants (+S123A, Δ27, Δ47, Δ59, and Δ71) (D); or Wee1A (Wt) and various mutants as indicated (E) were coexpressed with HA-tagged β-TrCP2 and SKP1 in HtTA-1 cells. Cell lysates (10% of total input is shown) and anti-HA immunoprecipitates of the lysates were analyzed by immunoblotting with anti-Wee1A Ab. Upper (hyperphosphorylated) and lower bands of Wee1A doublets are indicated by solid and open arrowheads, respectively, in A and B. (F) N-terminal region of human Wee1A [Wee1Hu; U10564; Top) (1) and two sequences of frog somatic, Wee1 (Wee2:AF358869; frog+; Wee1B:AB071983; frog++) (17, 30) were aligned. Identical amino acids among the three sequences are indicated by boxes. #, residues important for binding to β-TrCP in Δ71 Wee1A; *, residues important for binding to β-TrCP in S123A Wee1A. Positions of amino acids of human Wee1A are shown. (G) Phosphodegrons in Wee1A sequence are aligned with known consensus-recognition sequences for β-TrCP (boxed). The important amino acids shown in F are underlined.
Although either E116/117 or S123 was essential for binding of Δ71 Wee1A to β-TrCP, these mutations did not affect the binding of full-length Wee1A to β-TrCP, indicating that other binding motif(s) exist(s) in the N-terminal 71 aa of Wee1A (Fig. 2 C and D, lanes 1, 2, 7, and 8). By examining the binding of N-terminal Wee1A-deletion mutants in the E116/117A or S123A mutant background to β-TrCP, the region between residues 27 and 59 was found to be important for binding (Fig. 2 C and D). Analysis of several point mutations within this region revealed that five residues (S48, D52, S53, E57, and S60) significantly decreased binding to β-TrCP when mutated singly to alanine in S123A Wee1A (Fig. 2 E and F). Among these residues, S53 seemed to be the most important because the binding of the S53/123A double mutant to β-TrCP was almost undetectable (Fig. 2E). Although the role of S48 and S60 remains unclear, we suggest that 52DS(p)AFQE57 may mimic the common binding motif for β-TrCP, DS(p)GX2–3S(p) (Fig. 2G). Collectively, there are at least two important regions in Wee1A for the binding to β-TrCP. The results of the serine-to-alanine substitutions in these two regions suggest that phosphorylation of serines 53 and 123 is important for the binding to β-TrCP and may serve as phospho-degrons for SCFβ-TrCP ubiquitin ligase recognition, leading to proteasome degradation.
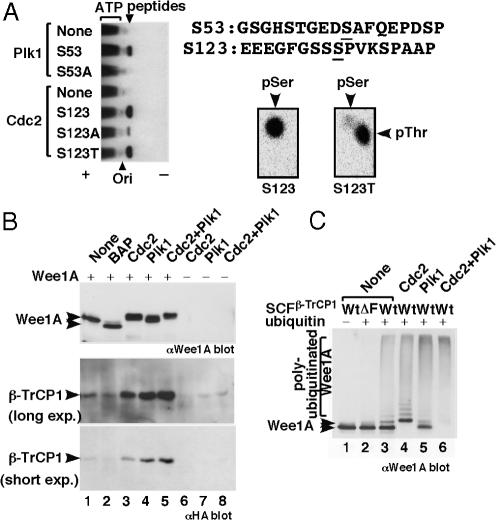
S53 and S123 Are Phosphorylated by M-Phase Kinases Plk1 and Cdc2, Respectively. Which protein kinase(s) phosphorylate S53 and S123? S53 (EDSAF) lies in a consensus for Plk1 phosphorylation (D/E-X-S/T) (31), whereas S123 (SPVK) lies in a consensus for Cdc2 phosphorylation (S/T-P-X-K/R). Because Plk1 and Cdc2 cyclin A- or cyclin B-associated Cdc2 are activated at the G2/M-phase, these are good candidates for the protein kinases that mediate phosphorylation-dependent ubiquitination of Wee1A at the onset of M-phase. We found that a synthetic S53-containing peptide was phosphorylated efficiently by Plk1 and that a S123 peptide was phosphorylated efficiently by Cdc2 (Fig. 3A). Because peptides in which either S53 or S123 was changed to alanine were not phosphorylated, we concluded that Plk1 can phosphorylate S53 and that Cdc2 can phosphorylate S123, at least in vitro. The reproducibly higher expression level of the S53A mutant, compared with that of wild-type Wee1A in transfected cells (Fig. 2E, lanes 1 and 2), and the effect of overexpressed Plk1 on Wee1A turnover (see below) strongly suggest that S53 is phosphorylated by Plk1 in vivo as well. Because the S123A mutation inhibited the characteristic large mobility shift of Wee1A (Fig. 2 D, lanes 1 and 2, and E, lanes 1 and 3), which occurs at late S/G2 phase (1), and because treatment of cells with butyrolactone I (a specific cyclin-dependent kinase inhibitor) (32) inhibited this mobility shift (data not shown), we suspect that, in vivo, S123 is phosphorylated by cyclin A- or B-associated Cdc2.
Fig. 3.
Plk1 and Cdc2 cooperate to phosphorylate Wee1A and induce its binding to SCFβ-TrCP, ubiquitination, and proteasome-dependent degradation. (A) Synthetic peptides corresponding to sequences around S53 or S123 (and their derivatives) were phosphorylated by Plk1 or Cdc2. The weak phosphorylation of the S123A peptide by Cdc2 is presumably due to phosphorylation of S127, which is also succeeded by proline. Phosphorylation of S123 was also confirmed by the detection of phosphothreonine (pThr) in phosphoamino acid analysis of the S123T peptide (Right). Plk1 and Cdc2 did not phosphorylate S123 and S53, respectively (data not shown). (B) GST-Wee1A (K328R) expressed and purified from insect cells was treated with bacterial alkaline phosphatase (BAP) or the indicated protein kinases, mixed with cell lysates of 293T cells expressing HA-tagged β-TrCP1, and reisolated on glutathione agarose beads. Isolated GST-Wee1A (Top) and bound β-TrCP (Middle and Bottom) were detected by immunoblotting using anti-Wee1A and anti-HA Abs, respectively. No significant binding of β-TrCP1 was detected by using kinase reactions without GST-Wee1A(–). (C) GST-Wee1A (K328R) expressed and purified from insect cells was phosphorylated by the indicated protein kinases and ubiquitinated in vitro by using purified SCFβ-TrCP complexes together with E1, E2 (UbcH5a), and ATP in the presence (+) or absence (–) of ubiquitin, and it was analyzed by immunoblotting using anti-Wee1A Abs. No significant ubiquitination was detected when β-TrCP1 lacking the F-box domain (ΔF) was used.
Plk1 and Cdc2 Cooperatively Stimulate the Binding and Ubiquitination of Wee1A by SCFβ-TrCP. Next, we examined the effect of phosphorylation of Wee1A by the Plk1 and Cdc2 M-phase kinases on β-TrCP binding. GST-Wee1A expressed and purified from insect cells was phosphorylated or treated with phosphatase, mixed with cell lysates expressing β-TrCP, and repurified on glutathione beads, and the levels of associated β-TrCP were examined (Fig. 3B). Both untreated and phosphatase-treated Wee1A bound only weakly to β-TrCP (Fig. 3B Middle and Bottom, lanes 1 and 2). In contrast, after phosphorylation by Cdc2 or Plk1, bound β-TrCP levels were increased significantly, indicating that these protein kinases created β-TrCP recognition signals (Fig. 3B, lanes 3 and 4). When Wee1A was phosphorylated by both protein kinases, the level of bound β-TrCP was increased further (Fig. 3B, lane 5). Thus, these M-phase kinases work cooperatively in creating binding signals, and this finding is consistent with the existence of two independent β-TrCP binding motifs in Wee1A. Because S53/123A mutant Wee1A did not bind to β-TrCP even after phosphorylation by Plk1 and Cdc2, phosphorylation of these two serines by these kinases creates the major binding signals (data not shown).
Untreated GST-Wee1A was ubiquitinated by SCFβ-TrCP in a ubiquitin- and F-box domain-dependent manner in vitro (Fig. 3C, lanes 1–3). When Wee1A was prephosphorylated by Cdc2, polyubiquitinated Wee1A was increased significantly and non-ubiquitinated GST-Wee1A was decreased (Fig. 3C, lane 4). A similar or stronger effect was observed when Wee1A was prephosphorylated by Plk1 (Fig. 3C, lane 5). Consistent with Cdc2 and Plk1 phosphorylation cooperatively enhancing β-TrCP binding, phosphorylation of Wee1A by both Cdc2 and Plk1 stimulated Wee1A ubiquitination further, and almost all of the GST-Wee1A was ubiquitinated (Fig. 3C, lane 6). Thus, we concluded that recognition and ubiquitination of Wee1A by SCFβ-TrCP is regulated by phosphorylation and that the Plk1 and Cdc2 M-phase kinases are responsible for this regulation by the phosphorylation of S53 and S123, respectively. Although these two serines are the most important for the binding, it is possible that phosphorylation of Wee1A at other sites by these M-phase kinases may contribute to the association of Wee1A and β-TrCP, as is the case for association of Cdc4 with Sic1, which has nine suboptimal binding sites for Cdc4 (33). Because phosphorylation at S123 by Cdc2 creates the recently identified polo-box domain binding-motif consensus (34) [S-phospho-S/T-(P)], Cdc2 phosphorylation may stimulate the recognition and phosphorylation of Wee1A by Plk1 in addition to directly promoting binding to β-TrCP.
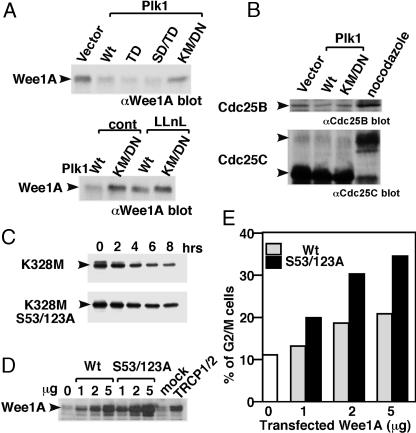
Proteasome-Dependent Degradation of Wee1A by Overexpressed Plk1 in Vivo. Plx1 (polo-like kinase in Xenopus) is reported to be responsible for activation of Cdc25 and triggering mitosis in embryonic cells (35). In somatic cells, however, the role of Plk1 in the onset of mitosis is not clear (36–38). When active Plk1 was overexpressed, the level of endogenous Wee1A was decreased dependently on Plk1 kinase and proteasome activities (Fig. 4A). Because we did not detect any effect of overexpressed Plk1 on Cdc25B or Cdc25C levels, we suspect that the increase in Plk1 activity in G2 has a direct effect on stimulating proteasome-dependent degradation of Wee1A at the onset of mitosis in vivo (Fig. 4B).
Fig. 4.
Phosphorylation of two serines (S53 and S123) is important for ubiquitination and proteasome-dependent degradation of Wee1A in vivo, and it is required for the G2–M-phase transition. (A) Various Plk1 expression constructs [wild-type (Wt), constitutively active mutants T210D (TD) or S137D/T210D (SD/TD) and the kinase-negative mutant K82M/D194N (KM/DN)] were transfected into HeLa cells. Levels of endogenous Wee1A were examined by immunoblotting using anti-Wee1A Abs (Upper). In some experiments, transfected cells were treated with 50 μM N-acetyl-leucyl-leucyl-norleucinal (LLnL) for 6 h before harvesting (Lower). (B) Effect of Plk1 overexpression on endogenous Cdc25B and Cdc25C levels was analyzed by immunoblotting using anti-Cdc25B (upper) and anti Cdc25C (lower) Abs, respectively. An increased level of Cdc25B and a mobility retardation of Cdc25C were detected in nocodazole-treated M-phase cells. (C) Kinase negative mutant Wee1A (K328M) or the phosphorylation site mutant (K328M, S53/123A) was expressed in HeLa cells, and turnover was examined after inhibition of protein synthesis by using CHX (25 μg/ml) for the indicated times. (D and E) Various amounts of expression plasmids encoding wild-type or S53/123A Wee1A were transfected with GFP in HtTA1 cells. At 2 days after transfection, levels of Wee1A expression were compared with those in β-TrCP siRNA-treated cells (D), and cell-cycle distribution of GFP positive cells (≈100% of the transfected cells) was determined. The percentage of G2/M cells is shown in E. Because round-up mitotic cells were not increased in Wee1A-transfected cells under examination with microscope, we concluded that arrest at G2-phase rather than M-phase is responsible for the increased G2/M population (data not shown).
Stabilization of the S53/123A Mutant Wee1A in Vivo. The effect of phosphorylation of Wee1A by Plk1 and Cdc2 on Wee1A turnover in vivo was examined by using CHX. Although S53/123A mutation did not completely block Wee1A degradation, turnover of S53/123A Wee1A was significantly slower than that of wild-type Wee1A, indicating that phosphorylation of these serines is important for rapid turnover of Wee1A in vivo (Fig. 4C).
Physiological Role of SCFβ-TrCP-Dependent Ubiquitination and Degradation of Wee1A on G2/M Transition. Finally, we examined the role of phosphorylation-dependent degradation of Wee1A on the onset of mitosis. We could not detect any significant effect of β-TrCP depletion with siRNA on the cell-cycle progression in synchronized HeLa cells when analyzed by fluorescence-activated cell sorting and microscopy (see Fig. 5, which is published as supporting information on the PNAS web site, and data not shown). The apparent absence of any effect of β-TrCP depletion suggests the existence of other substrate(s) for β-TrCP in which accumulation can counteract the effect of accumulated Wee1A on the G2/M transition. In fact, Cdc25A, which reverses the action of Wee1A, has been shown recently (39, 40) to be a substrate for β-TrCP. An increase in Cdc25A level, also caused by β-TrCP siRNA treatment, might artificially counteract the action of Wee1A accumulation. To overcome this problem, we examined the effect of transfecting 2 μg of Wee1A plasmid, which gave an expression level almost equivalent to that achieved with TRCP1/2 siRNA treatment (Fig. 4D). When analyzed by fluorescence-activated cell sorting (Fig. 4E), a significant increase in the G2/M population was observed, indicating the requirement of β-TrCP-dependent Wee1A degradation for G2/M transition. Because one-half of the amount of the S53/123A Wee1A expression plasmid led to a similar level of Wee1A protein and induced accumulation of G2/M population, we concluded that these phosphorylations are important for the regulation of Wee1A level and G2/M transition at physiological condition.
Concluding Remarks. The turnover of budding yeast Wee1 (Swe1p) was reported to require SCFMet-30 and cyclin-dependent kinase activity (5, 12). However, the decreased rate of Swe1p turnover proves to be an indirect consequence of Met30p deficiency (13). In the present study, we have shown that the degradation of human Wee1A is directly dependent on the SCF complex containing the F-box protein β-TrCP, whose closest relative in budding yeast is Met30p. Further studies are necessary to elucidate whether a similar pathway exists in yeast. The recently identified genetic link between Cdc5p (Plk1 in yeast) and Swe1p may be also on this pathway (41). In addition to Wee1A (our data) and Cdc25A (39, 40), Emi1, a negative regulator of APC activity, was also recently reported (27, 42) to be degraded by SCFβ-TrCP in mitosis. Thus, SCFβ-TrCP is turning out to be important for mitotic progression by regulating the protein levels of many critical targets.
Wee1, the Cdc2 inhibitory kinase, needs to be down-regulated at the onset of mitosis for maximal Cdc2 activation. We show that Cdc2 itself is responsible for the down-regulation by creating a phospho-degron on Wee1. Thus, the negative feedback loop between Cdc2 and Wee1 is mediated by phosphorylation-dependent degradation rather than direct inhibition by phosphorylation. Interestingly, the down-regulation of Wee1 by protein degradation is specific for somatic cells because frog embryonic Wee1 is stable at M-phase (16, 17). Another important difference from embryonic cells is the present finding that Plk1 triggers mitosis by means of the degradation of Wee1 in somatic cells, whereas Plx1 triggers mitosis by activating Cdc25 in embryonic cells (35). Because Plk1 has an important role in checkpoint signaling in somatic cells (43), Wee1 may be a critical checkpoint-pathway target.
Supplementary Material
Acknowledgments
We thank Kazusa DNA Research Institute (T. Nagase), W. Jiang, T. Kamura, K. Kitagawa, M. Nakanishi, K. I. Nakayama, R. Poon, and T. Sudo for reagents; members of the RIKEN Antibiotics Laboratory for discussion; Y. Ichikawa and R. Nakazawa for DNA sequencing; N. Hirotani for peptide synthesis; and M. Watanabe and Y. Ikawa for encouragement. T.H. is a Frank and Else Schilling American Cancer Society Research Professor.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SCF, SKP1/Cul1/F-box protein; β-TrCP, β-transducin repeat-containing protein; Plk1, polo-like kinase 1; CHX, cycloheximide; HA, hemagglutinin; siRNA, small interfering RNA.
References
- 1.Watanabe, N., Broome, M. & Hunter, T. (1995) EMBO J. 14, 1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu, F., Stanton, J. J., Wu, Z. & Piwnica-Worms, H. (1997) Mol. Cell. Biol. 17, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booher, R. N., Holman, P. S. & Fattaey, A. (1997) J. Biol. Chem. 272, 22300–22306. [DOI] [PubMed] [Google Scholar]
- 4.Izumi, T., Walker, D. H. & Maller, J. L. (1992) Mol. Biol. Cell 3, 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sia, R. A., Bardes, E. S. G. & Lew, D. J. (1998) EMBO J. 17, 6678–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu, L. & Russell, P. (1993) Nature 363, 738–741. [DOI] [PubMed] [Google Scholar]
- 7.Coleman, T. R., Tang, Z. & Dunphy, W. G. (1993) Cell 72, 919–929. [DOI] [PubMed] [Google Scholar]
- 8.Parker, L. L., Walter, S. A., Young, P. G. & Piwnica-Worms, H. (1993) Nature 363, 736–738. [DOI] [PubMed] [Google Scholar]
- 9.Tang, Z., Coleman, T. R. & Dunphy, W. G. (1993) EMBO J. 12, 3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boddy, M. N., Furnari, B., Mondesert, O. & Russell, P. (1998) Science 280, 909–912. [DOI] [PubMed] [Google Scholar]
- 11.Christensen, P. U., Bentley, N. J., Martinho, R. G., Nielsen, O. & Carr, A. M. (2000) Proc. Natl. Acad. Sci. USA 97, 2579–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser, P., Sia, R. A., Bardes, E. G. S., Lew, D. J. & Reed, S. I. (1998) Genes Dev. 12, 2587–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMillan, J. N., Theesfeld, C. L., Harrison, J. C., Bardes, E. G. S. & Lew, D. J. (2002) Mol. Biol. Cell 13, 3560–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michael, W. M. & Newport, J. (1998) Science 282, 1886–1889. [DOI] [PubMed] [Google Scholar]
- 15.Ayad, N. G., Rankin, S., Murakami, M., Jebanathirajah, J., Gygi, S. & Kirschner, M. W. (2003) Cell 113, 101–113. [DOI] [PubMed] [Google Scholar]
- 16.Mueller, P. R., Coleman, T. R. & Dunphy, W. G. (1995) Mol. Biol. Cell 6, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto, K., Nakajo, N. & Sagata, N. (2002) EMBO J. 21, 2472–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maniatis, T. (1999) Genes Dev. 13, 505–510. [DOI] [PubMed] [Google Scholar]
- 19.Margottin, F., Bour, S. P., Durand, H., Selig, L., Benichou, S., Richard, V., Thomas, D., Strebel, K. & Benarous, R. (1998) Mol. Cell 1, 565–574. [DOI] [PubMed] [Google Scholar]
- 20.Kitagawa, M., Hatakeyama, S., Shirane, M., Matsumoto, M., Ishida, N., Hattori, K., Nakamichi, I., Kikuchi, A., Nakayama, K. & Nakayama, K. (1999) EMBO J. 18, 2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu, G., Xu, G., Schulman, B. A., Jeffrey, P. D., Harper, J. W. & Pavletich, N. P. (2003) Mol. Cell 11, 1445–1456. [DOI] [PubMed] [Google Scholar]
- 22.Gossen, M. & Bujard, H. (1992) Proc. Natl. Acad. Sci. USA 89, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells, N. J., Watanabe, N., Tokusumi, T., Jiang, W., Verdecia, M. A. & Hunter, T. (1999) J. Cell. Sci. 112, 3361–3371. [DOI] [PubMed] [Google Scholar]
- 24.Strohmaier, H., Spruck, C. H., Kaiser, P., Won, K. A., Sangfelt, O. & Reed, S. I. (2001) Nature 413, 316–322. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi, M., Ando, H., Watanabe, N., Kitamura, K., Ito, K., Okayama, H., Miyamoto, T., Agui, T. & Sasaki, M. (2000) Genes Cells 5, 839–847. [DOI] [PubMed] [Google Scholar]
- 26.Koepp, D. M., Schaefer, L. K., Ye, X., Keyomarsi, K., Chu, C., Harper, J. W. & Elledge, S. J. (2001) Science 294, 173–177. [DOI] [PubMed] [Google Scholar]
- 27.Guardavaccaro, D., Kudo, Y., Boulaire, J., Barchi, M., Busino, L., Donzelli, M., Margottin-Goguet, F., Jackson, P. K., Yamasaki, L. & Pagano, M. (2003) Dev. Cell 4, 799–812. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, H., Chiba, T., Kobayashi, M., Takeuchi, M., Suzuki, T., Ichiyama, A., Ikenoue, T., Omata, M., Furuichi, K. & Tanaka, K. (1999) Biochem. Biophys. Res. Commun. 256, 127–132. [DOI] [PubMed] [Google Scholar]
- 29.Yaron, A., Hatzubai, A., Davis, M., Lavon, I., Amit, S., Manning, A. M., Andersen, J. S., Mann, M., Mercurio, F. & Ben-Neriah, Y. (1998) Nature 396, 590–594. [DOI] [PubMed] [Google Scholar]
- 30.Leise, W., III & Mueller, P. R. (2002) Dev. Biol. 249, 156–173. [DOI] [PubMed] [Google Scholar]
- 31.Kelm, O., Wind, M., Lehmann, W. D. & Nigg, E. A. (2002) J. Biol. Chem. 277, 25247–25256. [DOI] [PubMed] [Google Scholar]
- 32.Kitagawa, M., Okabe, T., Ogino, H., Matsumoto, H., Suzuki-Takahashi, I., Kokubo, T., Higashi, H., Saitoh, S., Taya, Y., Yasuda, H., et al. (1993) Oncogene 8, 2425–2432. [PubMed] [Google Scholar]
- 33.Nash, P., Tang, X., Orlicky, S., Chen, Q., Gertler, F. B., Mendenhall, M. D., Sicheri, F., Pawson, T. & Tyers, M. (2001) Nature 414, 514–521. [DOI] [PubMed] [Google Scholar]
- 34.Elia, A. E. H., Cantley, L. C. & Yaffe, M. B. (2003) Science 299, 1228–1231. [DOI] [PubMed] [Google Scholar]
- 35.Kumagai, A. & Dunphy, W. G. (1996) Science 273, 1377–1380. [DOI] [PubMed] [Google Scholar]
- 36.Toyoshima-Morimoto, F., Taniguchi, E. & Nishida, E. (2002) EMBO Rep. 3, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan, J., Eckerdt, F., Bereiter-Hahn, J., Kurunci-Csacsko, E., Kaufmann, M. & Strebhardt, K. (2002) Oncogene 21, 8282–8292. [DOI] [PubMed] [Google Scholar]
- 38.Jackman, M., Lindon, C., Nigg, E. A. & Pines, J. (2003) Nat. Cell Biol. 5, 143–148. [DOI] [PubMed] [Google Scholar]
- 39.Busino, L., Donzelli, M., Chiesa, M., Guardavaccaro, D., Ganoth, D., Dorrello, N. V., Hershko, A., Pagano, M. & Draetta, G. F. (2003) Nature 426, 87–91. [DOI] [PubMed] [Google Scholar]
- 40.Jin, J., Shirogane, T., Xu, L., Nalepa, G., Qin, J., Elledge, S. J. & Harper, J. W. (2003) Genes Dev. 17, 3062–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park, C. J., Song, S., Lee, P. R., Shou, W., Deshaies, R. J. & Lee, K. S. (2003) Genetics 163, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margottin-Goguet, F., Hsu, J. Y., Loktev, A., Hsieh, H. M., Reimann, J. D. R. & Jackson, P. K. (2003) Dev. Cell 4, 813–826. [DOI] [PubMed] [Google Scholar]
- 43.Smits, V. A. J., Klompmaker, R., Arnaud, L., Rijksen, G., Nigg, E. A. & Medema, R. H. (2000) Nat. Cell Biol. 2, 672–676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.