Abstract
To study the regulation of cyclin-dependent kinase (CDK) activity during mitotic exit in mammalian cells, we constructed murine cell lines that constitutively express a stabilized mutant of cyclin A (cyclin A47). Even though cyclin A47 was expressed throughout mitosis and in G1 cells, its associated CDK activity was inactivated after the transition from metaphase to anaphase. Cyclin A47 associated with both p21 and p27 during mitotic exit, implicating these proteins in CDK inactivation. However, cyclin A47 was fully inhibited during the M-to-G1 transition in p21–/– p27–/– fibroblasts. Also, the CDKs associated with cyclin A47 were not inactivated by phosphorylation at tyrosines. The protein responsible for CDK inactivation during mitotic exit in p21/p27 null cells was the Rb family member, p107. p107 bound to cyclin A47 when p21 and p27 were absent, and cyclin A47–CDK activity was not inactivated during the M-to-G1 transition in p21–/– p27–/– p107–/– null fibroblasts. Enforced expression of cyclin A in cells lacking all three CDK inhibitors induced rapid tetraploidization, indicative of mitotic failure/endoreduplication. We concluded that cyclin proteolysis and CDK inhibitors constitute redundant pathways that control cyclin A–CDK activity during mitotic exit in mammalian cells and that loss of these pathways can cause genetic instability.
Oscillation in cyclin-dependent kinase (CDK) activity is a basic mechanism controlling progression through the eukaryotic cell cycle (1, 2). Beginning in late G1, rising amounts of CDK activity first trigger DNA replication and, later, mitosis. Then, at the conclusion of anaphase, CDK activity is extinguished to allow for mitotic exit, reassembly of preinitiation complexes at replication origins, and establishment of a G1 state (3).
Three mechanisms for controlling CDK activity during mitotic exit have been identified in organisms ranging from yeast to humans: cyclin degradation, tyrosine phosphorylation, and CDK inhibitor (CKI) binding. The anaphase-promoting complex (APC) is a multisubunit E3 ubiquitin–protein ligase that initiates the proteasomal degradation of cyclins (4–6). Both the A and B type cyclins contain a motif, the D box (destruction box) (7), that is required for its ubiquitination by the APC (8–10). Cyclin destruction is initiated at metaphase by one form of the APC, APC-Cdc20, and completed at anaphase by another form, APC-Cdh1 (11). APC-Cdh1 remains active after mitotic exit has been completed, creating a persistent state of low CDK activity, called G1. At the end of G1, APC-Cdh1 is inactivated, thereby allowing the reaccumulation of cyclin A and the onset of DNA replication.
CDKs can also be inhibited by Wee1-related tyrosine kinases. Phosphorylation of Cdk2 on tyrosine 15 contributes to the timing of cyclin B degradation and the rate of mitotic exit in the early cleavage cycles of Xenopus embryos (12, 13). This pathway does not seem to be important in budding yeast (14), and its role in other organisms is not known.
CKIs have been shown to regulate CDK activity during mitosis in yeast and Drosophila. In yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe, CKIs Sic1 and Rum1, respectively, bind to and inactivate mitotic cyclin–CDK complexes (15, 16). These CKIs accumulate and persist during mid-M-to-G1 phase until they are phosphorylated by Sic1/Rum1-resistant G1 cyclin-CDKs, which initiates their ubiquitin-dependent degradation at the G1-to-S phase transition (17–19). Thus, Sic1 and Rum1 constitute a switch that controls the transition from a state of low CDK activity to that of high CDK activity, thereby regulating mitotic exit and S phase entry. This parallels the activity of APC-Cdh1, and indeed these two pathways constitute redundant oscillators that dictate the timing of CDK activation and inactivation during the cell cycle (20). For example, a nondegradable mutant of Clb5 does not disrupt cell-cycle progression, although it is lethal when expressed in sic1-null yeast (21, 22). In Drosophila, the inhibitory protein Roughex (Rux) regulates the activity of mitotic cyclin–CDK complexes. Rux binds to and inactivates cyclin A- and B-CDK1 (23). It contributes to mitotic exit in Drosophila embryo (24) and to G1 arrest of the cells in the morphogenetic furrow of developing eye disk (25). Like Sic1, Rux prevents a nondegradable cyclin A mutant from inducing S phase (26).
It is not known whether there are pathways in addition to cyclin degradation that control CDK activity during mitotic exit in mammalian cells. To examine this, we have constructed murine cell lines that express a stabilized mutant of cyclin A at nearly physiological levels. This has enabled us to identify new pathways that regulate cyclin A–CDK activity during mitotic exit and to explore how cell-cycle regulation is disrupted when these pathways are inactivated.
Methods
Mice. p21–/– and p107–/– mice were provided by T. Jacks (Massachusetts Institute of Technology, Cambridge).
Cells. Mouse embryonic fibroblasts (MEFs) were isolated and propagated as described (27). NIH 3T3 cells (clone 7) were provided by C. Sherr (St. Jude Children's Hospital, Memphis, TN). Human cyclin A2 cDNA was provided by B. Clurman (Fred Hutchinson Cancer Research Center, Seattle) and mutagenized (28). Cyclin A2 cDNAs were N-terminally tagged with 6× myc epitope and transduced into passage 3–5 MEFs by using retroviral vectors (29) at a multiplicity of infection of >2, and stably infected cells were pooled (>105 clones). For synchronization at M phase, cells were treated with 2 mM thymidine for 16–20 h and transferred into medium containing no thymidine and 0.1 μg/ml nocodazol for 8–10 h; mitotic cells were then harvested by shake-off and released into nocodazol-free medium.
Microscopy. For immunofluorescence microscopy (30), cells were fixed with 4% paraformaldehyde in PBS for 10 min and permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature. Cells were stained with anti-myc tag monoclonal antibody PL14 (Medical and Biological Laboratories, Nagoya, Japan) and FITC-conjugated anti-mouse IgG antibody (Roche Diagnostics).
Preparation of Cell Extracts and Immunoprecipitation. Cells were rinsed once with TBS (pH 7.4) and lysed on ice in RIPA buffer (10 mM Tris·HCl, pH 7.4/0.15 M NaCl/0.5% Nonidet P-40/50 mM NaF/1 mM Na-vanadate/10 mg/ml each of aprotinin, leupeptin, and pepstatin). Immunoprecipitation and histone H1 kinase assays were performed as described (31, 32) by using the following antibodies: anti-myc tag 9E10 (31), anti-cyclin A (33), anti-Cdk2 (32), and anti-p21 (C-19), anti-p27 (N-20), anti-Cdc2 (clone 17), and anti-p107 (C-18) from Santa Cruz Biotechnology.
In Vivo Radiolabeling and Phosphoamino Acid Analysis. Cells were synchronized with nocodazol, and mitotic cells (4 × 106) were rinsed twice with phosphate-free medium plus 10% FBS dialyzed against 0.15 M NaCl and split (2 × 106 cells each) into 4 ml of the same medium containing 37 MBq/ml [32P]orthophosphate (ICN), with and without 0.1 μg/ml nocodazol. When >95% of the cells completed cytokinesis (3–4 h after the release from nocodazol), they were rinsed twice with TBS (pH 7.4), and extracts were prepared. CDK proteins in the anti-Cdk2 and anti-Cdc2 immunoprecipitates, and corresponding proteins in the anti-myc tag immunoprecipitates, were extracted from the gel and subjected to phosphoamino acid analysis (34).
Transfection and Flow Cytometry. Cyclin cDNAs were subcloned into pCS2MT (35) and myc-tagged and cotransfected with pCMVCD20 plasmid expressing cell-surface antigen CD20 (36) by using the modified Ca-phosphate method (31), with 10 μg each of the pCS2MT-cyclin plasmids and 2 μg of pCMVCD20 per 4 × 105 cells in 100-mm dishes. Sixteen hours after the transfection, cells were refed and cultured for an additional 24 h. Harvested cells were incubated with FITC-conjugated anti-CD20 antibody (BD Biosciences, San Jose, CA), fixed with 80% ethanol, and stained with propidium iodide (36). Flow cytometry was performed by using FACSCalibur cytometer and cellquest software (BD Biosciences), and data were gated for FITC (CD20)-positive cells.
Results
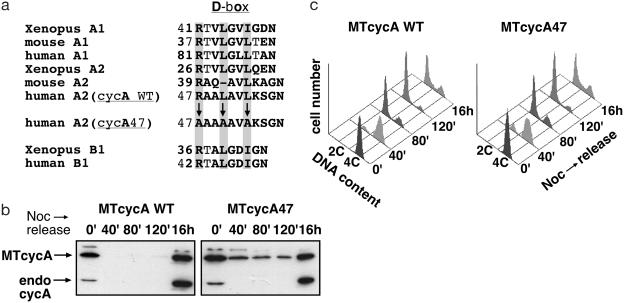
Constitutive Expression of Cyclin A During Mitotic Exit. We have focused on regulation of the activity of human cyclin A2, which is essential for normal mitotic cell cycles. To prevent cyclin destruction during mitotic exit, and thereby determine whether there are additional pathways for regulating cyclin A-associated kinase activity, we constructed human cyclin A2 mutants that contained alanine substitution or deletion mutations in the D box region. One of these, cyclin A47 (cycA47), contained three alanine substitutions at amino acid residues which are most conserved in the D boxes of A- and B-type cyclins (Fig. 1a). CycA47 and wild-type cyclin A were expressed in mouse NIH 3T3 fibroblasts as myc epitope-tagged proteins by retroviral transduction. The levels of expression of these exogenous cyclins closely approximated the amount of endogenous cyclin A2 in S/G2 phase cells (Fig. 1b, 16-h time point). D box-dependent degradation of cyclin A commences at metaphase and continues through G1. When the established cell lines were synchronized at prometaphase with the microtubule destabilizing agent nocodazole, cycA47 accumulated to a substantially higher level than endogenous cyclin A (Fig. 1b). Moreover, the cycA47 protein was inefficiently degraded after the cells were released from nocodazole-induced prometaphase arrest, and it remained present throughout the G1 phase of the cell cycle. In contrast, both the endogenous cyclin A2 and wild-type exogenous cyclin A2 proteins were completely degraded and absent from G1 phase cells.
Fig. 1.
A stabilized D box mutant of human cyclin A2. (a) D box sequences in various cyclin A and cyclin B1 proteins. (b and c) NIH 3T3-derived cell lines stably expressing myc-tagged (MT) wild-type (cycA WT) or the mutant (cycA47) cyclin A protein were released from nocodazole block. At the indicated times, endogenous and exogenous cyclin A proteins were detected by anti-cyclin A immunoblotting (b), and the cell-cycle distribution was analyzed by flow cytometry (c).
Cells expressing the cycA47 protein exited mitosis normally. By 80 min after release from the metaphase block, most cells expressing either cycA47 or wild-type cyclin A2 had a G1 DNA content (Fig. 1c), and there was no evidence that binucleated cells accumulated (data not shown). Thus, mitotic exit seemed unaffected by the constitutive presence of cyclin A2 during M and G1, although overexpression of superphysiological amounts of stabilized cyclin A2 can cause mitotic abnormalities and even a block to cell-cycle progression (37–40).
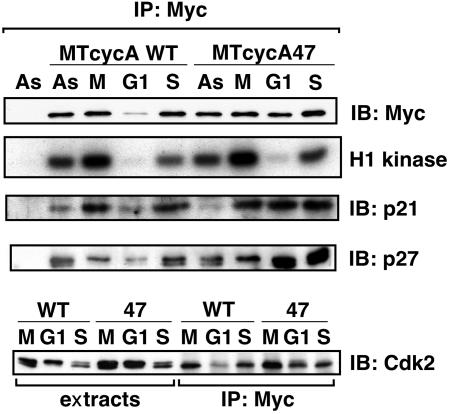
Regulation of Cyclin A-Associated Kinase Activity During Mitotic Exit. The observation that mitotic exit proceeded normally despite constitutive cyclin A expression suggested that cyclin A-associated kinase activity might be regulated independently of cyclin destruction. To address this, we immunoprecipitated the exogenous cyclin A proteins and measured their associated CDK activity. Wild-type cyclin A-associated CDK activity was high in metaphase and decreased to an undetectable level in early G1 coincidentally with cyclin degradation (Fig. 2). The cycA47-associated CDK activity was also extinguished in early G1 cells, even though a substantial amount of cyclin A protein remained and was bound to Cdk2 (Fig. 2). Immunofluorescence showed that the cycA47 protein was localized in nuclei of newly divided G1 cells (data not shown). These results showed that mechanisms other than cyclin destruction were able to down-regulate CDK activity during mitotic exit in mammalian cells.
Fig. 2.
Cyclin A-associated proteins and CDK activity. The NIH 3T3 derivatives were grown asynchronously (As) or synchronously as in Fig. 1, and the extracts from cells under nocodazole block (M), 120 min after the release (G1), and 16 h after the release (S) were immunoprecipitated (IP) with anti-myc antibodies and then immunoblotted (IB) for the indicated proteins or assayed for the histone H1 kinase activity.
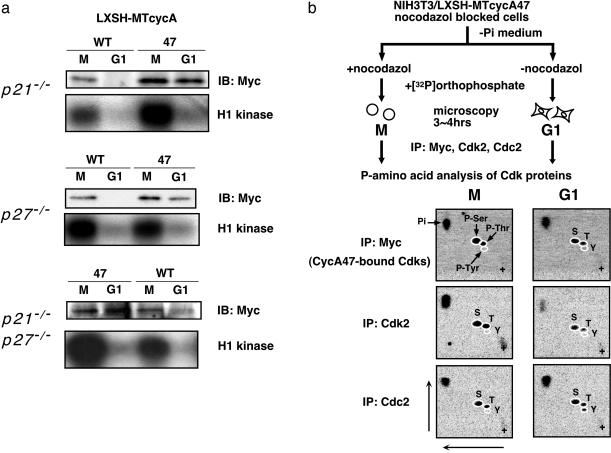
In addition to cyclin destruction, mechanisms of CDK inhibition include association with CKIs and tyrosine phosphorylation (41). We observed that both the p21 and p27 CKIs were associated with cyclin A in metaphase cells and that the portion of cyclin A that escaped proteolysis was associated with these CKIs in G1 cells (Fig. 2). Indeed, the amount of p27 associated with stabilized cycA47 further increased in G1 cells when cyclin A-associated kinase activity became inactive (Fig. 2). We could not detect any p57 associated with cyclin A in these cells (data not shown). These results showed that cyclin A associated with p21 and p27 during mitotic exit and suggested that these CKIs might be responsible for the repression of the cycA47-associated CDK activity in G1 cells. This was tested by using early passage MEFs derived from p21–/–, p27–/–, and p21–/– p27–/– knockout mice. Wild-type cyclin A2 and cycA47 were introduced as myc-tagged proteins by retroviral transduction. As we had observed in NIH 3T3 cells, the cycA47 protein was relatively stable during the M-to-G1 transition in MEFs of all three genotypes, whereas the wild-type cyclin A2 protein was degraded. CycA47-associated CDK activity was inactivated in early G1 in MEF strains of all three genotypes (Fig. 3a) and in MEFs from p21+/+ p27+/+ littermates (data not shown). Therefore, neither p21 nor p27 was essential for the repression of the cycA47-associated CDK activity during the transition from M to G1.
Fig. 3.
Regulation of cyclin A-associated CDK activity in G1 phase. (a) CycA47 abundance and its associated H1 kinase activity were analyzed during the M-to-G1 transition in p21–/–, p27–/–, and p21–/– p27–/– MEFs. The extracts from nocodazole-blocked (M) and early G1-phase (G1) cells were immunoprecipitated and analyzed as in Fig. 2. (b Upper) Scheme of the in vivo 32P-labeling procedure (see Methods for details). (Lower) Phosphoamino acid analysis of the CDK proteins recovered from the immunoprecipitates after the in vivo 32P labeling. Horizontal and vertical arrows represent the first and second electrophoresis, respectively.
We also investigated the contribution of tyrosine phosphorylation to CDK regulation during mitotic exit by determining the in vivo phosphorylation state of the cycA47-associated CDKs. NIH 3T3 cells stably expressing the myc-tagged cycA47 protein were synchronized in prometaphase and released into G1 in the continuous presence of [32P]orthophosphate. The phosphoamino acid composition of the CDK proteins coimmunoprecipitated with the cycA47 protein indicated that they were phosphorylated at serine and threonine but not at tyrosine residues (Fig. 3b). Parallel phosphoamino acid analysis on total Cdk2 and Cdc2 proteins immunoprecipitated with their specific antibodies revealed some phosphotyrosine in Cdc2 in early G1 cells (Fig. 3b), presumably representing either free Cdc2 or Cdc2 associated with residual cyclin B. This was consistent with previous observations (42, 43). Our results suggested that inhibitory phosphorylation on tyrosine does not make a major contribution to CDK inactivation in cyclin A complexes during mitotic exit.
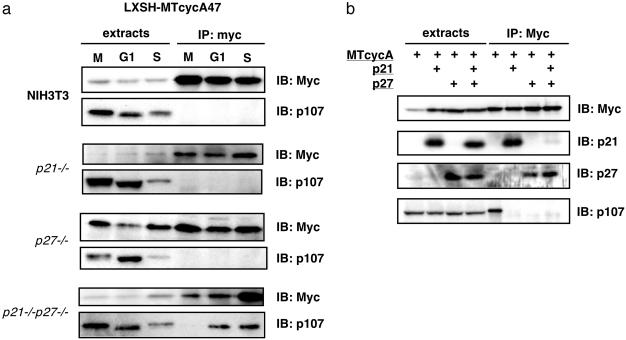
Regulation of Cyclin A Activity by the Rb-Related Protein p107. Previous results have shown that the Rb-related proteins p107 and p130 can bind to and inhibit CDKs (44). Because p130 has a physiological role in regulating Cdk2 activity in G0 cells that express low amounts of p27 (45), we asked whether p107 might have a parallel role in CDK regulation in G1 cells. CycA47 was immunoprecipitated from M, G1, and S phase cells that were p21–/–, p27–/–, or p21–/– p27–/– (Fig. 4a). There was little p107 stably associated with cycA47 in control NIH 3T3 cells and in MEFs that lacked either p21 or p27. In contrast, a complex of p107 and cycA47 was readily detected in G1 cells that lacked both p21 and p27 and was also present at lower stoichiometry in S phase p21–/– p27–/– cells (Fig. 4a).
Fig. 4.
p107 binds to cyclin A in the absence of p21 and p27. (a) NIH 3T3- and the indicated MEF-derived cell lines, stably expressing the myc-tagged cycA47 protein, were synchronized with nocodazole, and the extracts from nocodazole-blocked (M), early G1-phase (G1), and S-phase (S) cells were immunoprecipitated with anti-myc antibodies and immunoblotted for myc-tagged cycA47 and p107. (b) HEK293 cells were transfected with the combinations of myc-tagged wild-type cyclin A-, p21-, and p27-expressing vectors as indicated, and the cell extracts were immunoprecipitated with anti-myc tag antibodies and immunoblotted for the indicated proteins.
The mutually exclusive interaction between cyclin A and either p21/p27 or p107 was confirmed in transfected cells. Cyclin A was transiently overexpressed in HEK293 cells, and its association with endogenous p107 was measured by coimmunoprecipitation. A complex between p107 and cyclin A was detected, but formation of this complex was prevented by simultaneous overexpression of either p21 or p27 (Fig. 4b). Similar observations have been reported previously (46).
These observations suggested that p107 might regulate CDK activity during mitotic exit and perhaps be the primary regulator in cells lacking p21 and p27. To test this, we studied CDK regulation in MEFs lacking all three inhibitory proteins. p21–/–, p27–/–, and p107–/– mice were interbred, and MEFs were prepared from triple knockout progeny. However, using retroviral vectors, we were unable to establish any triple knockout cell lines that stably expressed either wild-type cyclin A or cycA47, suggesting that even relatively moderate overexpression of cyclin A protein might be lethal in this genetic background.
To circumvent this problem, we exposed these triple knockout MEFs to high titer retroviral stocks at an multiplicity of infection of >5, such that nearly 100% of the recipient cells were successfully transduced and expressed exogenous cyclin A protein without the need for subsequent selection (data not shown). Under these conditions, the abundance and activity of wild-type cyclin A was down-regulated during mitotic exit as we had seen in MEFs of other genotypes. However, the CDK activity associated with cycA47 was not effectively down-regulated in G1 cells of p21–/– p27–/– p107–/– MEFs (Fig. 5a). Therefore, p107 is required for repression of the cycA47-associated CDK activity when both p21 and p27 are absent.
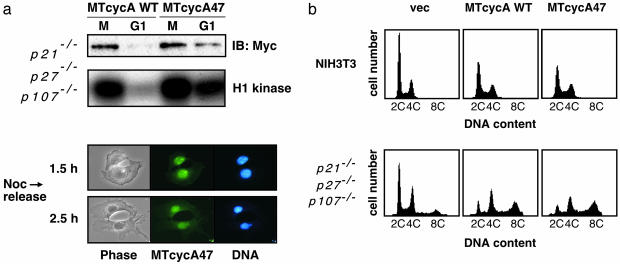
Fig. 5.
p107 regulates cycA47-associated CDK activity. (a Upper) The p21–/– p27–/– p107–/– MEF cells, infected with the myc-tagged cyclin A-carrying retroviruses, were synchronized with nocodazole, and the extracts from nocodazol blocked cells (M) and 2.5 h after release from arrest (G1) were analyzed as in Fig. 3. (Lower) Anti-myc tag immunostaining (green) of MEFs expressing myc-tagged cycA47. MEFs were fixed 1.5 and 2.5 h after the release from nocodazole block. DNA was stained with Hoechst 33342 (blue). (b) NIH 3T3 cells and the p21–/– p27–/– p107–/– MEFs were transiently cotransfected with the CD20-expressing vector and the expression vectors carrying no cyclin (vec), myc-tagged wild-type cyclin A (MTcycA WT), or the cycA47 mutant (MTcycA47). Cell-cycle distribution of the transfected (CD20-positive) cells was analyzed by flow cytometry.
Despite the constitutive activation of cycA47, >90% of the cells completed cytokinesis after release from a nocodazole-induced prometaphase arrest (Fig. 5a). However, stable activation of cyclin A in p21–/– p27–/– p107–/– MEFs was associated with genetic instability. Whereas transient overexpression of wild-type cyclin A or cycA47 in NIH 3T3 cells (Fig. 5b) or in wild-type MEFs (data not shown) caused only a modest increase in the percentage of S and G2/M phase cells, transient overexpression of either wild-type or cycA47 in p21–/– p27–/– p107–/– MEFs resulted in a remarkable decrease in diploid G1 cells and an increase in tetraploid cells (Fig. 5b). This was not accompanied by an increase in binucleated cells (data not shown), suggesting that the rapid appearance of tetraploid cells was due to mitotic failure and/or endoreduplication.
Discussion
Destruction of mitotic cyclins by ubiquitin-dependent proteolysis is a universal mechanism for regulating CDK activity and mitotic exit (11). In addition, CKIs Rux and Sic1 collaborate with cyclin destruction to ensure timely CDK inactivation during mitotic exit in Drosophila and yeast, respectively (21, 22, 24–26). We now show that CKIs also perform this function in mammalian cells. Our major conclusion is that cyclin destruction and CDK inhibition are redundant pathways for controlling cyclin A activity during mitotic exit in mammalian cells. Either pathway alone is sufficient, as no mitotic abnormalities were observed when only one or the other was disabled. However, simultaneous inactivation of both pathways causes rapid and severe genetic instability.
We probed the mechanisms that regulate CDK activity during mitosis in murine cells by constructing cell lines that expressed a mutant version of cyclin A, cycA47, that was relatively stable compared to endogenous cyclin A. CycA47 contained three alanine substitution mutations within the D box, which did not completely prevent cyclin A proteolysis but sufficiently disabled it so that some cyclin A protein was present and bound to CDK throughout mitosis and in G1 cells. The amount of exogenous cycA47 expressed by these cells was not greater than endogenous cyclin A, which likely accounted for our success in producing these viable cell lines, whereas previous studies employing cyclin A overexpression observed cyclin A-dependent mitotic arrest (37–40).
In wild-type fibroblasts, the kinase activity associated with cycA47 was inactivated during mitotic exit. This correlated with its assembly into complexes containing the CKIs p21 and p27 and not with CDK phosphorylation on tyrosine. These results suggest that the p21 and p27 participate in CDK regulation during mitosis. However, genetic experiments designed to confirm this showed that p21 and p27 were not essential mitotic CKIs. Thus, the kinase activity associated with cycA47 was just as effectively inhibited in p21–/– p27–/– cells as it was in control cells.
Previous work showed that the Rb-related proteins p107 and p130 not only regulate E2F-related transcription factors but can also bind to and inhibit CDKs (44). It has also been shown that p130 can substitute for p27 and inhibit Cdk2 in mitogen-starved, p27–/– fibroblasts (45). We now show that p107 has a parallel function in regulating CDK activity during the M-to-G1 transition. In cells lacking both p21 and p27, but not in wild-type cells, p107 was associated with cyclin A during mitotic exit. Moreover, whereas deletion of p21 and p27 alone did not cause constitutive activation of cycA47-CDK in M and G1 phase cells, deletion of p107 in combination with p21 and p27 was sufficient to deregulate cycA47–CDK activity. In MEFs, p107 seemed to perform this function only when p21 and p27 were absent because no p107 was bound to cycA47 in M-phase p21–/– or p27–/– MEFs. It remains to be determined whether p107 might normally perform this function in somatic cells that express lesser amounts of p21 and p27. Another possibility is that the role of p107 in controlling CDK activity may become critically important in pathophysiological settings, such as tumor cells, in which cyclins are overexpressed and p21 and p27 are present in aberrantly low amounts.
In yeast and Drosophila, the CKIs Sic1 and Rux, respectively, play crucial roles in regulating mitotic exit, and loss of those pathways results in genetic instability (24, 47, 48). Neither Rux nor Sic1 is essential because their roles in CDK regulation during mitotic exit are at least partially redundant with APC-mediated cyclin destruction, but both sic1 and rux mutant cells display mitotic abnormalities. rux mutant cells are delayed at metaphase (24), and sic1 mutant cells are delayed in mid-anaphase and display a high frequency of chromosome nondisjunction and breakage (47, 48). p21, p27, and p107 are also nonessential, and we are continuing to explore whether their loss, alone or in combination, causes mitotic defects. Initial observations did not reveal a substantial delay in the timing of mitotic exit in triple knockout cells compared to wild-type MEFs (see Fig. 5a).
The mitotic abnormalities associated with sic1 and rux mutations are greatly exacerbated if the functionally overlapping cyclin destruction pathway is also disabled, for example by expression of a nondegradable cyclin mutant or by ectopic overexpression of a wild-type mitotic cyclin. For instance, expression of a D box mutant of cyclin A in rux mutant cells causes the cells to exit mitosis without separation of sister chromatids (24). We observed a similar phenotype in MEFs lacking p21, p27, and p107. Overexpression of even modest amounts of cyclin A was lethal in p21–/– p27–/– p107–/– MEFs. Moreover, transient and ectopic cyclin A expression in the triple knockout MEFs caused the cells to rapidly become tetraploid, suggestive of a failure to separate sister chromatids during mitotic exit or endoreduplication of DNA. This does not seem to be due to a deregulation of the E2F transcription program (49), as we observed no difference between p21–/– p27–/– and p21–/– p27–/– p107–/– MEFs in the expression of E2F-regulated genes, such as DNA polymerase α and cyclin E (data not shown).
In conclusion, we show that two redundant pathways control cyclin A–CDK activity during mitotic exit in mammalian cells: cyclin destruction and CKIs. The CKIs responsible for mitotic CDK regulation have all been implicated in tumor suppression. Their roles in mitotic exit may contribute to their tumor suppressor function, as corruption of this pathway can cause genetic instability.
Acknowledgments
We thank M. Ohtsubo, B. Clurman, E. Firpo, E. Randel, M. Fero, D. Miller, J. Kato, and members of the Roberts laboratory for helpful discussions, technical assistance, and materials. This work was supported by grants from the National Institutes of Health and from the Japan Society for the Promotion of Science (11660328). J.M.R. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: CDK, cyclin-dependent kinase; D box, destruction box; MEF, mouse embryonic fibroblast; APC, anaphase-promoting complex; CKI, cyclin-dependent kinase inhibitor.
References
- 1.Nurse, P. (1990) Nature 344, 503–508. [DOI] [PubMed] [Google Scholar]
- 2.Hartwell, L. H. (1991) Genetics 129, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts, J. M. (1999) Cell 98, 129–132. [DOI] [PubMed] [Google Scholar]
- 4.Irniger, S., Piatti, S., Michaelis, C. & Nasmyth, K. (1995) Cell 81, 269–278. [DOI] [PubMed] [Google Scholar]
- 5.King, R. W., Peters, J. M., Tugendreich, S., Rolfe, M., Hieter, P. & Kirschner, M. W. (1995) Cell 81, 279–288. [DOI] [PubMed] [Google Scholar]
- 6.Sudakin, V., Ganoth, D., Dahan, A., Heller, H., Hershko, J., Luca, F. C., Ruderman, J. V. & Hershko, A. (1995) Mol. Biol. Cell 6, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glotzer, M., Murray, A. W. & Kirschner, M. W. (1991) Nature 349, 132–138. [DOI] [PubMed] [Google Scholar]
- 8.Schwab, M., Lutum, A. S. & Seufert, W. (1997) Cell 90, 683–693. [DOI] [PubMed] [Google Scholar]
- 9.Pfleger, C. M. & Kirschner, M. W. (2000) Genes Dev. 14, 655–665. [PMC free article] [PubMed] [Google Scholar]
- 10.Yeong, F. M., Lim, H. H., Padmashree, C. G. & Surana, U. (2000) Mol. Cell 5, 501–511. [DOI] [PubMed] [Google Scholar]
- 11.Peters, J. M. (2002) Mol. Cell 9, 931–943. [DOI] [PubMed] [Google Scholar]
- 12.Kim, S. H., Li, C. & Maller, J. L. (1999) Dev. Biol. 212, 381–391. [DOI] [PubMed] [Google Scholar]
- 13.D'Angiolella, V., Costanzo, V., Gottesman, M. E., Avvedimento, E. V., Gautier, J. & Grieco, D. (2001) Curr. Biol. 11, 1221–1226. [DOI] [PubMed] [Google Scholar]
- 14.Rudner, A. D., Hardwick, K. G. & Murray, A. W. (2000) J. Cell Biol. 149, 1361–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendenhall, M. D., al-Jumaily, W. & Nugroho, T. T. (1995) Prog. Cell Cycle Res. 1, 173–185. [DOI] [PubMed] [Google Scholar]
- 16.Martin-Castellanos, C. & Moreno, S. (1996) Prog. Cell Cycle Res. 2, 29–35. [DOI] [PubMed] [Google Scholar]
- 17.Schwob, E., Bohm, T., Mendenhall, M. D. & Nasmyth, K. (1994) Cell 79, 233–244. [DOI] [PubMed] [Google Scholar]
- 18.Schneider, B. L., Yang, Q. H. & Futcher, A. B. (1996) Science 272, 560–562. [DOI] [PubMed] [Google Scholar]
- 19.Benito, J., Martin-Castellanos, C. & Moreno, S. (1998) EMBO J. 17, 482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan, D. O. & Roberts, J. M. (2002) Nature 418, 495–496. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson, M. D., Gray, S., Yuste-Rojas, M. & Cross, F. R. (2000) Mol. Cell. Biol. 20, 4483–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasch, R. & Cross, F. R. (2002) Nature 418, 556–562. [DOI] [PubMed] [Google Scholar]
- 23.Foley, E., O'Farrell, P. H. & Sprenger, F. (1999) Curr. Biol. 9, 1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foley, E. & Sprenger, F. (2001) Curr. Biol. 11, 151–160. [DOI] [PubMed] [Google Scholar]
- 25.Avedisov, S. N., Krasnoselskaya, I., Mortin, M. & Thomas, B. J. (2000) Mol. Cell. Biol. 20, 8220–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprenger, F., Yakubovich, N. & O'Farrell, P. H. (1997) Curr. Biol. 7, 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fero, M. L., Rivkin, M., Tasch, M., Porter, P., Carow, C. E., Firpo, E., Polyak, K., Tsai, L. H., Broudy, V., Perlmutter, R. M., et al. (1996) Cell 85, 733–744. [DOI] [PubMed] [Google Scholar]
- 28.Kunkel, T. A., Roberts, J. D. & Zakour, R. A. (1987) Methods Enzymol. 154, 367–382. [DOI] [PubMed] [Google Scholar]
- 29.Miller, A. D. & Rosman, G. J. (1989) BioTechniques 7, 980–990. [PMC free article] [PubMed] [Google Scholar]
- 30.Ohtsubo, M., Theodoras, A. M., Schumacher, J., Roberts, J. M. & Pagano, M. (1995) Mol. Cell. Biol. 15, 2612–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clurman, B. E., Sheaff, R. J., Thress, K., Groudine, M. & Roberts, J. M. (1996) Genes Dev. 10, 1979–1990. [DOI] [PubMed] [Google Scholar]
- 32.Koff, A., Giordano, A., Desai, D., Yamashita, K., Harper, J. W., Elledge, S., Nishimoto, T., Morgan, D. O., Franza, B. R. & Roberts, J. M. (1992) Science 257, 1689–1694. [DOI] [PubMed] [Google Scholar]
- 33.Marraccino, R. L., Firpo, E. J. & Roberts, J. M. (1992) Mol. Biol. Cell 3, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyle, W. J., van der Geer, P. & Hunter, T. (1991) Methods Enzymol. 201, 110–149. [DOI] [PubMed] [Google Scholar]
- 35.Turner, D. L. & Weintraub, H. (1994) Genes Dev. 8, 1434–1447. [DOI] [PubMed] [Google Scholar]
- 36.van den Heuvel, S. & Harlow, E. (1993) Science 262, 2050–2054. [DOI] [PubMed] [Google Scholar]
- 37.Luca, F. C., Shibuya, E. K., Dohrmann, C. E. & Ruderman, J. V. (1991) EMBO J. 10, 4311–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigrist, S., Jacobs, H., Stratmann, R. & Lehner, C. F. (1995) EMBO J. 14, 4827–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Elzen, N. & Pines, J. (2001) J. Cell Biol. 153, 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geley, S., Kramer, E., Gieffers, C., Gannon, J., Peters, J. M. & Hunt, T. (2001) J. Cell Biol. 153, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan, D. O. (1995) Nature 374, 131–134. [DOI] [PubMed] [Google Scholar]
- 42.Krek, W. & Nigg, E. A. (1991) EMBO J. 10, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu, Y., Rosenblatt, J. & Morgan, D. O. (1992) EMBO J. 11, 3995–4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grana, X., Garriga, J. & Mayol, X. (1998) Oncogene 17, 3365–3383. [DOI] [PubMed] [Google Scholar]
- 45.Coats, S., Whyte, P., Fero, M. L., Lacy, S., Chung, G., Randel, E., Firpo, E. & Roberts, J. M. (1999) Curr. Biol. 9, 163–173. [DOI] [PubMed] [Google Scholar]
- 46.Zhu, L., Harlow, E. & Dynlacht, B. D. (1995) Genes Dev. 9, 1740–1752. [DOI] [PubMed] [Google Scholar]
- 47.Lengronne, A. & Schwob, E. (2002) Mol. Cell 9, 1067–1078. [DOI] [PubMed] [Google Scholar]
- 48.Nugroho, T. T. & Mendenhall, M. D. (1994) Mol. Cell. Biol. 14, 3320–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dyson, N. (1998) Genes Dev. 12, 2245–2262. [DOI] [PubMed] [Google Scholar]