Abstract
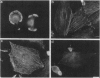
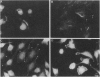
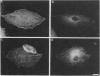
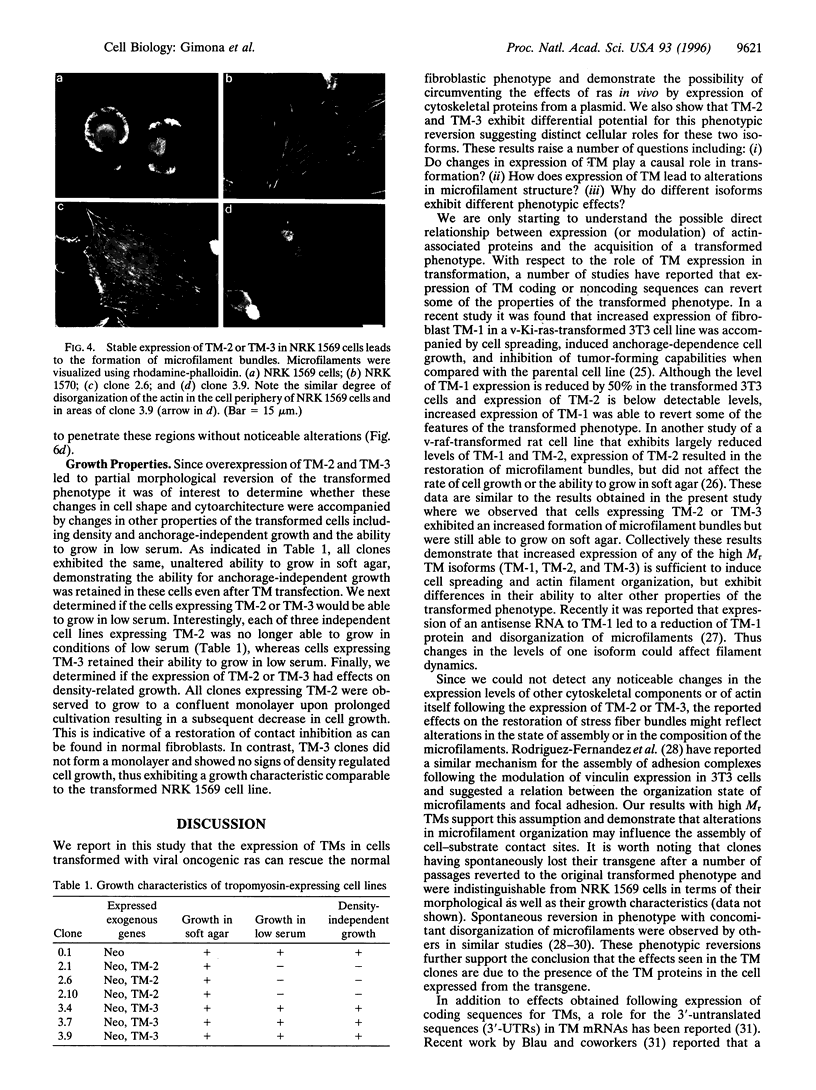
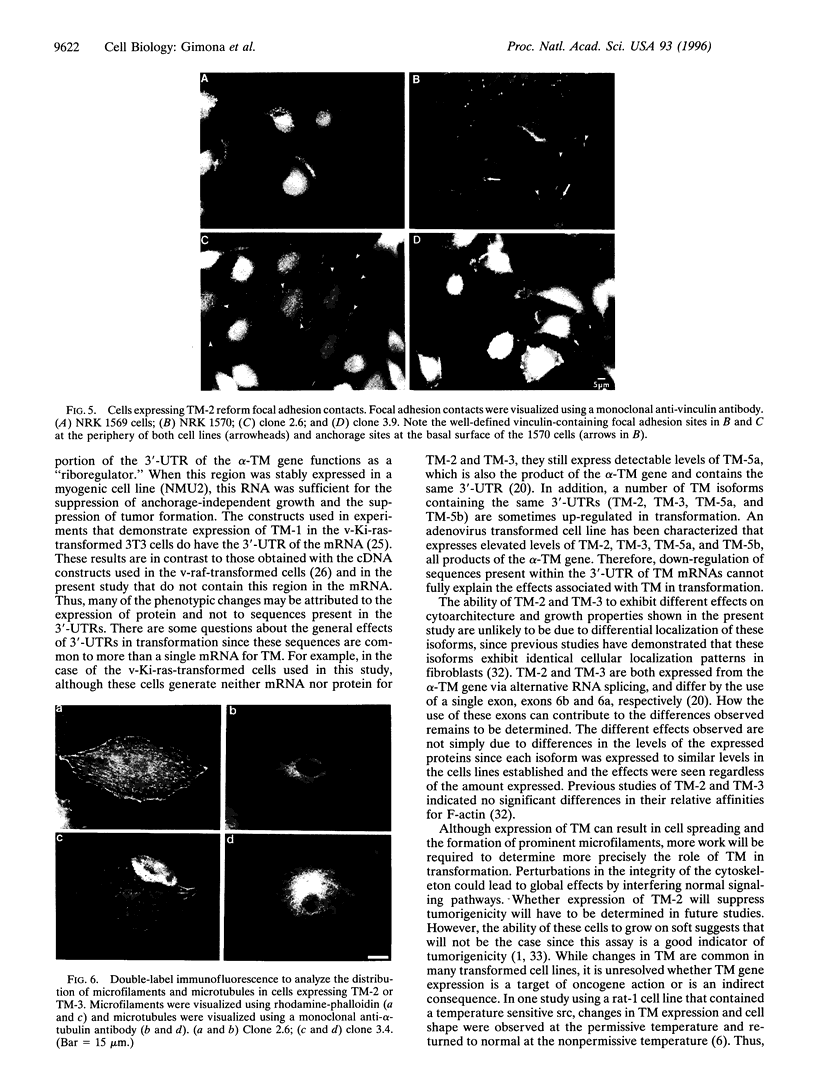
Transformation of cells in tissue culture results in a variety of cellular changes including alterations in cell growth, adhesiveness, motility, morphology, and organization of the cytoskeleton. Morphological and cytoskeletal changes are perhaps the most readily apparent features of transformed cells. Although a number of studies have documented a decrease in the expression of specific tropomyosin (TM) isoforms in transformed cells, it remains to be determined if the suppression of TM synthesis is essential in the establishment and maintenance of the transformed pheno-type. To address the roles of different TM isoforms in transformed cells we have examined the effects of expressing specific TM isoforms in transformed cells using a Kirsten virus-transformed cell line (ATCC NRK1569) as a model system. In contrast to normal fibroblasts, the NRK 1569 cells contain reduced levels of TM-1 and undetectable levels of TM-2 and TM-3. These cells have a rounded morphology and are devoid of stress fibers. Employing expression plasmids for TM-2 and TM-3, stable cell lines were established from the NRK 1569 cells that express these isoforms individually. We demonstrate that expression of TM-2 or TM-3 leads to increased cell spreading accompanied by the formation of identifiable microfilament bundles, as well as significant restoration of well-defined vinculin-containing focal adhesion plaques, although expression of each isoform exhibited distinct properties. In addition, cells expressing TM-2, but not TM-3, exhibited contact-inhibited cell growth and a requirement for serum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J., Risinger J. I., Wiseman R. W., Merrick B. A., Selkirk J. K., Barrett J. C. Regulation of microfilament organization and anchorage-independent growth by tropomyosin 1. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11534–11538. doi: 10.1073/pnas.92.25.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button E., Shapland C., Lawson D. Actin, its associated proteins and metastasis. Cell Motil Cytoskeleton. 1995;30(4):247–251. doi: 10.1002/cm.970300402. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Bhattacharya B., Bassin R. H., Salomon D. S. Suppression of synthesis and utilization of tropomyosin in mouse and rat fibroblasts by transforming growth factor alpha: a pathway in oncogene action. Cancer Res. 1987 Aug 15;47(16):4493–4500. [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H., Suzuki H., Kuzumaki N., Müllauer L., Ogiso Y., Oda A., Ebisawa K., Sakurai T., Nonomura Y., Kijimoto-Ochiai S. A specific protein, p92, detected in flat revertants derived from NIH/3T3 transformed by human activated c-Ha-ras oncogene. Exp Cell Res. 1990 Jan;186(1):115–121. doi: 10.1016/0014-4827(90)90217-x. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Quantitative two-dimensional gel electrophoresis of proteins. Methods Enzymol. 1983;100:411–423. doi: 10.1016/0076-6879(83)00070-1. [DOI] [PubMed] [Google Scholar]
- Giometti C. S., Anderson N. L. Tropomyosin heterogeneity in human cells. J Biol Chem. 1984 Nov 25;259(22):14113–14120. [PubMed] [Google Scholar]
- Glück U., Ben-Ze'ev A. Modulation of alpha-actinin levels affects cell motility and confers tumorigenicity on 3T3 cells. J Cell Sci. 1994 Jul;107(Pt 7):1773–1782. doi: 10.1242/jcs.107.7.1773. [DOI] [PubMed] [Google Scholar]
- Glück U., Kwiatkowski D. J., Ben-Ze'ev A. Suppression of tumorigenicity in simian virus 40-transformed 3T3 cells transfected with alpha-actinin cDNA. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):383–387. doi: 10.1073/pnas.90.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin L. O., Lees-Miller J. P., Leonard M. A., Cheley S. B., Helfman D. M. Four fibroblast tropomyosin isoforms are expressed from the rat alpha-tropomyosin gene via alternative RNA splicing and the use of two promoters. J Biol Chem. 1991 May 5;266(13):8408–8415. [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Multiple tropomyosin polypeptides in chicken embryo fibroblasts: differential repression of transcription by Rous sarcoma virus transformation. Mol Cell Biol. 1984 Sep;4(9):1823–1833. doi: 10.1128/mcb.4.9.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Shin S. I. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J Cell Biol. 1979 Jul;82(1):1–16. doi: 10.1083/jcb.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Latter G., Lutomski L., Goldstein D., Burbeck S. Tropomyosin isoform switching in tumorigenic human fibroblasts. Mol Cell Biol. 1986 Jul;6(7):2721–2726. doi: 10.1128/mcb.6.7.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller J. P., Helfman D. M. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991 Sep;13(9):429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- Leonardi C. L., Warren R. H., Rubin R. W. Lack of tropomyosin correlates with the absence of stress fibers in transformed rat kidney cells. Biochim Biophys Acta. 1982 Apr 29;720(2):154–162. doi: 10.1016/0167-4889(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Helfman D. M., Hughes S. H., Chou C. S. Tropomyosin isoforms in chicken embryo fibroblasts: purification, characterization, and changes in Rous sarcoma virus-transformed cells. J Cell Biol. 1985 Mar;100(3):692–703. doi: 10.1083/jcb.100.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Matsumura F., Yamashiro-Matsumura S. Tropomyosin-enriched and alpha-actinin-enriched microfilaments isolated from chicken embryo fibroblasts by monoclonal antibodies. J Cell Biol. 1984 Jan;98(1):116–127. doi: 10.1083/jcb.98.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S., Lin J. J. Isolation and characterization of tropomyosin-containing microfilaments from cultured cells. J Biol Chem. 1983 May 25;258(10):6636–6644. [PubMed] [Google Scholar]
- Patterson S. D., Latter G. I. Evaluation of storage phosphor imaging for quantitative analysis of 2-D gels using the Quest II system. Biotechniques. 1993 Dec;15(6):1076–1083. [PubMed] [Google Scholar]
- Pittenger M. F., Helfman D. M. In vitro and in vivo characterization of four fibroblast tropomyosins produced in bacteria: TM-2, TM-3, TM-5a, and TM-5b are co-localized in interphase fibroblasts. J Cell Biol. 1992 Aug;118(4):841–858. doi: 10.1083/jcb.118.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger M. F., Kazzaz J. A., Helfman D. M. Functional properties of non-muscle tropomyosin isoforms. Curr Opin Cell Biol. 1994 Feb;6(1):96–104. doi: 10.1016/0955-0674(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Prasad G. L., Fuldner R. A., Cooper H. L. Expression of transduced tropomyosin 1 cDNA suppresses neoplastic growth of cells transformed by the ras oncogene. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7039–7043. doi: 10.1073/pnas.90.15.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastinejad F., Conboy M. J., Rando T. A., Blau H. M. Tumor suppression by RNA from the 3' untranslated region of alpha-tropomyosin. Cell. 1993 Dec 17;75(6):1107–1117. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- Rodríguez Fernández J. L., Geiger B., Salomon D., Ben-Ze'ev A. Overexpression of vinculin suppresses cell motility in BALB/c 3T3 cells. Cell Motil Cytoskeleton. 1992;22(2):127–134. doi: 10.1002/cm.970220206. [DOI] [PubMed] [Google Scholar]
- Rodríguez Fernández J. L., Geiger B., Salomon D., Ben-Ze'ev A. Suppression of vinculin expression by antisense transfection confers changes in cell morphology, motility, and anchorage-dependent growth of 3T3 cells. J Cell Biol. 1993 Sep;122(6):1285–1294. doi: 10.1083/jcb.122.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Fernández J. L., Geiger B., Salomon D., Sabanay I., Zöller M., Ben-Ze'ev A. Suppression of tumorigenicity in transformed cells after transfection with vinculin cDNA. J Cell Biol. 1992 Oct;119(2):427–438. doi: 10.1083/jcb.119.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. A., Goldman R. D. Microfilaments and tropomyosin of cultured mammalian cells: isolation and characterization. J Cell Biol. 1980 Dec;87(3 Pt 1):633–642. doi: 10.1083/jcb.87.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V. The actin cytoskeleton. Electron Microsc Rev. 1988;1(1):155–174. doi: 10.1016/s0892-0354(98)90010-7. [DOI] [PubMed] [Google Scholar]
- Takenaga K., Masuda A. Restoration of microfilament bundle organization in v-raf-transformed NRK cells after transduction with tropomyosin 2 cDNA. Cancer Lett. 1994 Nov 25;87(1):47–53. doi: 10.1016/0304-3835(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Takenaga K., Nakamura Y., Sakiyama S. Suppression of synthesis of tropomyosin isoform 2 in metastatic v-Ha-ras-transformed NIH3T3 cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1111–1116. doi: 10.1016/s0006-291x(88)80988-4. [DOI] [PubMed] [Google Scholar]
- Talbot K., MacLeod A. R. Novel form of non-muscle tropomyosin in human fibroblasts. J Mol Biol. 1983 Feb 15;164(1):159–174. doi: 10.1016/0022-2836(83)90091-8. [DOI] [PubMed] [Google Scholar]
- Temm-Grove C. J., Guo W., Helfman D. M. Low molecular weight rat fibroblast tropomyosin 5 (TM-5): cDNA cloning, actin-binding, localization, and coiled-coil interactions. Cell Motil Cytoskeleton. 1996;33(3):223–240. doi: 10.1002/(SICI)1097-0169(1996)33:3<223::AID-CM6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]