Abstract
Both SRC-1 and TIF2 are members of the p160 steroid receptor coactivator family. Genetic analyses have shown that inactivation of TIF2, but not SRC-1, reduces postnatal survival, growth, and male reproductive function. Here, we demonstrate that, through analyses of SRC-1/TIF2 compound mutant mice, SRC-1 can partially compensate for the effects of a loss of TIF2 on mouse survival and growth, whereas SRC-1 and TIF2 are dispensable for primary organogenesis. The highly variable onset of defects observed in TIF2–/– testes due to the absence of TIF2 in Sertoli cells, including abnormal spermiogenesis, age-dependent degeneration of seminiferous epithelium, and disorder of cholesterol homeostasis, is uniformly accelerated upon inactivation of SRC-1 alleles in the TIF2 null genetic background, thus demonstrating that TIF2 and SRC-1 can perform redundant functions in Sertoli cells. Massive desquamation of immature germ cells together with an increase in germ cell apoptosis and a decrease in germ cell proliferation may be responsible for the early onset of the severe seminiferous epithelial degeneration observed in SRC-1+/–/TIF2–/– testes. Interestingly, the overall abnormal features displayed by the SRC-1+/–/TIF2–/– and SRC-1–/–/TIF2–/– mutant testes, including spermatid maturation defects, increase in Sertoli cell lipid stores, loss of immature germ cells, and formation of giant multinucleated spermatids, are commonly detected in testes of elderly men, suggesting that deficiencies in molecular pathways involving TIF2 and SRC-1 in Sertoli cells could participate in testicular senescence.
Nuclear receptors (NRs) are hormone-dependent transcription factors that modulate gene expression and regulate various biological processes including development, homeostasis, reproduction, and environmental adaptation (1). Recent in vitro studies have demonstrated that multiple coactivator complexes mediate NR-dependent transcriptional activation. The switch defective/sucrose nonfermenter complex contains AT-Pase activity and plays an important role in ATP-dependent chromatin remodeling when recruited by NRs to specific chromatin regions (2). The thyroid receptor-associated protein/vitamin D receptor-interacting protein complex interacts with NRs in a ligand-dependent manner and enhances transcription through direct interactions with general transcription factors (GTFs) (2). The activating signal cointegrator 2 complex contains a subset of trithorax group proteins, which can methylate histone H3 when recruited to chromatin by NRs (3). The SRC complex contains acetyltransferases including cAMP response element binding protein (CEBP) binding protein (CBP), p300 and p300/CBP associated factor and methyltransferases including coactivator-associated arginine methyltransferase 1 and protein arginine methyltransferase 1. These chromatin-remodeling enzymes are recruited to promoters through interaction between NRs and SRC family members in a ligand-dependent manner and consequently facilitate the assembly and stabilization of GTFs (4). These coactivator complexes also may provide platforms targeted by multiple signaling pathways such as hormones, growth factors, and cytokines in a tissue-specific manner for transcriptional control (2, 5).
The SRC/p160 family consists of three homologous proteins with 50–55% similarity among them, including SRC-1 (NCoA-1), TIF2 (GRIP1), and SRC-3 (p/CIP/RAC3/ACTR/AIB1/TRAM-1) (reviewed in ref. 6). Each SRC family member displays a specific expression patterns in vivo (7–12), which may explain, in part, why mice deficient in SRC genes exhibit distinct phenotypes. SRC-1–/– mice are partially resistant to steroid and thyroid hormones and exhibit a delayed development of cerebellar Purkinje cells (9, 13, 14). Peroxisome proliferator-activated receptor γ function is partially impaired in brown fat of SRC-1–/– mice causing lower energy expenditure and higher sensitivity to diet-induced obesity (15, 16). Unlike SRC-1–/– mice, TIF2–/– mice exhibit higher energy expenditure and are resistant to diet-induced obesity (16). In addition, TIF2–/– mice are hypofertile because of restricted growth of the placenta in females and partial impairment of spermatogenesis in males (17). SRC-3–/– mice are growth-retarded, have lower levels of insulin-like growth factor 1 and estradiol, and display a delayed puberty and reduced female reproductive function (11, 18). The vasoprotective functions of estrogen receptor after vascular injury also are partially impaired in SRC-3–/– mice (12). Altogether, these genetic studies indicate that SRC family members have unique biological roles. However, the facts that SRCs coactivate gene transcription with little selectivity among NRs in transfected cells and that the defects observed in SRC-1, TIF2, and SRC-3 single null mutants are restricted to a small subset of the tissues normally expressing these genes (4, 11, 13, 17) also suggests that SRC family members may perform overlapping functions. Furthermore, these data raise the possibility that certain functions normally performed by a given SRC family member in WT mice may not be unveiled in the corresponding single null mutants because of artificial functional compensation of the deleted gene by another member of the SRC family. The occurrence of such functional compensations in multigene families leads to a underestimation of phenotypic defects emanating from the gene deletion (19). Therefore, with the aim to gain further insights into the functions of SRC-1 and TIF2 in vivo, we have analyzed herein the phenotype of SRC-1/TIF2 compound mutant mice.
Materials and Methods
Mice. SRC-1 and TIF2 knockout mouse lines have been described (13, 17). Mutant mice with all combinations of deleted SRC-1 and TIF2 alleles were generated from SRC-1 +/–/TIF2 +/– parents or from mating of SRC-1+/–/TIF2+/– males and SRC-1–/–/TIF2+/– females. All mice were genotyped by PCR, as described (13, 17). In vivo fertilization tests were carried out as described (17).
Histological and Ultrastructural Analyses. For histological analyses, testes were either fixed in Bouin's fixative before paraffin embedding, or in 2.5% glutaraldehyde in PBS before embedding in epon. Stages of the seminiferous epithelium cycle were identified according to well established morphological criteria (20). Ultrastructural analyses were performed as described (17).
Sperm Number and Quality. Mouse spermatozoa were collected by pulverizing the caudal epididymides in human tubal fluid (HTF) medium (Irvine Scientific, Santa Ana, CA) supplemented with 5mg/ml BSA. The number of spermatozoa was determined after incubating the samples in HTF medium at 37°C for 15 min in a tissue culture incubator. Sperm motility was analyzed after 1 h of incubation and presented as percentage of motile sperm to total sperm number. To analyze sperm morphology, samples were washed with PBS, fixed in 4% paraformaldehyde for 20 min, and smeared onto glass slides. Spermatozoa on air-dried slides were stained with hematoxylin, and the percentage of abnormal sperm was calculated.
Detection of Apoptotic Cells and Immunohistochemistry. Testes were fixed in 4%paraformaldehyde in PBS for 16 h at 4°C and embedded in paraffin. Terminal deoxynucleotidytransferase-mediated dUTP-biotin nick end-labeling (TUNEL) assays were carried out by using an In Situ Cell Death Detection kit (Roche Diagnostics). For detecting the proliferation cell nuclear antigen (PCNA) by immunohistochemistry, sections were treated with 1% H2O2 in methanol, blocked with 5% goat serum in PBS, and incubated overnight at 4°C with antibodies against PCNA (1:500 of FL261, Santa Cruz Biotechnology). After washing in PBS, sections were incubated with peroxidase-labeled anti-rabbit IgG (1:200). For detecting SRC-1, sections were boiled for 10 min in 10 mM sodium citrate buffer (pH 6.0), treated with 1% H2O2, blocked with 5% goat serum, and incubated with antibodies against SRC-1 (1:1,000 of M341, Santa Cruz Biotechnology). The antigen-bound primary antibodies were detected by using biotinylated anti-rabbit IgG (1:500) and avidin-conjugated peroxidase (Vector Laboratories). In all immunohistochemical analyses, peroxidase activity was visualized by using 3,3′-diaminobenzidine as a substrate. Substitution of the primary antibodies with nonimmune IgG was performed as negative controls. After immunostaining, sections were counterstained with methylgreen.
Results
SRC-1/TIF2 Double Null Mutants Die at Birth. Functional redundancies between SRC-1 and TIF2 in vivo were evaluated by analyzing the phenotypes of SRC-1/TIF2 compound mutants generated by either intercrossing SRC-1+/–/TIF2+/– mice (Table 1) or crossing SRC-1+/–/TIF2+/– males with SRC-1–/–/TIF2+/– females (Fig. 1b). SRC-1–/–, TIF2–/–, SRC-1+/–/TIF2+/–, and SRC-1–/–/TIF2+/– mice genotyped between 2 and 3 weeks postpartum were found at normal Mendelian ratios. In contrast, only ≈60% of the expected SRC-1+/–/TIF2–/– mice and <10% of the expected SRC-1–/–/TIF2–/– mice were recovered at these ages (Table 1). Less than 10% of the expected SRC-1–/–/TIF2–/– mice were recovered at weaning stage in the offspring of SRC-1–/–/TIF2+/– mice (data not shown). However, SRC-1–/– /TIF2–/– mutants (as well as SRC-1+/–/TIF2–/– mutants), collected at embryonic day 18.5 (E18.5) by cesarean delivery, were obtained at a normal Mendelian ratio from similar intercrosses and crosses (obtained, 15; expected, 15). These E18.5 double null mutants were on average 11% lighter than their WT littermates (SRC-1–/–/TIF2–/– mutants, 0.98 ± 0.09 g; WT, 1.10 ± 0.10 g; P < 0.001; n = 14 in each group), whereas the weights of their littermates with other genotypes were not significantly altered. Analysis of serial histological sections through five E18.5 double null mutants did not reveal any congenital malformation (data not shown). When left on their own after cesarean delivery, all became rapidly cyanotic and died within 45 min, in contrast to E18.5 WT mice, which survived for >15 h. Double null mutant lungs were not, or only very poorly, inflated in comparison to WT lungs (Fig. 1 a and b, compare structures indicated by A). Lung mRNA levels of surfactant proteins SPA, SPB, SPC, and SPD were similar in SRC-1–/–/TIF2 –/– mutants and in their WT littermates (data not shown), making it is unlikely that the cause of the respiratory distress of SRC-1–/–/TIF2–/– mutants was a decrease in pulmonary surfactant production. These results indicate that inactivation of SRC-1 alleles in a TIF2–/– genetic background decreases postnatal viability in a dose-dependent manner. Moreover, SRC-1 or TIF2 are necessary for prenatal growth, whereas they are not indispensable for organogenesis.
Table 1. Viability of compound SRC-1/TIF2 mutant mice.
| SRC-1+/- | SRC-1-/- | SRC-1+/- | SRC-1-/- | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotypes | WT | SRC-1+/- | TIF2+/- | TIF2+/- | SRC-1-/- | TIF2-/- | TIF2+/- | TIF2-/- | TIF2-/- |
| SRC-1+/-/TIF2+/- × SRC-1+/-/TIF2+/- | |||||||||
| Observed | 15 | 35 | 36 | 68 | 22 | 18 | 37 | 21 | 1 |
| Expected | 17 | 34 | 34 | 68 | 17 | 17 | 34 | 34 | 17 |
| SRC1+/-/TIF2+/- × SRC-1-/-/TIF2+/- | |||||||||
| Observed | 39 | 74 | 39 | 78 | 24 | 4 | |||
| Expected | 38 | 76 | 38 | 76 | 38 | 38 |
Offspring derived from SRC-1+/-/TIF2+/- parents or from mating of SRC-1+/-/TIF2+/- males and SRC-1-/-/TIF2+/- females were genotyped between 2 and 3 weeks of age. Expected numbers were calculated on the basis of the assumption that heterozygosis for SRC-1 and/or TIF2 null alleles does not impair viability, as demonstrated in previous studies (17, 21).
Fig. 1.
Failure of lung alveoli expansion (a and b) at birth and reproductive defects (c–m) in SRC-1/TIF2 compound mutants. (a and b) Histological sections from E18.5 WT (a) and SRC-1–/–/TIF2–/– (b) lungs: 30 min after cesarian delivery, mutant alveoli are still collapsed. (c–m) Histological sections of epididymides (c–e) and testes (f–m) of 9-week-old WT, TIF2–/–, and SRC-1+/–/TIF2–/– males. Oligozoospermia and testicular degeneration are observed only in compound mutants. Mild forms of the spermiation failure and Sertoli cell lipid accumulation, which are consistently observed in compound mutants are occasionally seen in their TIF2–/– littermates. A, alveoli; B, bronchi; L, Leydig cells; P, pachytene spermatocytes; R, retained, mature spermatids; RS, round spermatids; S, Sertoli cells; St9, elongating, step 9, spermatids; SY, symplasts; T, seminiferous tubules; T*, Sertoli cell-only seminiferous tubule; V, vacuole; Z, spermatozoa. IX, stage nine of the seminiferous epithelium cycle. The arrowheads point to lipid droplets which are blackened by osmium fixation of the tissues. Shown are Mallory's trichrome stain (a and b) and osmium tetroxide fixation with (c–g and k–m) or without (h–j) toluidine blue stain. (Bars are 100 μmin a and b, 10 μmin c–e and h–m, and 30 μmin f and g.)
SRC-1+/–/TIF2–/– Males Are Sterile Due to Severe Oligo- and Teratozoospermia. Fertility is normal in SRC-1–/– males, but decreased in TIF2–/– males (13, 17, 21). To investigate the possibility that SRC-1 could partially compensate for the loss of TIF2 in male reproductive functions, we tested the fertility of single and compound SRC-1 and/or TIF2 mutant males by mating them with WT females over 6 months. Crosses using SRC-1–/–, SRC-1–/–/TIF2+/–, and SRC-1+/–/TIF2+/– males yielded numbers of litters and numbers of pups per litter similar to those obtained when WT males were used (data not shown). TIF2–/– males appeared hypofertile in this assay, in agreement with previous observations (ref. 17 and data not shown). SRC-1+/–/TIF2–/– males produced vaginal plugs, but they failed to generate any offspring; furthermore, in vivo fertilization assays (17) carried out with five SRC-1+/–/TIF2–/– males showed an absence of egg fertilization (data not shown). Thus, SRC-1+/–/TIF2–/– males are completely sterile.
Both in TIF2–/– and SRC-1+/–/TIF2–/– young males, the testis to body weight ratio was reduced by about one-third, the motility of epididymal spermatozoa was 3- to 4-fold lower than in WT males, and the percentage of abnormal spermatozoa was increased (Table 2). However, spermatozoa numbers were significantly reduced only in SRC-1+/–/TIF2–/– males (to <1% of WT numbers; Table 2). In accordance with the results of sperm counts, histological sections showed that spermatozoa filled the caudal epididymides of the young TIF2–/– males (Z, compare Fig. 1 c and d), whereas SRC-1+/–/TIF2–/– epididymides contained most round degenerating spermatids (RS, Fig. 1e), and rare spermatozoa (Z, Fig. 1e). Thus, inactivation of one SRC-1 allele in a TIF2–/– background causes a dramatic reduction in the number of spermatozoa (i.e., a severe oligozoospermia), which accounts at least in part for the SRC-1+/–/TIF2–/– male sterility. On the other hand, because testis to body weight ratios and sperm parameters were normal in SRC-1–/–/TIF2+/– males, a single TIF2 allele appears to be sufficient to support normal spermatid maturation, even in the absence of SRC-1 (Table 2 and data not shown).
Table 2. Testis weights and number, mobility, and shapes of epididymal spermatozoa in SRC-1 and TIF2 single and compound mutants at 5 and 9 weeks of age.
| WT | TIF2-/- | SRC-1+/-/TIF2-/- | SRC1-/-/TIF2+/- | |
|---|---|---|---|---|
| 5 weeks | ||||
| TW/BW × 103 | 4.1 ± 0.2 (5) | 3.1 ± 0.2** (5) | 2.7 ± 0.8** (5) | 4.4 ± 0.7 (5) |
| 9 weeks | ||||
| TW/BW × 103 | 4.2 ± 0.6 (5) | 2.6 ± 0.7* (5) | 2.6 ± 0.1** (5) | 4.1 ± 0.5 (5) |
| SN × 107 | 6.81 ± 2.57 (4) | 3.40 ± 1.68 (3) | 0.06 ± 0.04** (3) | 3.34 ± 0.74 (3) |
| SM, % | 57.8 ± 6.4 (4) | 17.0 ± 5.7** (3) | 13.8 ± 4.3** (3) | 49.1 ± 5.9 (3) |
| AS, % | 13.4 ± 3.0 (4) | 46.5 ± 3.2** (3) | 59.4 ± 5.1** (3) | 9.5 ± 2.6 (3) |
TW, testis weight; BW, body weight; SN, number of spermatozoa per ml of cauda epididymal fluid; SM, percentage of mobile spermatozoa; AS, percentage of abnormal spermatozoa displaying looped tails and/or triangular head shapes. The number of mice that were analyzed is indicated in parentheses. Note that no significant changes in number and morphology of spermatozoa were observed in SRC-1-/-/TIF2+/- mice and that, although the number of spermatozoa in TIF2-/- epididymides was apparently lower than in their WT counterparts, this difference was not statistically significant. *, P < 0.05 vs. WT in unpaired t test. **, P < 0.01 vs. WT in unpaired t test.
Spermiogenesis and Spermiation Are Severely Affected in SRC-1+/–/TIF2–/– Males. In WT and SRC-1–/– testes, mature (i.e., fully condensed and elongated) spermatids were released into the lumen of the tubules at stages VII and VIII of the seminiferous epithelium cycle, and thus were no longer seen in this epithelium at stage IX (Fig. 1k). In contrast, most of the SRC-1+/–/TIF2–/– mature spermatids were retained in the epithelium (R, Fig. 1m) and subsequently phagocytosed by Sertoli cells (data not shown). This massive failure of spermiation (i.e., spermatid release) and the decreased germ cell production (see below) both account for the oligozoospermia. Retained spermatids were rare in young TIF2–/– males, but their number markedly increased with aging (data not shown).
Because failure of spermiation is often associated with abnormal spermiogenesis (i.e., spermatid maturation), we next analyzed germ cell morphology by using electron microscopy. In all 9-week-old SRC-1+/–/TIF2–/– males (n = 3), ≈80% of the elongating spermatids appeared abnormal: their acrosomes were vacuolated, partially detached from the nuclear envelope (Fig. 2 a and b, compare acrosomes indicated by A), and/or formed invaginations into the nuclei (Fig. 2 e and c); their nuclei were asymmetrically elongated (compare N in Fig. 2 b with a), and often showed deep indentations containing cytoplasmic constituents (N in Fig. 2 d and e, compare with c); and their manchettes were either lacking or were ectopic. The normal manchette, a conical aggregate of microtubules located around the postacrosomal portion of the spermatid nucleus (M, Fig. 2 a and c), is thought to play a instrumental role in its shaping (22, 23). Therefore, topographically misplaced manchettes, developing along the plasma membrane (M in Fig. 2b), or along areas of the nucleus that normally do not display a manchette (M in Fig. 2 d and e), could account for the abnormal nuclear shapes of SRC-1+/–/TIF2–/– spermatids.
Fig. 2.
Elongating spermatids are abnormal in SRC-1+/–/TIF2–/– males. Analysis by electron microscopy of WT (a and c) and SRC-1+/–/TIF2–/– (b, d, and e) step 12 spermatids at 9 weeks of age. A, acrosome; M, manchette; MI, mitochondria; MS, spermatocyte in metaphase; N, nucleus. (Bars are 2 μmin a and b and 0.6 μmin c–e.)
The observation in epididymides of all sexually mature TIF2–/– mutants of numerous spermatozoa displaying acrosomes detached from their nuclei has suggested that the adhesion of these two structures, which takes place in the testis during spermiogenesis, may be deficient (17). Unexpectedly, elongating spermatids within testes of 9-week-old TIF2–/– males appeared ultrastructurally normal. However, in TIF2–/– males older than 6 months, a small percentage of these cells displayed maturation defects identical to those of young SRC-1+/–/TIF2–/– mutants (data not shown). Thus, certain alterations of spermiogenesis may occur early in TIF2–/– mutants and worsen with aging. In any event, the present results indicate that the defects in spermiogenesis and spermiation seen in TIF2–/– mutants are much more severe in SRC-1+/–/TIF2–/– mutants.
Testis Degeneration in SRC-1+/–/TIF2–/– and SRC-1–/–/TIF2–/– Males Is Early and Severe. All SRC-1+/–/TIF2–/– testes analyzed at 5 and 9 weeks of age (n = 4 and 11, respectively) displayed signs of degeneration manifested by (i) the presence of seminiferous tubules showing various degrees of germ cell depletion (T in Fig. 1 g, compare with f), and eventually containing only Sertoli cells (T*, Fig. 1g and data not shown), (ii) the occurrence between Sertoli cells of large empty vacuoles (V, Fig. 1 g and j), (iii) the desquamation of immature germ cells (RS, Fig. 1g), and (iv) the formation of multinucleated giant cells (SY, symplasts, Fig. 1g) by expansion of the cytoplasmic bridges that connect clones of spermatocytes or spermatids. The only SRC-1–/–/TIF2–/– testis that was analyzed displayed identical features of degeneration at 9 weeks of age.
SRC-1–/– testes at 12 months of age were undistinguishable from WT testes (data not shown). In contrast, TIF2–/– mutants displayed degeneration features similar to those described in the testes from young SRC-1+/–/TIF2–/– males; degeneration was rarely observed before 3 months of age and became fully penetrant only by 9 months (17). Degeneration affected all SRC-1+/–/TIF2–/– testes by the completion of puberty (24), i.e., at a much earlier stage than in TIF2–/– males. These data indicate that the late and variable onset of testis degeneration in TIF2–/– mutants is caused by a partial functional compensation of TIF2 inactivation by SRC-1.
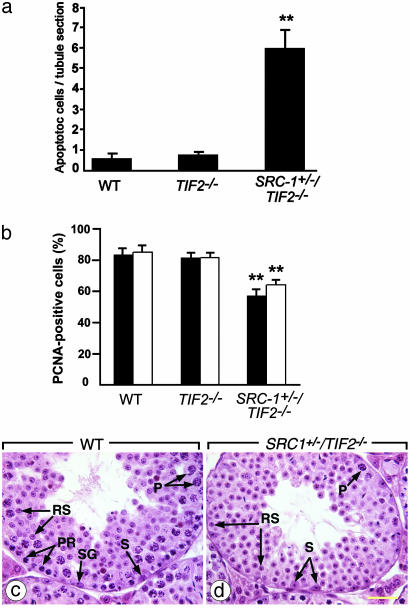
To gain further insight into the causes of the early SRC-1+/– /TIF2–/– testicular degeneration, germ cell apoptosis and proliferation were analyzed and compared to the situation in age-matched TIF2–/– males. In SRC-1+/–/TIF2–/– seminiferous tubules, ≈6% of the germ cells were positive in TUNEL assays versus <1% in WT and in TIF2–/– tubules (Fig. 3a). Symplasts were all positive in TUNEL assays. In contrast, the vast majority of immature germ cells detaching from the SRC-1+/–/TIF2–/– seminiferous epithelium were negative in these assays, thus indicating that apoptosis was not the cause of their desquamation (data not shown; see also ref. 17). The cell proliferation marker PCNA was detected in only 60% of spermatogonia and young spermatocytes in SRC-1+/–/TIF2–/– tubules, versus 80% in WT and in TIF2–/– tubules (Fig. 3b). Recent arrests of spermatogonial proliferation were readily diagnosed on hematoxylin and eosin-stained histological sections of SRC-1+/–/TIF2–/– testes in the form of tubules showing normal complements of round spermatids, but depleted of all spermatids precursors (i.e., spermatogonia and spermatocytes) (compare Fig. 3 c and d). Therefore, the massive desquamation of immature germ cells together with germ cell apoptosis and decreased cell proliferation, can account for the earlier onset of testicular degeneration in SRC-1+/–/TIF2–/– when compared to single TIF2–/– mutants.
Fig. 3.
Increased apoptosis and decreased germ cell proliferation in testes from young SRC-1+/–/TIF2–/– males. (a) Analysis of apoptotic cell death at 9 weeks of age. TUNEL-positive cells were counted on 30 seminiferous tubule cross sections from five mice. Data are presented as the average number of TUNEL-positive cells per cross section. **, P < 0.01 vs. the other two groups in unpaired t test. (b) Analysis of cell proliferation by immunohistochemical detection of PCNA at 5 (black bars) and 9 (white bars) weeks of age. PCNA-positive and -negative cells in contact with basement membranes of the seminiferous tubules (which include spermatogonia and early-stage spermatocytes) were counted were counted on 10 tubule cross sections from four mice, and data are presented as mean percentage of PCNA-positive cells. **, P < 0.01 vs. the other two groups in unpaired t test. (c and d) Histological sections of seminiferous tubules of 9-week-old mice; d provides an example of abnormal tubular cross section displaying a normal amount of round spermatids but lacking almost all spermatogonia and spermatocytes. P, pachytene spermatocytes; PR, preleptotene spermatocytes; RS, round spermatids; S, Sertoli cells; SG, spermatogonia. A hematoxylin and eosin stain was used. (Bar is 40 μm.)
Early Lipid Accumulation in SRC-1+/–/TIF2–/– Sertoli Cells. Lipid droplets that are normally present at the base of Sertoli cells (arrowheads in Fig. 1 h and k; ref. 20) markedly enlarged during aging in TIF2–/–, but not in WT and SRC1–/– testes analyzed at up to 1 year of age (data not shown). This abnormal lipid accumulation affected all TIF2–/– males by 5 months of age (ref. 17 and data not shown). In contrast, large lipid droplets were detected in the Sertoli cell cytoplasm in all SRC-1+/–/TIF2–/– testes at 9 weeks (n = 3) (arrowheads in Fig. 1 j and m), whereas only one of the three age-matched TIF2–/– males showed a slight enlargement of these structures (arrowheads in Fig. 1 i and l). Therefore, lipid accumulation begins earlier in SRC-1+/–/TIF2–/– Sertoli cells than in those lacking only TIF2.
It has been proposed that the small lipid droplets normally observed in WT seminiferous tubules (Fig. 1 h and k) arise through the Sertoli cell phagocytic activity that is normally responsible for elimination of apoptotic germ cells and cyclic degradation of lipid-rich residual bodies originating from mature spermatids. It also was suggested that their enlargement in testis degenerations could be secondary to increased phagocytosis of dying germ cells (20). However, SRC-1+/–/TIF2–/– mutants, similarly to TIF2–/– mutants (17), display large lipid droplets in Sertoli cells from degenerating tubules as well as from apparently healthy tubules that showed normal germ cell associations (compare arrowheads in stage IX tubules of Fig. 1m, and data not shown). Therefore, these abnormally large lipid droplets seen in TIF2–/– and SRC-1+/–/TIF2–/– are unlikely to arise from an overload of the Sertoli cell metabolic machinery by germ cell degradation products, but rather, may reflect a primary defect of Sertoli cell lipid homeostasis in these mutants.
SRC-1 Is Localized in Sertoli Cell Nuclei but Not in Germ Cells. In the seminiferous epithelium of WT mice, a weak but clear SRC-1 immunoreactivity was detected by immunohistochemistry in Sertoli cell nuclei but not in germ cells (Fig. 4b). These Sertoli cells were identified based on their locations at the periphery of the seminiferous tubules and by triangular shapes of their nuclei (20). A similar level of SRC-1 immunoreactivity was also detected in Leydig cells (data not shown). In SRC-1–/– testes, no SRC-1 immunoreactivity was observed (Fig. 4a), confirming that the SRC-1 immunostaining in WT mice was specific. Because TIF2 protein is only localized in Sertoli cell nuclei in the testis (17), we can conclude that TIF2 and SRC-1 are coexpressed in Sertoli cells.
Fig. 4.
Immunohistochemical detection of SRC-1 in the seminiferous epithelium. Histological sections from SRC-1–/– (a) and WT (b) testes were immunostained with antibodies to SRC-1. SRC-1 immunoreactivity in Sertoli cell nuclei (arrows) was detected in WT, but not in SRC-1–/– mice. Sections were counterstained with methylgreen. (Bar is 50 μm.)
Discussion
SRC1–/– mice are fully viable (13, 21), whereas a small fraction of TIF2–/– mice do not survive beyond the first month of life (17). Because a large fraction of SRC-1+/–/TIF2–/– and almost all SRC-1–/–/TIF2–/– mutants die before weaning and at birth, these results suggest that SRC-1 can fulfil some TIF2 functions involved in postnatal survival. TIF2, but not SRC-1, is required for postnatal growth during the suckling period, but TIF2–/– fetuses have normal weights at the end of gestation (13, 17, 21). Our present results suggest that TIF2 may also play a role in prenatal growth, which is functionally compensated by SRC-1 in TIF2–/– fetuses. On the other hand, SRC-1 and TIF2 are dispensable for the primary organogenesis.
Spermatogenesis in SRC-1+/–/TIF2–/– and SRC-1–/–/TIF2–/– males display several abnormalities, including severe impairment in the release of mature spermatids and defective morphogenesis of the spermatid heads. The increase in germ cell apoptosis, decrease in germ cell proliferation, and desquamation of immature germ cells lead to accelerated degeneration of the seminiferous epithelium. These defects are similar to those observed in the TIF2–/– testes (17), but they affect a much higher percentage of germ cells and also occur much earlier in the life of these compound mutant mice. On the other hand, the testes of old SRC-1–/– males are histologically normal (ref. 21 and this report). Together, these data indicate that SRC-1 is dispensable for spermatogenesis in a WT genetic background, but can nevertheless partially compensate for the loss of TIF2 in male reproductive functions, including fertility, spermatid maturation and release, and maintenance of the structural integrity of the seminiferous epithelium.
Lipid droplet accumulation in Sertoli cells represents another early hallmark of the SRC-1+/–/TIF2–/– phenotype, which also is manifested in TIF2–/– testes, albeit at later ages (17). The large lipid droplets formed in TIF2–/– Sertoli cells contain large amounts of cholesteryl esters. Accumulations of cholesteryl esters are observed in Sertoli cells from liver X receptor β (LXRβ) null mutants and in those from retinoid X receptor β (RXRβ) loss-of-function mutants, but are always apparent before the completion of puberty, i.e., much earlier than their counterparts in TIF2–/– mutants (ref. 25 and data not shown). Our present data show that the delayed appearance of abnormal lipid droplets in TIF2–/– testes, when compared to testes that are impaired in LXRβ or RXRβ signaling, is due to a partial functional compensation by SRC-1. These data further support our hypothesis that SRC family members are required for the functions of LXRβ/RXRβ heterodimers in respect to Sertoli cell cholesterol homeostasis.
Because TIF2 and SRC-1 are both localized in Sertoli cell nuclei and are absent from germ cells (ref. 17 and Fig. 4), the Sertoli cells appear to be the primary target of the double mutations in the seminiferous epithelium. The abnormalities observed in SRC-1+/–/TIF2–/– and SRC-1–/–/TIF2–/– mutant testes strongly support the view that Sertoli cells are instrumental for germ cell proliferation and survival, control cell adhesion mechanisms for cohesiveness of the seminiferous epithelium and participate in the shaping of spermatid head components as well as in the integrity of cytoplasmic bridges connecting germ cells (26–28). Interestingly, the overall abnormal features of compound mutant testes, including the high proportion of mature spermatids with malformed nuclei and acrosomes, increased Sertoli cells lipid stores, loss of germ cells, and formation of giant multinucleated spermatids, are commonly detected in the testes of elderly men (29), suggesting that deficiencies in molecular pathways involving TIF2 and SRC-1 in Sertoli cells might be involved in testicular senescence.
Acknowledgments
We thank Drs. F. J. DeMayo, M. L. Meistrich, D. J. Lamb, S. Y. Tsai, and A. J. Cooney for help and discussions and N. Messaddeq, A. Gansmuller, B. Weber, and S. Zhou for technical assistance. This work was supported by a Mellon Foundation Award (to J.X.), National Institutes of Health grants (to B.W.O. and J.X.), and grants from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Hôpital Universitaire de Strasbourg, the Collège de France, and the Institut Universitaire de France (to M.M. and P.C.).
Abbreviations: NR, nuclear receptor; PCNA, proliferation cell nuclear antigen; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling.
References
- 1.Wilson, J. D. & Foster, D. W. (1995) Williams Textbook of Endocrinology (Saunders, New York).
- 2.Rosenfeld, M. G. & Glass, C. K. (2001) J. Biol. Chem. 276, 36865–36868. [DOI] [PubMed] [Google Scholar]
- 3.Goo, Y. H., Sohn, Y. C., Kim, D. H., Kim, S. W., Kang, M. J., Jung, D. J., Kwak, E., Barlev, N. A., Berger, S. L., Chow, V. T., et al. (2003) Mol. Cell. Biol. 23, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu, J. & O'Malley, B. W. (2002) Rev. Endocr. Metab. Disord. 3, 185–192. [DOI] [PubMed] [Google Scholar]
- 5.Wu, R. C., Qin, J., Hashimoto, Y., Wong, J., Xu, J., Tsai, S. Y., Tsai, M. J. & O'Malley, B. W. (2002) Mol. Cell. Biol. 22, 3549–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu, J. & Li, Q. (2003) Mol. Endocrinol. 17, 1681–1692. [DOI] [PubMed] [Google Scholar]
- 7.Misiti, S., Koibuchi, N., Bei, M., Farsetti, A. & Chin, W. W. (1999) Endocrinology 140, 1957–1960. [DOI] [PubMed] [Google Scholar]
- 8.Meijer, O. C., Steenbergen, P. J. & De Kloet, E. R. (2000) Endocrinology 141, 2192–2199. [DOI] [PubMed] [Google Scholar]
- 9.Nishihara, E., Yoshida-Komiya, H., Chan, C. S., Liao, L., Davis, R. L., O'Malley, B. W. & Xu, J. (2003) J. Neurosci. 23, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puustinen, R., Sarvilinna, N., Manninen, T., Tuohimaa, P. & Ylikomi, T. (2001) Eur. J. Endocrinol. 145, 323–333. [DOI] [PubMed] [Google Scholar]
- 11.Xu, J., Liao, L., Ning, G., Yoshida-Komiya, H., Deng, C. & O'Malley, B. W. (2000) Proc. Natl. Acad. Sci. USA 97, 6379–6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan, Y., Liao, L., Tulis, D. A. & Xu, J. (2002) Circulation 105, 2653–2659. [DOI] [PubMed] [Google Scholar]
- 13.Xu, J., Qiu, Y., DeMayo, F. J., Tsai, S. Y., Tsai, M. J. & O'Malley, B. W. (1998) Science 279, 1922–1925. [DOI] [PubMed] [Google Scholar]
- 14.Weiss, R. E., Xu, J., Ning, G., Pohlenz, J., O'Malley, B. W. & Refetoff, S. (1999) EMBO J. 18, 1900–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi, C., Zhu, Y., Pan, J., Yeldandi, A. V., Rao, M. S., Maeda, N., Subbarao, V., Pulikuri, S., Hashimoto, T. & Reddy, J. K. (1999) Proc. Natl. Acad. Sci. USA 96, 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picard, F., Gehin, M., Annicotte, J., Rocchi, S., Champy, M. F., O'Malley, B. W., Chambon, P. & Auwerx, J. (2002) Cell 111, 931–941. [DOI] [PubMed] [Google Scholar]
- 17.Gehin, M., Mark, M., Dennefeld, C., Dierich, A., Gronemeyer, H. & Chambon, P. (2002) Mol. Cell. Biol. 22, 5923–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, Z., Rose, D. W., Hermanson, O., Liu, F., Herman, T., Wu, W., Szeto, D., Gleiberman, A., Krones, A., Pratt, K., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 13549–13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kastner, P., Mark, M. & Chambon, P. (1995) Cell 83, 859–869. [DOI] [PubMed] [Google Scholar]
- 20.Russell, L. D., Ettlin, R. A., Sinha-Hikim, A. P. & Clegg, E. D. (1990) Histological and Histopathological Evaluation of the Testis (Cache River, Clearwater, FL).
- 21.Weiss, R. E., Gehin, M., Xu, J., Sadow, P. M., O'Malley, B. W., Chambon, P. & Refetoff, S. (2002) Endocrinology 143, 1554–1557. [DOI] [PubMed] [Google Scholar]
- 22.Russell, L. D., Russell, J. A., MacGregor, G. R. & Meistrich, M. L. (1991) Am. J. Anat. 192, 97–120. [DOI] [PubMed] [Google Scholar]
- 23.Kierszenbaum, A. L. (2002) Mol. Reprod. Dev. 63, 1–4. [DOI] [PubMed] [Google Scholar]
- 24.Oakberg, E. F. (1956) Am. J. Anat. 99, 507–516. [DOI] [PubMed] [Google Scholar]
- 25.Kastner, P., Mark, M., Leid, M., Gansmuller, A., Chin, W., Grondona, J. M., Decimo, D., Krezel, W., Dierich, A. & Chambon, P. (1996) Genes Dev. 10, 80–92. [DOI] [PubMed] [Google Scholar]
- 26.Russell, L. D. (1993) in The Sertoli Cell, eds. Russell, L. D. & Griswold, M. D. (Cache River, Clearwater, FL), pp. 269–303.
- 27.Sharpe, R. M. (1993) in The Sertoli Cell, eds. Russell, L. D. & Griswold, M. D. (Cache River, Clearwater, FL), pp. 391–418.
- 28.Byers, S., Pelletier, R.-M. & Suarez-Quian, C. (1993) in The Sertoli Cell, eds. Russell, L. D. & Griswold, M. D. (Cache River, Clearwater, FL), pp. 431–446.
- 29.Holstein, A. F., Roosen-Runge, E. C. & Schirren, C. (1988) Illustrated Pathology of Human Spermatogenesis (Grosse, Berlin).