Abstract
Mental simulations of future experiences are often concerned with emotionally arousing events. Although it is widely believed that mental simulations enhance future behavior, virtually nothing is known about the mnemonic fate of these simulations over time or whether emotional simulations are especially well-remembered. We used a novel paradigm, combining recently developed methods for generating future event simulations and well-established memory testing procedures, to examine the retention of positive, negative, and neutral simulations over multiple delays. We found that with increasing delay, details associated with negative simulations become more difficult to remember than details associated with positive and neutral simulations. We suggest that these delay-by-emotion interactions reflect the mnemonic influence of fading affect bias, where negative reactions fade more quickly than positive ones, resulting in a tendency to remember a rosy simulated future. We also discuss implications for affective disorders such as depression and anxiety.
Over the last several years, research from a diverse set of perspectives – ranging from cognitive and social psychology to psychopathology and neuropsychology as well as cognitive and affective neuroscience – has revealed that memory plays an important role in mentally simulating future events (for reviews, see Schacter, Addis, & Buckner, 2008; Szpunar, 2010). For example, many brain regions that support remembering past events are similarly involved during simulation of possible future events (e.g., Addis, Wong, & Schacter, 2007; Okuda et al., 2003; Szpunar, Watson, & McDermott, 2007).
This growing literature has highlighted the adaptive value of memory in allowing us to prepare for the future (Schacter & Addis, 2007; Suddendorf & Corballis, 2007). But little is known about the functional significance of simulating future events, including the fate of simulated future events over time: is there any benefit to remembering the details of simulated future events that may or may not come to pass? Ingvar (1985) suggested that such “memories for the future” represent an important adaptation: remembering planned actions, reactions, and the like makes future behavior more efficient. Indeed, the little data available suggest that simulated future events are well remembered (Klein, Robertson, & Delton, 2010; McDonough & Gallo, 2010). Nonetheless, simulations of the future can take many forms and, to our knowledge, no study has examined whether certain kinds of simulations are better remembered than others.
One important characteristic of a future simulation concerns whether or not the to-be-simulated event is emotionally arousing. D’Argembeau, Renaud, and Van der Linden (2010) reported that nearly two-thirds of everyday future thoughts are emotionally charged (i.e., positive or negative). Moreover, these emotional simulations were rated higher in terms of personal importance than non-emotional simulations. Given the frequency and importance of emotional simulations, a critical and as yet unexplored question concerns whether emotional simulations are remembered especially well over time compared with neutral simulations, and therefore potentially more available to influence future behavior. No data exist that address this issue. While the literature on emotion and memory generally supports the idea that positive and negative events are better remembered than neutral events (for reviews, see Kensinger, 2009, Phelps, 2006), there are conditions in which emotional arousal can impair memory (Kensinger, 2009; Mather & Sutherland, 2011).
Moreover, few studies have examined the fate of emotional memories at multiple points in time, and those that have provide the basis for competing predictions. On the side of future utility, some types of negative stimuli (e.g., highly arousing words or photos) are more resistant to forgetting than neutral stimuli (e.g., non-arousing words or photos; e.g., Sharot & Phelps, 2004; Sharot & Yonelinas, 2008). Conversely, from the standpoint of psychological well-being (Taylor, 1991), the affect associated with negative experiences tends to fade more quickly than it does for positive experiences (for review, see Walker & Skowronski, 2009). Although it is unclear whether this “fading affect bias” represents a loss in memory for details associated with negative experiences, conditions exist in which negative experiences are remembered less well over time than positive experiences (Holmes, 1970; see also Stagner, 1931; Thompson, 1930).
We set out to (1) directly examine, for the first time, the relative memorability of positive, negative, and neutral simulated future events, and (2) present a new paradigm for approaching such questions that combines recently developed methods for generating future event simulations (Addis et al., 2009, 2010) with well-established memory testing procedures.
Experiments 1a and 1b
METHOD
Participants
Forty-eight Boston University students were recruited via the Boston University Job Service and provided informed written consent in a manner approved by the Harvard Institutional Review Board.
Stimulus collection and preparation
One week before the future simulation session, participants visited the laboratory and generated a set of 110 familiar people, places, and objects. Participants in Experiment 1a accomplished this task via an adapted version of the experimental recombination procedure (Addis et al., 2009, 2010). This procedure required participants to generate a list of 110 personal memories from the last ten years of their lives that were specific in time and place and that lasted no more than one day. For each memory, participants provided a brief description that included: a person other than themselves, the location where the event occurred, and a salient object (Table 1a). Each person, place, and object could only be mentioned once in the context of the entire set of 110 memories. Participants were provided with an extensive list of common experiences to help them generate these memories. To ensure that generation of personal memories did not interfere with later memory for future simulations, participants in Experiment 1b listed the names of 110 familiar people (using their Facebook friends list), places, and objects without reactivating detailed personal memories. The stimulus collection phase lasted approximately two (Experiment 1b) to three (Experiment 1a) hours.
Table 1.
Experimental design for Experiments 1a and 1b.
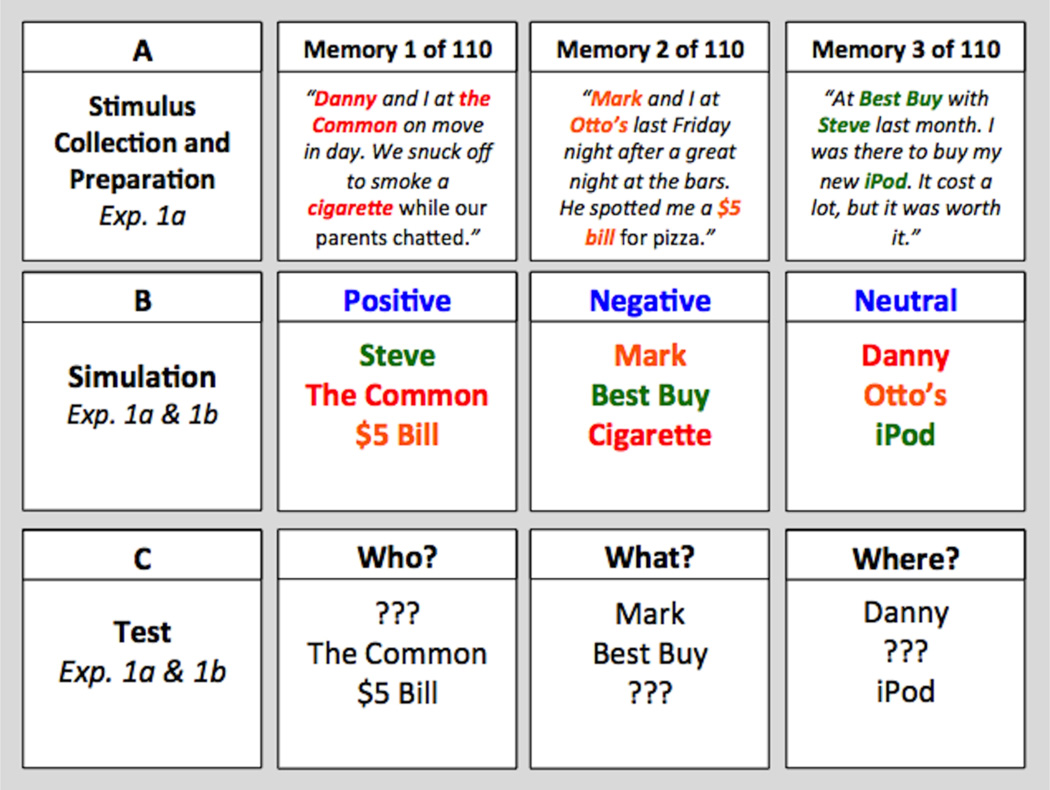 |
The resulting lists of 110 familiar people, places, and objects were subsequently examined for quality and the 93 best examples were selected and randomly recombined in a manner such that 93 simulation cues (i.e., novel arrangements of person, place, and object) were created (lower panels of Table 1b).
Simulation
One week later, participants returned to simulate 30 positive, 30 negative, and 30 neutral future events (presented in random order). Each trial consisted of a simulation cue (person, place, and object; lower panels of Table 1b) that was accompanied by one of three emotional tags (positive, negative, or neutral; upper panels of Table 1b). In each instance, participants were allotted 12.5 seconds to generate a plausible future event that might take place within the next 5 years and that would evoke the emotion indicated by the emotional tag. After each trial, participants rated the simulation they had generated on a series of three five-point scales and had five seconds to make each rating: (1) valence (1 = very negative, 5 = very positive), (2) arousal (1 = very exciting, 5 = very relaxing), and (3) detail (1 = few details, 5 = many details). The order of ratings was counterbalanced across participants for a total of six possible orders. To ensure that participants understood all instructions and that they simulated future events within the stipulated time period, three practice trials were completed, during which participants were asked to describe the content of their simulations to the experimenter. During experimental trials, no verbal descriptions were collected so as to make the task of engaging in mental simulation as natural as possible. Importantly, after the experimental trials, all participants reported that their simulations were novel (i.e., they had not thought about or experienced them before). Study materials were presented via E-Prime 1.0 software on a Dell desktop computer and participants made their responses (valence, arousal, and detail ratings) using a keyboard. The simulation phase lasted approximately 40 minutes.
Test
Immediately following the simulation phase, participants worked through a set of logic puzzles for ten minutes and were told that they would complete an additional set of simulation trials after the puzzles or one day later (between-participants; crossed with method of stimulus collection). Subsequently, participants were presented with a surprise cued recall memory test. None of the participants reported expecting this test. The test consisted of 90 trials. On each trial, one detail (i.e., person, place, or object) of a previously encountered simulation cue was missing and participants were asked to think back to the future event they had generated and fill in the missing detail (Table 1c; cf. Jones, 1976). Each detail was missing an equal number of times for cues that had previously been associated with positive, negative, and neutral simulations (emotion tags were not re-presented during testing). Participants were told that they were allowed to produce guesses, but only if they were reasonably certain of the correct answer. Test materials were presented via E-Prime 1.0 software on a Dell desktop computer and participants made their responses using a keyboard. The test phase was self-paced and lasted approximately 30 minutes.
RESULTS
Phenomenological Ratings
Table 2 summarizes the phenomenological ratings for positive, negative, and neutral simulations. Ratings did not differ as a function of experiment (1a or 1b) or delay (largest Z = 1.73; Mann-Whitney U tests) and so are collapsed across these factors. Friedman tests demonstrated that valence, detail, and arousal ratings differed significantly as a function of emotion, χ2(2) = 92.17, P < .001, χ2(2) = 27.83, P < .001, and χ2(2) = 60.76, P < .001, respectively. Wilcoxon sign tests further showed that: (1) positive and negative simulations were respectively rated as more positive (Z = 6.03, P < .001) and negative (Z = 6.03, P < .001) than neutral simulations; (2) positive simulations were rated as more detailed than negative (Z = 3.68, P < .001) and neutral (Z = 5.26, P < .001) simulations, and negative simulations were rated as more detailed than neutral simulations (Z = 2.06, P = .04); and (3) negative simulations were rated as more arousing than positive (Z = 5.76, P < .001) and neutral (Z = 6.00, P < .001) simulations, whereas positive and neutral simulations did not differ in this regard (Z = 1.03, P = .30).
Table 2.
Phenomenological Ratings for Experiments 1a and 1b.
| Positive | Negative | Neutral | |
|---|---|---|---|
| Valence | 4.11 (0.30) | 1.89 (0.51) | 3.22 (0.38) |
| Detail | 3.79 (0.46) | 3.58 (0.56) | 3.45 (0.61) |
| Arousal | 2.91 (0.63) | 1.97 (0.46) | 2.97 (0.31) |
Note. Standard deviations are presented in parentheses.
Memory performance
Cued recall performance was subjected to a 2 (experiment)×2 (delay)×3 (emotion)×3 (cue-type) mixed ANOVA, with experiment and delay entered as between-subject factors and emotion and cue-type entered as within-subject factors.
For the between-subject factors: (1) there was no main effect of experiment, F(1,44) = 1.10, p = .30; (2) there was a main effect of delay, F(1,44) = 31.33, p < .001, ηp2 = .42, such that simulations were better remembered after ten minutes (M = .63) than after one day (M = .32); and (3) there was no experiment-by-delay interaction (F < 1).
For the within-subject factors: (1) there was a main effect of emotion, F(2,88) = 7.36, p = .001, ηp2 = .14, and post-hoc paired-samples t-tests showed that positive simulations (M = .51) were better remembered than negative (M = .46), t(47) = 2.90, p = .006, d = .42, and neutral (M = .46), t(47) = 3.66, p = .001, d = .53, simulations; (2) there was a main effect of cue-type, F(2,88) = 48.36, p < .001, ηp2 = .53, and post-hoc paired-samples t-tests showed that participants performed best when cued to remember people (M = .58) as opposed to objects (M = .43), t(47) = 7.70, p < .001, d = 1.12, and places (M = .42), t(47) = 9.53, p < .001, d = 1.38; and (3) there was no emotion-by-cue-type interaction, F (4,176) = 1.65, p = .17.
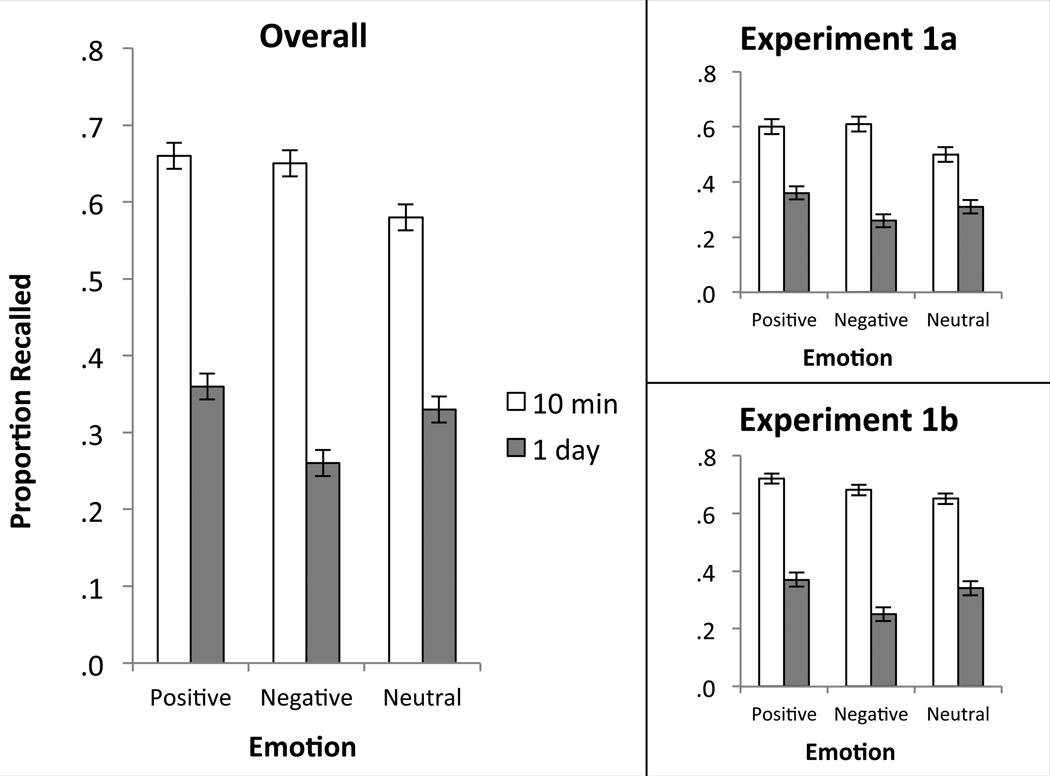
Importantly, for interactions among between- and within-subject factors, only delay interacted with emotion, F(2,88) = 7.73, p < .001, ηp2 = .15 (see Figure 1; notably, this interaction was similar for participants in Experiments 1a and 1b, suggesting that the reactivation of personal memories had little or no influence on later memory for simulations of future events). This interaction was further characterized by three additional 2 (delay)×2 (emotion) mixed ANOVAs and their associated paired-samples t-tests which demonstrated that: (1) there was a significant interaction between positive and negative simulations, F(1,46) = 4.94, p = .031, ηp2 = .10, such that positive and negative simulations were remembered equally well after ten minutes and negative simulations were remembered worse after one day, t(23) = 3.68, p = .001, d = .80; (2) there was a significant interaction between negative and neutral simulations, F(1,46) = 16.80, p < .001, ηp2 = .27, such that negative simulations were remembered better than neutral simulations after ten minutes, t(23) = 2.74, p = .012, d = .57, but worse after one day, t(23) = 3.17, p = .004, d = .69; and (3) there was no interaction between positive and neutral simulations, F(1,46) = 2.28, p = .14 (although positive simulations were remembered better than neutral simulations after ten minutes, t(23) = 4.21, p < .001, d = .88). All other higher-order interactions were non-significant (all but one F < 1).
Figure 1.
Cued recall memory performance for Experiments 1a and 1b. Left panel presents delay-by-emotion interaction collapsed across both experiments. Right panels demonstrate consistency of the delay-by-emotion interaction in both experiments.
DISCUSSION
The experiments reported here demonstrate that, over time, details associated with negative simulations of future events become more difficult to remember than details associated with positive and neutral simulations. The present data fit well with previous studies showing that emotional reactions fade more quickly for negative than positive life experiences. Although most demonstrations of this fading affect bias lack objective measures of memory (for discussion, see Walker & Skowronski, 2009), there is experimental evidence that negative experiences are sometimes remembered less well over time than positive experiences (Holmes, 1970; Stagner, 1931; Thompson, 1930).
Why are details associated with simulations of negative future events difficult to remember over time? We suggest a hypothesis that links fading affect bias with binding of, and memory for, event details. Mather and colleagues have argued that emotional arousal facilitates binding of event details when people attempt to integrate those details into a coherent mental representation (for review, see Mather & Sutherland, 2011). Extending this proposal, we suggest that the affect associated with a mental simulation of a future event serves to link together the components of that simulation. As the affective component dissipates, so too does the integrity of the associated mental simulation. If, as shown by work on fading affect bias, negative affect fades more quickly over time than positive affect, then negative simulations should be more adversely affected by retention interval than positive or neutral simulations.1
While this account fits well with the results of the present experiments, it remains to be determined how broadly the hypothesis can be extended. For example, it is unclear how the present hypothesis could explain why traumatic experiences are resistant to forgetting (Porter & Peace, 2007). Moreover, simulations of emotional future events often have to do with the distant future (e.g., retirement) and it will be interesting to test whether a similar pattern of data emerges using extended time intervals. Finally, although participants were instructed to simulate future events at encoding and think back to those simulations at retrieval, associations among person-place-object cues formed during encoding probably influenced memory performance.
Nonetheless, the present data fit nicely into theories that emphasize the importance of adaptive cognitive processes that promote psychological well-being (Taylor, 1991). With respect to future-directed cognition, healthy adults often think about their futures in an overly positive light (Sharot, Riccardi, Raio, Phelps, 2007; Weinstein, 1980). The present data suggest that optimistic views of the future can conspire with fading affect bias such that the “remembered future” is extremely rosy. In light of these considerations, studies examining memory for emotional simulations in patients with affective disorders, such as depression and anxiety, would be of considerable interest. Previous studies have revealed that future simulations tend to be negatively biased in such patients (e.g., MacLeod, Tata, Kentish, & Jacobsen, 1997; Williams et al., 1996), but nothing is known about the nature of patients’ memories for those simulations. Understanding when the remembered future is rosy and not-so-rosy should increase our understanding of the relations among emotion, memory, and future thinking.
Acknowledgments
The authors thank Stefanie McCartney and Norah Liang for help with data collection. This research was supported by grants from the National Institute on Aging (AG008441) and the National Institute of Mental Health (5R01MH60941-11) awarded to Daniel L. Schacter.
Footnotes
Although neutral simulations may not have benefitted from strong emotional arousal after a short delay, the positively biased nature of neutral simulations may have benefitted their long-term retention relative to negative simulations.
References
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Addis DR, Musicaro R, Pan L, Schacter DL. Episodic simulation of past and future events in older adults: Evidence from an experimental recombination task. Psychology and Aging. 2010;25:369–376. doi: 10.1037/a0017280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau AD, Renaud O, Van der Linden M. Frequency, characteristics, and functions of future-oriented thoughts in daily life. Applied Cognitive Psychology. 2011;25:96–103. [Google Scholar]
- Holmes DS. Differential change in affective intensity and the forgetting of unpleasant personal experiences. Journal of Personality and Social Psychology. 1970;15:234–239. doi: 10.1037/h0029394. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. "Memory of the future": An essay on the temporal organization of conscious awareness. Human Neurobiology. 1985;4:127–136. [PubMed] [Google Scholar]
- Jones GV. A fragmentation hypothesis of memory: Cued recall of pictures and of sequential position. Journal of Experimental Psychology: General. 1976;105:277–293. [Google Scholar]
- Kensinger EA. Remembering the details: Effects of emotion. Emotion Review. 2009;1:99–113. doi: 10.1177/1754073908100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, Robertson TE, Delton AW. Facing the future: Memory as an evolved system for planning future acts. Memory & Cognition. 2010;38:13–22. doi: 10.3758/MC.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod AK, Tata P, Kentish J, Jacobsen H. Retrospective and prospective cognitions in anxiety and depression. Cognition and Emotion. 1997;11:467–479. [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, Gallo DA. Separating past and future autobiographical events in memory: Evidence for a reality monitoring asymmetry. Memory & Cognition. 2010;38:3–12. doi: 10.3758/MC.38.1.3. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, et al. Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. NeuroImage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Porter S, Peace KA. The scars of memory: A prospective, longitudinal investigation of the consistency of traumatic and positive emotional memories in adulthood. Psychological Science. 2007;18:435–441. doi: 10.1111/j.1467-9280.2007.01918.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London: B. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Sharot T, Phelps EA. How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience. 2004;4:294–306. doi: 10.3758/cabn.4.3.294. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA. Neural mechanisms mediating optimism bias. Nature. 2007;450:102–106. doi: 10.1038/nature06280. [DOI] [PubMed] [Google Scholar]
- Sharot T, Yonelinas AP. Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition. 2008;106:538–547. doi: 10.1016/j.cognition.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Stagner R. The redintegration of pleasant and unpleasant experiences. American Journal of Psychology. 1931;43:463–468. [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Szpunar KK. Episodic future thought: An emerging concept. Perspectives on Psychological Science. 2010;5:142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proceedings of the National Academy of Sciences USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Asymmetrical effects of positive and negative events: The mobilization-minimization hypothesis. Psychological Bulletin. 1991;110:67–85. doi: 10.1037/0033-2909.110.1.67. [DOI] [PubMed] [Google Scholar]
- Thompson RH. An experimental study of memory as influenced by feeling tone. Journal of Experimental Psychology. 1930;13:462–468. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Walker WR, Skowronski JJ. The fading affect bias: But what the hell is it for? Applied Cognitive Psychology. 2009;23:1122–1136. [Google Scholar]
- Weinstein ND. Unrealistic optimism about future life events. Journal of Personality and Social Psychology. 1980;39:806–820. [Google Scholar]
- Williams JMG, Ellis NC, Tyers C, Healy H, Rose G, MacLeod AK. The specificity of autobiographical memory and imaginability of the future. Memory and Cognition. 1996;24:116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]