Abstract
Evidence supporting the impact of therapeutic zinc supplementation on the duration and severity of diarrhea among children under five is largely derived from studies conducted in South Asia. China experiences a substantial portion of the global burden of diarrhea, but the impact of zinc treatment among children under five has not been well documented by previously published systematic reviews on the topic. We therefore conducted a systematic literature review, which included an exhaustive search of the Chinese literature, in an effort to update previously published estimates of the effect of therapeutic zinc. We conducted systematic literature searches in various databases, including the China National Knowledge Infrastructure (CNKI), and abstracted relevant data from studies meeting our inclusion and exclusion criteria. We used STATA 12.0 to pool select outcomes and to generate estimates of percentage difference and relative risk comparing outcomes between zinc and control groups. We identified 89 Chinese and 15 non-Chinese studies for the review, including studies in 10 countries from all WHO geographic regions, and analyzed a total of 18,822 diarrhea cases (9469 zinc and 9353 control). None of the included Chinese studies had previously been included in published pooled effect estimates. Chinese and non-Chinese studies reported the effect of therapeutic zinc supplementation on decreased episode duration, stool output, stool frequency, hospitalization duration and proportion of episodes lasting beyond three and seven days. Pooling Chinese and non-Chinese studies yielded an overall 26% (95% CI: 20%−32%) reduction in the estimated relative risk of diarrhea lasting beyond three days among zinc-treated children. Studies conducted in and outside China report reductions in morbidity as a result of oral therapeutic zinc supplementation for acute diarrhea among children under five years of age. The WHO recommendation for zinc treatment of diarrhea episodes should be supported in all low- and middle-income countries.
Keywords: zinc, children, global health, China
1. Introduction
In response to mounting evidence supporting the efficacy and effectiveness of therapeutic zinc supplementation for diarrhea among children under five years of age, the World Health Organization (WHO) and the United Nation’s Children Fund (UNICEF) issued a global recommendation in 2004, which advised zinc supplementation in addition to oral rehydration solution (ORS) for the treatment of all diarrhea episodes among children <5 years of age [1,2]. Systematic reviews have quantified the association between therapeutic zinc supplementation and a reduction in the duration and severity of childhood diarrhea episodes in low- and middle-income countries (LMICs) [1,3,4]. Many of the studies contributing to this body of evidence were conducted in South Asia [5,6,7], but literature stemming from East Asia has not been included in past reviews. In 2011, Zhang published a systematic review which identified 11 Chinese studies assessing zinc treatment for diarrhea and signified the need to update previous meta-analyses with literature published in languages other than English [8].
We sought to conduct an extensive search for studies of oral therapeutic zinc supplementation published in Chinese and any other language. We also aimed to combine evidence across regions in order to generate global estimates of the effect of oral therapeutic zinc supplementation on selected morbidity and mortality outcomes among children under five years of age.
2. Methods
We conducted a systematic literature search for studies published in any language between 1980 and November 2012 using the MeSH search terms “zinc” and “diarrhea” limited to “humans” in the following databases: Biosis, Cumulative Index to Nursing and Allied Health (CINAHL), Cochrane Central Register of Controlled Trials (CENTRAL), Embase, the WHO International Clinical Trials Registry Platform (ICTRP), Global Health, Latin American and Caribbean Health Sciences Literature (LILACS), PubMed, Scopus, Web of Science, IndMed, Egyptian Universities Library Consortium, Index Medicus for the Eastern Mediterranean Region (IMEMR), China National Knowledge Infrastructure (CNKI), WanFang, and Chinese BioMedical (CBM) database.
Titles and abstracts were reviewed by two independent reviewers, and complete manuscripts were obtained for further review of pertinent studies. Discrepancies were resolved in consultation with a third reviewer. We restricted inclusion to individually randomized controlled trials (RCTs) of children under five years of age with acute diarrhea, including dysentery, where diarrhea was defined as the passage of at least three loose or watery stools in a 24-h period. We excluded cluster RCTs, studies that exclusively enrolled a particular subgroup of children (e.g., HIV-infected children; preterm infants), and studies of persistent diarrhea. We included RCTs assessing oral zinc supplementation of any zinc salt in comparison to a control group receiving placebo supplement. For studies conducted in China, where placebo supplements may not have been readily available, we included trials in which cases received the same supportive therapy regardless of zinc allocation. For all studies, administration of minerals (excluding iron), vitamins, and supporting therapy beyond zinc were only considered acceptable if these were received by both the intervention and control groups. Studies that used supplements that included iron, zinc-fortified ORS, or zinc-fortified foods were excluded.
Included studies were reviewed for the following outcomes: diarrhea duration; the proportion of diarrhea episodes lasting >3 and >7 days; duration of hospitalization; duration of fever; duration of vomiting; proportion of cases vomiting; stool frequency (number per day); stool output (mL); and death from diarrhea or any cause. Two independent reviewers entered data into structured tables, and discrepancies were resolved in consultation with a third reviewer.
We conducted independent analyses for studies assessing diarrhea due to unspecified causes and those assessing specific pathogens (e.g., rotavirus) that were laboratory confirmed prior to enrollment. All data analyses were conducted in STATA 12.0 [9]. We fit Poisson and logistic regression models to continuous and binary outcomes, respectively, weighting all outcomes by sample size. These models generated pooled estimates and 95% confidence intervals lower bound by zero for all outcomes and upper bound by one for proportions.
For continuous outcomes, we calculated the overall percentage difference between the pooled estimates for the zinc and control groups. For binary outcomes, we calculated estimates of relative risk (RR) with placebo as the reference group and conducted random effects meta-analyses to combine RRs across studies [9].
We conducted hypothesis testing to assess the equivalence of pooled outcomes and of effect estimates by placebo and non-placebo controlled trials. To compare effect estimates, we tested the difference of mean percentage differences for continuous outcomes and the ratio of relative risks (RRR) for binary outcomes [10]. We subsequently pooled placebo and non-placebo controlled trials for outcomes with no statistically significant difference in effect size.
We assessed the association between the dose of oral zinc supplement and diarrhea duration by regressing the mean percentage difference in diarrhea duration comparing the zinc and control groups onto a categorical variable which indicated whether zinc dose was lower than, equal to, or greater than the WHO recommendation.
During the course of our analyses, we identified a zinc product called Licorzinc that appeared to be unique to China. To determine whether outcomes for Chinese studies were generalizable comparing Licorzinc to other better established zinc products, we conducted hypothesis testing to assess the equivalence of the mean percentage difference in episode duration between zinc and placebo. We also calculated the RRR to compare the RR of episodes lasting >3 days between studies using Licorzinc and other zinc products.
We plotted funnel plots to assess our primary outcomes for publication bias. We also employed the Child Health Epidemiology Reference Group (CHERG) grading system to assess the quality of evidence for each outcome on a four-point scale (“high”, “moderate”, “low”, “very low”) [11].
3. Results
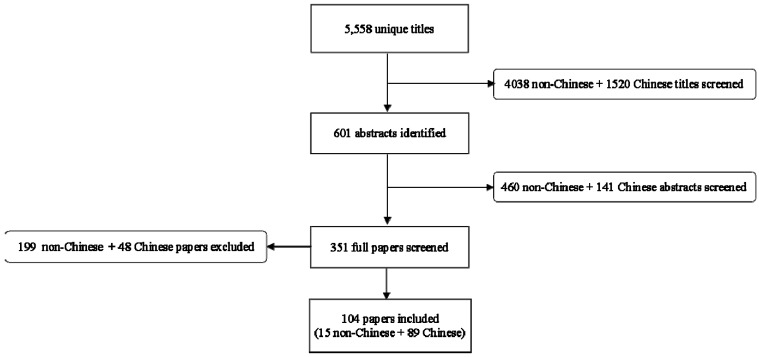
The systematic literature search of the non-Chinese databases uncovered 4038 titles, and 15 were included after subsequent review of abstracts and full manuscripts for inclusion and exclusion criteria (Figure 1) [5,6,7,12,13,14,15,16,17,18,19,20,21,22,23]. Of the included studies, 13 were conducted in a hospital setting and two assessed episodes occurring in the community. Included studies were conducted in sites located within 10 countries: India (n = 6); Bangladesh (n = 5); Nepal (n = 1); Turkey (n = 1); Brazil (n = 1); Pakistan (n = 1); Ethiopia (n = 1); Yemen (n = 1); and Poland (n = 1). These studies enrolled a total of 3271 zinc-allocated and 3314 placebo-allocated diarrhea cases. The systematic literature search for Chinese studies resulted in 1520 titles, of which 89 were included (Figure 1) [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]. All included studies were conducted in a hospital setting, and 33 studies focused on diarrhea attributable to laboratory confirmed rotavirus. None of the included studies identified through the Chinese database were placebo-controlled; for Chinese studies, zinc and control groups received a range of supportive treatments, including fluid infusion, probiotics and antivirals. The total enrolment of included Chinese studies was 6198 zinc group and 6039 control group diarrhea cases. Table 1 describes the trial setting, sample size, and zinc intervention for all included studies.
Figure 1.
Results of systematic literature search and review.
Table 1.
Characteristics of included studies.
| Author [Reference] | Year Published | Country | Trial Setting | Specific Causative Organisms | Age Group (months) | Sample Size | Zinc Salt | Tablet or Syrup | Daily Zinc Dose | Length of Supplementation (days) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zinc Group | Control Group | ||||||||||
| Al Sonboli [17] | 2003 | Brazil | Hospital | Unknown | 3–60 | 37 | 37 | Not Listed | Tablet | 3–5 mos: 22.5 mg 6–60 mos: 45 mg |
5 |
| Bahl [7] | 2002 | India | Community | Unknown | 6–35 | 404 | 401 | Zinc Gluconate | Syrup | 6–11 mos: 15 mg 12–35 mos: 30 mg |
14 |
| Brooks [16] | 2005 | Bangladesh | Hospital | Unknown | 1–6 | 91 | 93 | Zinc Acetate | Syrup | 20 mg | Duration of episode |
| Brooks [16] | 2005 | Bangladesh | Hospital | Unknown | 1–6 | 91 | 93 | Zinc Acetate | Syrup | 5 mg | Duration of episode |
| Dutta [23] | 2011 | India | Hospital | Unknown | 6–24 | 44 | 41 | Not Listed | Syrup | 40 mg | 14 |
| Elnemr [21] | 2007 | Yemen | Hospital | Unknown | 3–24 | 88 | 92 | Zinc Acetate | Syrup | 20 mg | 14 |
| Faruque [12] | 1999 | Bangladesh | Hospital | Unknown | 6–24 | 343 | 341 | Zinc Acetate | Syrup | 14.2 mg | 15 |
| Fischer Walker [19] | 2006 | Pakistan | Hospital | Unknown | 1–5 | 281 | 279 | Zinc Sulfate | Tablet | 10 mg | 14 |
| Fischer Walker [19] | 2006 | India | Hospital | Unknown | 1–5 | 186 | 187 | Zinc Sulfate | Tablet | 10 mg | 14 |
| Fischer Walker [19] | 2006 | Ethiopia | Hospital | Unknown | 1–5 | 87 | 90 | Zinc Sulfate | Tablet | 10 mg | 14 |
| Larson [18] | 2005 | Bangladesh | Hospital | Unknown | 3–59 | 267 | 266 | Zinc Sulfate | Tablet | 20 mg | 10 |
| Patel [20] | 2009 | India | Hospital | Unknown | 6–59 | 264 | 271 | Zinc Sulfate | Syrup | 20 mg | 14 |
| Patro [22] | 2010 | Poland | Hospital | Unknown | 3–48 | 81 | 79 | Zinc Sulfate | Syrup | 3–5 mos: 10 mg 6–48 mos: 20 mg |
10 |
| Polat [15] | 2003 | Turkey | Hospital | Unknown | 2–29 | 52 | 54 | Zinc Sulfate | Syrup | 20 mg | 10 |
| Roy [13] | 1999 | Bangladesh | Hospital | Unknown | 3–24 | 32 | 35 | Zinc Acetate | Syrup | 20 mg | 14 |
| Sachdev [5] | 1988 | India | Hospital | Unknown | 6–18 | 25 | 25 | Zinc Sulfate | Tablet | 40 mg | Not Listed |
| Sazawal [6] | 1995 | India | Hospital | Unknown | 6–35 | 456 | 481 | Zinc Gluconate | Syrup | 20 mg | Not Listed |
| Strand [14] | 2002 | Nepal | Community | Unknown | 6–35 | 442 | 449 | Not Listed | Syrup | 6–11 mos: 15 mg 12–35 mos: 30 mg |
From enrolment until 7 days after episode subsided |
| Zhao [24] | 2011 | China | Hospital | Unknown | 4–36 | 40 | 40 | Licorzinc | Tablet | 4–5 mos: 10.8 mg 6–12 mos: 14.4 mg 13–36 mos: 21.6 mg |
Not Listed |
| Zhang [25] | 2009 | China | Hospital | Rotavirus | 6–24 | 60 | 60 | Zinc Gluconate | Not Listed | 20 mg | Duration of episode |
| Lin [26] | 2010 | China | Hospital | Rotavirus | 1.5–36 | 58 | 58 | Zinc Gluconate | Syrup | 1.5–5 mos: 10 mg 6–36 mos: 20 mg |
Duration of episode |
| Zhou [27] | 2010 | China | Hospital | Rotavirus | 6–24 | 42 | 40 | Zinc Gluconate | Not Listed | 20 mg | 14 |
| Yang [28] | 2011 | China | Hospital | Unknown | 3–36 | 42 | 40 | Zinc Gluconate | Tablet | 3–5 mos: 10 mg 6–36 mos: 20 mg |
10–14 |
| Liu [29] | 2010 | China | Hospital | Unknown | 5–18 | 40 | 40 | Zinc Gluconate | Not Listed | 5 mos: 10 mg 6–18 mos: 20 mg |
10–14 |
| Chen [30] | 2006 | China | Hospital | Rotavirus | 0–24 | 30 | 30 | Zinc gluconate | Not Listed | 10 mg | Not Listed |
| Liu [31] | 2011 | China | Hospital | Unknown | 6.8–22 | 90 | 90 | Zinc Gluconate | Tablet | 20 mg | Not Listed |
| Liu [32] | 2009 | China | Hospital | Unknown | 6–36 | 112 | 108 | Zinc Gluconate | Tablet | 20 mg | 10 |
| Fu [33] | 2010 | China | Hospital | Rotavirus | 2–24 | 98 | 102 | Zinc Gluconate | Syrup | 5 mg | Not Listed |
| Zhou [34] | 2008 | China | Hospital | Unknown | 2–48 | 40 | 40 | Licorzinc | Not Listed | 2–5 mos: 7.5 mg 6–12 mos: 11.25 mg 13–48 mos: 18.75 mg |
10–14 |
| Chen [35] | 2008 | China | Hospital | Rotavirus | 4–48 | 60 | 60 | Licorzinc | Not Listed | 4–5 mos: 7.2 mg 6–48 mos: 10.8 mg |
Not Listed |
| Guan [36] | 2012 | China | Hospital | Rotavirus | 1.5–45.6 | 45 | 45 | Licorzinc | Not Listed | 1.5–5 mos: 7.5 mg 6–11 mos: 11.25 mg 12–45.6 mos: 18.75 mg |
10–14 |
| Wu [37] | 2010 | China | Hospital | Rotavirus | 4–13 | 46 | 46 | Licorzinc | Not Listed | 4–5 mos: 10 mg 6–13 mos: 20 mg |
Not Listed |
| Zhou [38] | 2010 | China | Hospital | Unknown | 6–24 | 65 | 60 | Licorzinc | Tablet | 20 mg | Not Listed |
| Luo [39] | 2009 | China | Hospital | rotavirus | 6–36 | 55 | 50 | Licorzinc | Tablet | 18.75 mg | Not Listed |
| Zhang [40] | 2010 | China | Hospital | Unknown | 5–48 | 50 | 50 | Licorzinc | Not Listed | Not Listed * | Not Listed |
| Ju [41] | 2007 | China | Hospital | Unknown | 6–36 | 40 | 38 | Licorzinc | Tablet | 6–12 mos: 11–25 mg 13–36 mos: 15 mg |
Not Listed |
| Wang [42] | 2012 | China | Hospital | Unknown | 6–36 | 30 | 30 | Licorzinc | Tablet | Not Listed * | 3 |
| Hong [43] | 2009 | China | Hospital | Rotavirus | 3–60 | 140 | 120 | Zinc Sulfate | Syrup | 3–11 mos: 20 mg 12–36 mos: 30 mg 37–60 mos: 40 mg |
Not Listed |
| Lin [44] | 1994 | China | Hospital | Unknown | 0.5–24 | 46 | 58 | Zinc Sulfate | Syrup | 10–14 mg/kg * | Not Listed |
| Yan [45] | 2011 | China | Hospital | Unknown | 5–36 | 70 | 50 | Zinc Sulfate | Syrup | 5 mos: 50 mg 6–36 mos: 100 mg |
Not Listed |
| He [46] | 1997 | China | Hospital | Unknown | 6–36 | 52 | 58 | Zinc Gluconate | Not Listed | 20 mg | Not Listed |
| Wei [47] | 2011 | China | Hospital | Unknown | 3–36 | 44 | 42 | Zinc Gluconate | Syrup | 3–5 mos: 10 mg 6–36 mos: 20 mg |
10–14 |
| Yang [48] | 2012 | China | Hospital | Unknown | 0–36 | 80 | 80 | Zinc Gluconate | Tablet | 0–5 mos: 10 mg 6–36 mos: 20 mg |
10 |
| Pu [49] | 2010 | China | Hospital | Rotavirus | 0–24 | 38 | 34 | Zinc Gluconate | Not Listed | 0–5 mos: 10 mg 6–24 mos: 20 mg |
Not Listed |
| Zhang [50] | 2011 | China | Hospital | Rotavirus | 3–36 | 53 | 53 | Zinc Gluconate | Not Listed | 3–5 mos: 10 mg 6–36 mos: 20 mg |
10 |
| Sun [51] | 2008 | China | Hospital | Unknown | 1.5–36 | 45 | 45 | Zinc Gluconate | Syrup | 1.5–5 mos: 10 mg 6–36 mos: 20 mg |
Not Listed |
| Zhang [52] | 2011 | China | Hospital | Unknown | 3–36 | 90 | 90 | Zinc Gluconate | Syrup | 3–5 mos: 10 mg 6–36 mos: 20 mg |
Not Listed |
| Lin [53] | 2010 | China | Hospital | Rotavirus | 6–54 | 28 | 20 | Zinc Gluconate | Tablet | 6–54 mos: 20 mg | 14 |
| Liu [54] | 2009 | China | Hospital | Unknown | 3–36 | 95 | 91 | Zinc Gluconate | Not Listed | 3–5 mos: 10 mg 6–36 mos: 20 mg |
10–14 |
| Qiao [55] | 2011 | China | Hospital | Unknown | 6–36 | 73 | 72 | Zinc Gluconate | Tablet | 6–36 mos: 20 mg | 14 |
| Zhang [56] | 2007 | China | Hospital | Unknown | 0–24 | 85 | 90 | Zinc Gluconate | Not Listed | 0–5 mos: 10 mg 6–24 mos: 20 mg |
10 |
| Zhao [57] | 2012 | China | Hospital | Unknown | 0–24 | 70 | 70 | Zinc Gluconate | Syrup | 0–5 mos: 10 mg 6–24 mos: 20 mg |
10–14 |
| Cai [58] | 2011 | China | Hospital | Unknown | 0–24 | 88 | 84 | Zinc Gluconate | Not Listed | 0–5 mos: 10 mg 6–24 mos: 20 mg |
14 |
| Zhang [59] | 2012 | China | Hospital | Rotavirus | 6–17 | 120 | 120 | Zinc Gluconate | Tablet | 20 mg | 10–14 |
| Qiao [60] | 2012 | China | Hospital | Unknown | 0–24 | 85 | 85 | Zinc Gluconate | Not Listed | 0–5 mos: 10 mg 6–24 mos: 20 mg |
10 |
| Zhong [61] | 2012 | China | Hospital | Rotavirus | 3–48 | 50 | 50 | Zinc Gluconate | Tablet | 3–5 mos: 10 mg 6–48 mos: 20 mg |
10 |
| Wang [62] | 2011 | China | Hospital | Rotavirus | 0–24 | 60 | 60 | Zinc Gluconate | Not Listed | 0–5 mos: 10 mg 6–24 mos: 20 mg |
10 |
| Yang [63] | 2008 | China | Hospital | Rotavirus | 0–36 | 164 | 168 | Zinc Gluconate | Not Listed | 0–5 mos: 10 mg 6–36 mos: 20 mg |
10 |
| Zhao [64] | 2012 | China | Hospital | Rotavirus | 6–36 | 60 | 60 | Zinc Gluconate | Syrup | 35 mg | 10 |
| Ma [65] | 2012 | China | Hospital | Rotavirus | 4–42 | 41 | 41 | Zinc Gluconate | Not Listed | 20 mg | Not Listed |
| Chen [66] | 2012 | China | Hospital | Rotavirus | 0–36 | 93 | 93 | Zinc Gluconate | Not Listed | 0–5 mos: 10 mg 6–36 mos: 20 mg |
10 |
| Hu [67] | 2009 | China | Hospital | Rotavirus | 4–36 | 60 | 60 | Zinc Gluconate | Tablet | 4–5 mos: 10 mg 6–36 mos: 20 mg |
10 |
| Yuan [68] | 2011 | China | Hospital | Unknown | 1–36 | 100 | 100 | Zinc Gluconate | Tablet | 1–12 mos: 70 mg 13–36 mos: 140 mg |
14 |
| Tan [69] | 2011 | China | Hospital | Unknown | 3–36 | 50 | 35 | Zinc Gluconate | Tablet | 3–5 mos: 10 mg 6–36 mos: 20 mg |
10–14 |
| Liu [70] | 2010 | China | Hospital | Unknown | 0–36 | 89 | 77 | Zinc Gluconate | Syrup | 0–5 mos: 10 mg 6–36 mos: 20 mg |
10 |
| Hu [71] | 2011 | China | Hospital | Unknown | 3–60 | 108 | 100 | Zinc Gluconate | Tablet | 3–5 mos: 10 mg 6–60 mos: 20 mg |
14 |
| Li [72] | 2008 | China | Hospital | Unknown | 6–36 | 40 | 38 | Zinc Gluconate | Tablet | 6–12 mos: 7.5 mg 13–36 mos: 15 mg |
3 |
| Gao [73] | 2012 | China | Hospital | Unknown | 3–36 | 74 | 74 | Zinc Gluconate | Not Listed | 3–5 mos: 10 mg 6–36 mos: 20 mg |
14 |
| Wu [74] | 2011 | China | Hospital | Unknown | 3–60 | 20 | 20 | Zinc Sulfate | Syrup | 10 mg | 10 |
| Wu [74] | 2011 | China | Hospital | Unknown | 3–60 | 20 | 20 | Zinc Sulfate | Not Listed | 10 mg | 10 |
| Liu [75] | 2011 | China | Hospital | Unknown | 3–60 | 54 | 53 | Zinc Gluconate | Tablet | 3–5 mos: 10 mg 6–60 mos: 20 mg |
3–5 |
| Chen [76] | 2010 | China | Hospital | Unknown | 5–36 | 42 | 20 | Zinc Gluconate | Not Listed | 5 mos: 10 mg 6–36 mos: 20 mg |
10–14 |
| Ma [77] | 2012 | China | Hospital | Unknown | 2–36 | 63 | 63 | Zinc Gluconate | Not Listed | 2–5 mos: 70 mg 6–36 mos: 140 mg |
10–14 |
| Lu [78] | 2012 | China | Hospital | Unknown | 6–18 | 120 | 140 | Zinc Gluconate | Not Listed | 140 mg | 10–14 |
| Ma [79] | 2012 | China | Hospital | Unknown | 6–36 | 58 | 52 | Zinc Gluconate | Syrup | 6–36 mos: 20 mg | 10 |
| Ao [80] | 2012 | China | Hospital | Rotavirus | 0–24 | 87 | 80 | Zinc Gluconate | Syrup | 0–5 mos: 10 mg 6–24 mos: 20 mg |
Not Listed |
| Gu [81] | 2011 | China | Hospital | Unknown | 3–60 | 56 | 60 | Zinc Gluconate | Syrup | 3–5 mos: 10 mg 6–60 mos: 20 mg |
10 |
| Wen [82] | 2006 | China | Hospital | Unknown | 0–24 | 30 | 29 | Zinc Gluconate | Not Listed | 20 mg | 10–14 |
| Wang [83] | 2011 | China | Hospital | Unknown | 3–36 | 60 | 60 | Licorzinc | Not Listed | 10–20 mg * | Duration of episode |
| Liu [84] | 2012 | China | Hospital | Rotavirus | 8–30 | 90 | 90 | Licorzinc | Not Listed | 8–30 mos: 20 mg | Not Listed |
| Liu [85] | 2012 | China | Hospital | Unknown | 3–60 | 100 | 100 | Licorzinc | Tablet | 3–5 mos: 10 mg 6–60 mos: 20 mg |
Not Listed |
| Tong [86] | 2011 | China | Hospital | Unknown | 2–36 | 98 | 98 | Licorzinc | Not Listed | 2–5 mos: 10 mg 6–36 mos: 20 mg |
Not Listed |
| Qiu [87] | 2010 | China | Hospital | Rotavirus | 1–24 | 53 | 52 | Licorzinc | Tablet | 1–5 mos: 10 mg 6–24 mos: 20 mg |
14 |
| Kong [88] | 2011 | China | Hospital | Unknown | 3–30 | 35 | 35 | Zinc Gluconate | Tablet | 3–5 mos: 10 mg 6–11 mos: 15 mg 12–30 mos: 20 mg |
14 |
| He [89] | 2007 | China | Hospital | Rotavirus | 5–22 | 60 | 63 | Zinc Gluconate | Not Listed | 20 mg | Not Listed |
| Kang [90] | 2010 | China | Hospital | Rotavirus | 6–36 | 92 | 80 | Zinc Gluconate | Tablet | 20 mg | 14 |
| Su [91] | 2012 | China | Hospital | Rotavirus | 6–36 | 97 | 97 | Zinc Gluconate | Not Listed | 20 mg | Not Listed |
| Huang [92] | 2010 | China | Hospital | Rotavirus | 2–36 | 100 | 100 | Not Listed | Tablet | 2–5 mos: 10 mg 6–36 mos: 20 mg |
Not Listed |
| Zhang [93] | 2006 | China | Hospital | Unknown | 0–36 | 83 | 63 | Licorzinc | Syrup | 0–5 mos: 10 mg 6–36 mos: 20 mg |
10–14 |
| Wang [94] | 2012 | China | Hospital | Unknown | 4–30 | 60 | 60 | Zinc Gluconate | Syrup | 10 mg | Not Listed |
| Lin [95] | 2008 | China | Hospital | Unknown | 0.5–34 | 60 | 60 | Zinc Gluconate | Tablet | 0.5–5 mos: 140 mg 6–34 mos: 280 mg |
10–14 |
| Yan [96] | 2011 | China | Hospital | Unknown | 6–60 | 57 | 57 | Zinc Gluconate | Tablet | 20 mg | 10 |
| Yu [97] | 2012 | China | Hospital | Unknown | 0–36 | 40 | 40 | Zinc Gluconate | Tablet | 0–5 mos: 10 mg 6–36 mos: 20 mg |
10–14 |
| Zhang [98] | 2011 | China | Hospital | Rotavirus | 4–36 | 128 | 128 | Zinc Gluconate | Syrup | 4–5 mos: 10 mg 6–36 mos: 20 mg |
14 |
| Xu [99] | 2010 | China | Hospital | Rotavirus | 2–36 | 84 | 83 | Zinc Gluconate | Not Listed | 2–5 mos: 10 mg 6–36 mos: 20 mg |
14 |
| Tan [100] | 2010 | China | Hospital | Unknown | 3.5–60 | 55 | 55 | Zinc Gluconate | Syrup | 3.5–5 mos: 10 mg 6–60 mos: 20 mg |
10–14 |
| Shen [101] | 2012 | China | Hospital | Rotavirus | 2.5–40 | 46 | 42 | Zinc Gluconate | Not Listed | 2.5–5 mos: 10 mg 6–40 mos: 20 mg |
Duration of episode |
| Wang [102] | 2010 | China | Hospital | Unknown | 6–48 | 52 | 51 | Zinc Gluconate | Tablet | 20 mg | Not Listed |
| Chen [103] | 2011 | China | Hospital | Unknown | 1–36 | 50 | 50 | Zinc Gluconate | Tablet | 1–5 mos: 5 mg 6–36 mos: 10 mg |
Not Listed |
| Meng [104] | 2012 | China | Hospital | Unknown | 0–24 | 90 | 90 | Zinc Gluconate | Tablet | 0–5 mos: 2.5 mg 6–12 mos: 5 mg 13–24 mos: 10 mg |
Not Listed |
| Zhong [105] | 2010 | China | Hospital | Unknown | 1–24 | 60 | 60 | Zinc Gluconate | Tablet | 1–5 mos: 2.5 mg 6–12 mos: 5 mg 13–24 mos: 7.5 mg |
5–7 |
| Xie [106] | 2010 | China | Hospital | Rotavirus | 6–36 | 128 | 124 | Zinc Gluconate | Tablet | 20 mg | Not Listed |
| Fan [107] | 2012 | China | Hospital | Unknown | 0–36 | 163 | 121 | Not Listed | Not Listed | 0–5 mos: 10 mg 6–36 mos: 20 mg |
10 |
| Zhou [108] | 2012 | China | Hospital | Rotavirus | 6–24 | 75 | 75 | Zinc Gluconate | Syrup | 20 mg | 10–14 |
| Zhao [109] | 2008 | China | Hospital | Unknown | 0–36 | 44 | 43 | Zinc Gluconate | Tablet | 0–5 mos: 10 mg 6–24 mos: 20 mg |
Not Listed |
| Wan [110] | 2006 | China | Hospital | Unknown | 6–36 | 26 | 24 | Not Listed | Not Listed | Not Listed | Not Listed |
| Yang [111] | 2012 | China | Hospital | Unknown | 6–60 | 60 | 60 | Not Listed | Not Listed | 20 mg | Not Listed |
| Luo [112] | 2012 | China | Hospital | Unknown | 0–36 | 168 | 196 | Not Listed | Not Listed | 0–5 mos: 10 mg 6–36 mos: 20 mg |
Not Listed |
* Study not included in dose analyses.
The results of the studies identified through non-Chinese databases are summarized in Table 2 and Table 3. Acute episodes were 4% (95% CI: 1%–8%) shorter in duration among children treated with zinc compared to those receiving placebo (Table 2). Among children hospitalized for diarrhea, the duration of hospitalization was reduced by 37% (95% CI: 21%–53%) comparing the zinc and control groups (Table 2). Stool frequency was decreased by 6% (95% CI: 2%–10%) among zinc-treated children. Zinc-treated children had a reduced relative risk (RR) of acute diarrhea lasting beyond three and seven days and an increased risk of vomiting (RR: 1.83; 95% CI: 1.40–2.39) (Table 3).
Table 2.
Pooled means of select outcomes for non-Chinese studies.
| Outcome | Study Sites 1 | Pooled Mean (95% CI) 2 | Percent Difference 3 | |
|---|---|---|---|---|
| N | Zinc Group | Control Group | (%) | |
| Duration of Episode (days) | 13 | 3.51 (3.43–3.60) | 3.67 (3.59–3.76) | −4.4 (−7.8, −1.0) |
| Duration of Hospitalization (days) | 1 | 2.00 (1.99–2.01) | 3.17 (2.38–3.96) | −36.9 (−52.6, −21.2) |
| Stool Output (mL) | 2 | 391.2 (388.5–393.8) | 388.8 (386.2–391.5) | 0.6 (−0.3, 1.6) |
| Stool Frequency (Number per day) | 6 | 5.04 (4.88–5.19) | 5.36 (5.20–5.52) | −6.0 (−9.9, −2.0) |
1 Individual studies may contribute more than one study site (N) to each estimate; 2 Estimates for ≥2 study sites generated by Poisson regression model weighted by sample size; 3 Percent difference calculated by: 100 × ((Pooled Zinc Estimate − Pooled Control Estimate)/Pooled Control Estimate); 95% CI calculated by: Percent Difference ± 1.96 × {|(meanzinc/meancontrol)| × sqrt[(std errorzinc)2/(meanzinc)2 + (std errorcontrol)2/(meancontrol)2]} × 100.
Table 3.
Pooled relative risk of select outcomes for non-Chinese studies.
| Outcome | Study Sites 1 | Pooled Estimate Percentage (95% CI) 2 | Pooled Relative Risk 3 | |
|---|---|---|---|---|
| N | Zinc Group | Control Group | RR (95% CI) | |
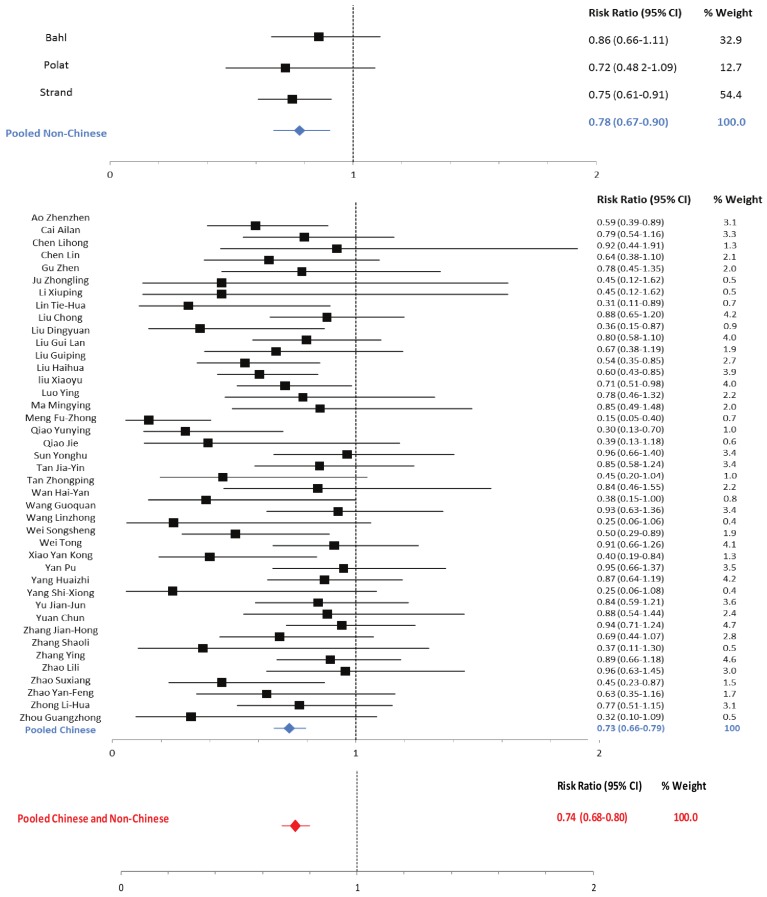
| Episodes > 3 days (%) | 3 | 29.7 (26.7–32.7) | 39.5 (36.3–42.7) | 0.78 (0.67–0.90) |
| Episodes > 7 days (%) | 6 | 10.3 (8.9–11.7) | 14.9 (13.2–16.5) | 0.74 (0.55–0.99) |
| Vomiting (%) | 3 | 18.8 (16.0–21.6) | 9.4 (7.3–11.4) | 1.83 (1.40–2.39) |
1 Individual studies may contribute more than one study site (N) to each estimate; 2 Estimates for ≥2 study sites generated by logistic regression model weighted by sample size; 3 Estimates for ≥2 studies generated by random effects meta-analysis.
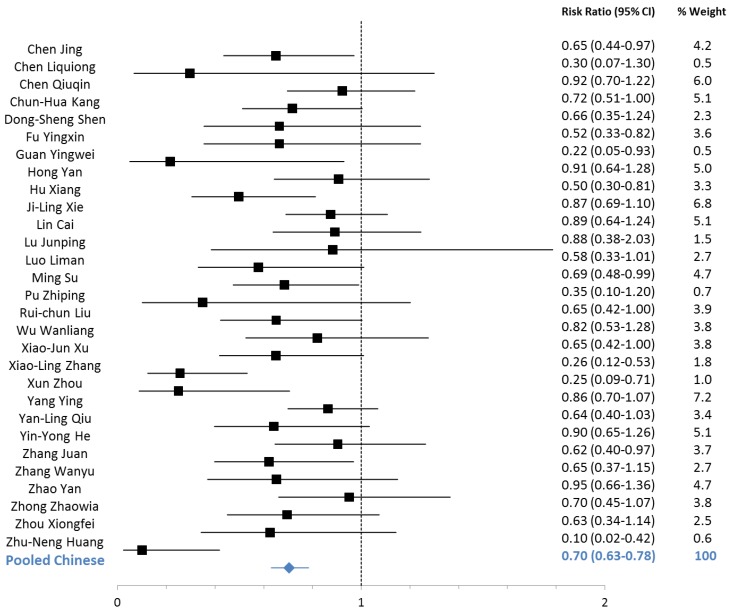
Outcomes pooled across studies conducted in China showed reductions in the duration of diarrhea, hospitalization, fever, vomiting, stool output and stool frequency among zinc-treated children with acute diarrhea attributable to rotavirus and to non-specific causes (Table 4). The reduction in the duration of diarrhea was 37% (95% CI: 35%–39%) among non-specific episodes and 31% (95% CI: 29%–34%) among rotavirus episodes (Table 4). The RR of diarrhea lasting beyond three days was reduced among zinc-treated patients with non-specific (RR: 0.73; 95% CI: 0.66–0.79) and rotavirus (RR: 0.70; 95% CI: 0.63–0.78) diarrhea (Table 5; Figure 2 and Figure 3).
Table 4.
Pooled means of select outcomes for Chinese studies.
| Outcome | Specific Causative Pathogens | Study Sites 1 | Pooled Mean (95% CI) 2 |
Percent Difference 3 | |
|---|---|---|---|---|---|
| N | Zinc Group | Control Group | (%) | ||
| Duration of Episode (days) | Unknown | 40 | 2.96 (2.90–3.03) | 4.68 (4.60–4.77) | −36.8 (−38.7, −34.8) |
| Rotavirus | 24 | 3.45 (3.36–3.54) | 5.01 (4.89–5.12) | −31.1 (−33.5, −28.8) | |
| Duration of Hospitalization (days) | Unknown | 10 | 4.65 (4.50–4.80) | 6.43 (6.25–6.61) | −27.7 (−30.8, −24.6) |
| Rotavirus | 2 | 4.15 (3.79–4.51) | 6.1 (5.66–6.54) | −32.0 (−39.6, −24.3) | |
| Duration of Fever (days) | Unknown | 13 | 1.90 (1.80–1.99) | 2.81 (2.70–2.92) | −32.4 (−36.5, −28.2) |
| Rotavirus | 4 | 1.96 (1.78–2.14) | 3.18 (2.95–3.41) | −38.4 (−45.6, −31.2) | |
| Duration of Vomiting (days) | Unknown | 6 | 1.15 (1.05–1.25) | 1.53 (1.41–1.64) | −24.8 (−33.3, −16.4) |
| Rotavirus | 3 | 1.84 (1.64–2.04) | 2.49 (2.26–2.72) | −26.1 (−36.6, −15.6) | |
| Stool Output (mL) | Unknown | 1 | 40 (38.1–41.9) | 70 (68.0–72.0) | −42.9 (−46.0, −39.7) |
| Rotavirus | 1 | 278.4 (256.8–300.0) | 425.4 (382.1–468.7) | −34.6 (−42.9, −26.2) | |
| Stool Frequency (Number per day) | Unknown | 1 | 4 (3.8–4.2) | 8 (7.6–8.4) | −50.0 (−53.5, −46.5) |
| Rotavirus | 2 | 3.74 (3.30–4.18) | 4.27 (3.77–4.77) | −12.4 (−27.0, 2.1) | |
1 Individual studies may contribute more than one study site (N) to each estimate; 2 Estimates for ≥2 study sites generated by Poisson regression model weighted by sample size; 3 Percent difference calculated by: 100 × ((Pooled Zinc Estimate − Pooled Control Estimate)/Pooled Control Estimate); 95% CI calculated by: Percent Difference ± 1.96 × {|(meanzinc/meancontrol)| × sqrt[(std errorzinc)2/(meanzinc)2 + (std errorcontrol)2/(meancontrol)2]} × 100.
Table 5.
Pooled relative risk of select outcomes for Chinese studies.
| Outcome | Specific Causative Pathogens | Study Sites 1 | Pooled Estimate Percentage (95% CI) 2 | Relative Risk 3 | |
|---|---|---|---|---|---|
| N | Zinc Group | Control Group | RR (95% CI) | ||
| Episodes > 3 days (%) | Unknown | 44 | 31.4 (29.4–33.5) | 49.2 (46.6–51.8) | 0.73 (0.66–0.79) |
| Rotavirus | 29 | 31.8 (29.5–34.1) | 50.3 (47.4–53.3) | 0.70 (0.63–0.78) | |
| Episodes > 7 days (%) | Unknown | 1 | 26.9 (-) | 39.2 (-) | 0.75 (0.42–1.37) |
1 Individual studies may contribute more than one study site (N) to each estimate; 2 Estimates for ≥2 study sites generated by Poisson regression model weighted by sample size; 3 Estimates for ≥2 studies generated by random effects meta-analysis.
Figure 2.
Forest plot for the effect of therapeutic zinc supplementation on Rotavirus diarrhea episodes >3 days.
Figure 3.
Forest plot for the effect of therapeutic zinc supplementation on non-specific diarrhea episodes lasting >3 days.
We did not identify any studies reporting diarrhea-specific or all-cause mortality for inclusion in this review. Nor did we identify non-Chinese studies reporting duration of fever or vomiting, or Chinese studies reporting the proportion of children vomiting.
The mean episode duration and proportion of episodes lasting >3 days were not statistically significantly different comparing zinc-treated children in Chinese and non-Chinese studies. There was no statistically significant difference between the estimated relative risk of an episode lasting >3 days (RRR: 1.07; 95% CI: 0.90–1.27) comparing Chinese and non-Chinese studies; therefore, we pooled this outcome across regions (RR: 0.74; 95% CI: 0.68–0.80) (Figure 3). The percentage difference between the mean episode duration of zinc-treated and control group children was statistically significantly larger for Chinese compared to non-Chinese studies (p < 0.05), so this outcome was not pooled across regions. We did not have sufficient power to compare other commonly reported outcomes by region.
Zinc dose was not associated with the mean percent difference in diarrhea duration comparing zinc and control children for non-Chinese (p = 0.50) or Chinese (p = 0.12) studies. Comparing Chinese studies that used Licorzinc to those that used other zinc supplements, there were no statistically significant differences in the mean percent difference in the duration of rotavirus episodes (p = 0.56), the RR of non-specific episodes lasting >3 days (RRR: 0.99; 95% CI: 0.72–1.35), or the RR of rotavirus episodes lasting >3 days (RRR: 0.93; 95% CI: 0.68–1.26). The percentage difference in the mean duration of non-specific episodes comparing zinc and control group children was statistically significantly higher for Licorzinc compared to “other zinc” studies (p = 0.01).
Our assessment of publication bias yielded largely symmetrical funnel plots for all outcomes.
Under the CHERG grading system, the studies included in this review were of moderate quality (Table 6) [11]. Effect estimates were largely consistent in directionality for all outcomes.
Table 6.
Quality assessment of studies measuring the association between therapeutic zinc supplementation and selected outcomes.
| Number of Studies | Design | Limitations | Consistency | Directness | |
|---|---|---|---|---|---|
| Generalizability to Population of Interest | Generalizability to Intervention of Interest | ||||
| Diarrhea Duration (mean): Moderate outcome-specific quality 1 | |||||
| 53 non-specific 24 Rotavirus |
RCT | Chinese studies not placebo-controlled (−0.5) | All but 4 studies showing decreased mean duration of diarrhea among zinc-treated children (+1) | Mostly South Asia and China (−0.5) | Generalizable |
| Diarrhea Duration (>3 days): Moderate outcome-specific quality 1 | |||||
| 47 non-specific 29 Rotavirus |
RCT | Chinese studies not placebo-controlled (−0.5) | All studies showing decreased risk of diarrhea duration >3 days among zinc-treated children (+1) | Mostly South Asia and China (−0.5) | Generalizable |
| Diarrhea Duration (>7 days): Moderate outcome-specific quality 1 | |||||
| 7 non-specific | RCT | Chinese studies not placebo-controlled (−0.5) | All but one study showing decreased risk of diarrhea duration >7 days among zinc-treated children (+1) | Mostly South Asia and China (−0.5) | Generalizable |
| Hospitalizations Duration: Moderate outcome-specific quality 1 | |||||
| 11 non-specific 2 Rotavirus |
RCT | Chinese studies not placebo-controlled (−0.5) | All studies showing decreased mean duration of hospitalization among zinc-treated children (+1) | Only one non-Chinese study (−0.5) | Generalizable |
| Stool Output: Moderate outcome-specific quality 1 | |||||
| 3 non-specific 1 Rotavirus |
RCT | Chinese studies not placebo-controlled (−0.5) | All but one study showing decreased stool output among zinc-treated children (+1) | Only South Asia and China (−0.5) | Generalizable |
| Stool Frequency: Moderate outcome-specific quality 1 | |||||
| 7 non-specific 2 Rotavirus |
RCT | Chinese studies not placebo-controlled (−0.5) | All but three studies showing decreased stool frequency among zinc-treated children (+1) | Mostly South Asia and China (−0.5) | Generalizable |
| Vomiting: Moderate outcome-specific quality 1 | |||||
| 3 non-specific | RCT | None | All studies showing increased vomiting among zinc-treated children (+1) | No Chinese studies (−0.5) | Generalizable |
| Vomiting Duration: Moderate outcome-specific quality 1 | |||||
| 6 non-specific 3 Rotavirus |
RCT | Chinese studies not placebo-controlled (−0.5) | All but one study showing decreased duration of vomiting among zinc-treated children (+1) | No non-Chinese studies (−0.5) | Generalizable |
| Fever Duration: Moderate outcome-specific quality 1 | |||||
| 13 non-specific 4 Rotavirus |
RCT | Chinese studies not placebo-controlled (−0.5) | All studies showing decreased duration of fever among zinc-treated children (+1) | No non-Chinese studies (−0.5) | Generalizable |
1 Quality assessment scoring based on previously published criteria [11].
4. Discussion
The findings of our systematic review confirm and highlight the benefits of therapeutic zinc supplementation for diarrhea among children under five years of age in low- and middle-income countries. The effects of zinc treatment, which include reductions in episode duration, stool output, stool frequency and length of hospitalization, were consistent across Chinese and non-Chinese studies and non-specific and rotavirus diarrhea. These results suggest that zinc therapy of diarrhea is largely beneficial and important in both low- and middle-income settings.
The results of the large number of Chinese trials in rotavirus diarrhea are a substantial addition to the global evidence base because there have been no non-Chinese trials. One study in India based on a post-hoc subgroup analysis suggested that zinc treatment was not beneficial for rotavirus diarrhea [113]; however, the evidence from China demonstrates that therapeutic zinc supplementation reduces the duration and severity of rotavirus episodes. As rotavirus is the predominant cause of severe acute diarrhea worldwide and most likely the leading cause of diarrhea mortality [114], zinc treatment of rotavirus diarrhea could potentially yield large reductions in hospitalizations and deaths.
In comparison to non-Chinese studies, the difference between the mean episode duration of zinc-treated and control group children was statistically significantly higher for Chinese studies (p < 0.05). It is possible that this difference resulted from lack of placebo-controlled groups and blinding among Chinese studies. However, estimates of the effects of therapeutic zinc supplementation on other outcomes were largely consistent across study locations and we were able to generate a pooled global effect size for the proportion of episodes >3days. The consistency of effect estimates between studies conducted in and outside China suggests that the lack of placebo-controlled groups in Chinese studies did not greatly bias the results.
Zinc dose did not affect the estimate of the effect of zinc supplementation on the duration of diarrhea for non-Chinese or Chinese studies. Although Licorzinc was associated with slightly greater reductions in the mean duration of non-specific diarrhea than other zinc products, zinc effect sizes were generally comparable across Chinese studies regardless of type of zinc preparation.
There is a dearth of literature meeting our inclusion criteria that assessed diarrhea-specific and all-cause mortality. Although a previous review published mortality effect estimates [4], the sole study reporting diarrhea-specific deaths was cluster-randomized and thus violated our inclusion criteria [115]. In addition, three studies of all-cause mortality were also excluded from our review; one was on persistent diarrhea [116], and two others were review papers [3,117].
Using previously published scoring criteria, the studies included in our review yielded pooled estimates of overall moderate quality [11]. The majority of studies contributing to this review were conducted in China and South Asia; however, studies conducted outside Asia were consistent in the directionality of effect estimates. The consistency and quality of all outcomes bolsters the evidence in support of oral zinc supplementation for the treatment of acute diarrhea among children under five in low- and middle-income countries.
5. Conclusions
Oral therapeutic zinc supplementation reduces the morbidity of acute diarrhea among children under five in and outside China. Global efforts should be made to support scale-up of the WHO recommended regimen of therapeutic zinc in all regions.
Acknowledgments
We would like to thank Wei-Ju Chen, Xun Luo and Wenze Zhang for reviewing and abstracting data from Chinese publications. Financial support for this review was provided by the Bill and Melinda Gates Foundation to the US Fund for UNICEF for the ongoing work of the Child Health Epidemiology Reference Group (CHERG).
Authors’ Contributions
LML conducted the systematic review of non-Chinese studies, analysis and led the initial manuscript preparation. CLFW assisted with the analysis and the manuscript preparation. KC and WYJ conducted the systematic review of Chinese studies. REB conceptualized the idea and assisted with the interpretation of the analysis and the final manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fischer-Walker C., Lamberti L., Roth D., Black R. Zinc and fectious Diseases. In: Rink L., editor. Zinc in Human Health. IOS Press; Amsterdam, The Netherlands: 2011. pp. 234–253. [Google Scholar]
- 2.WHO/UNICEF . Joint Statement: Clinical Management of Acute Diarrhoea. WHO/UNICEF; New York, NY, USA: 2004. [Google Scholar]
- 3.Lazzerini M., Ronfani L. Oral zinc for treating diarrhoea in children. Cochrane Database Syst. Rev. 2013;1 doi: 10.1002/14651858.CD005436. [DOI] [PubMed] [Google Scholar]
- 4.Fischer Walker C.L., Black R.E. Zinc for the treatment of diarrhoea: Effect on diarrhoea morbidity, mortality and incidence of future episodes. Int. J. Epidemiol. 2010;39:i63–i69. doi: 10.1093/ije/dyq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sachdev H.P., Mittal N.K., Mittal S.K., Yadav H.S. A controlled trial on utility of oral zinc supplementation in acute dehydrating diarrhea in infants. J. Pediatr. Gastroenterol. Nutr. 1988;7:877–881. doi: 10.1097/00005176-198811000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Sazawal S., Black R.E., Bhan M.K., Bhandari N., Sinha A., Jalla S. Zinc supplementation in young children with acute diarrhea in India. N. Engl. J. Med. 1995;333:839–844. doi: 10.1056/NEJM199509283331304. [DOI] [PubMed] [Google Scholar]
- 7.Bahl R., Bhandari N., Saksena M., Strand T., Kumar G.T., Bhan M.K., Sommerfelt H. Efficacy of zinc-fortified oral rehydration solution in 6- to 35-month-old children with acute diarrhea. J. Pediatr. 2002;141:677–682. doi: 10.1067/mpd.2002.128543. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J.S. Efficacy and effectiveness of 20 child health interventions in China: Systematic review of Chinese literature. J. Glob. Health. 2011;1:87–95. [PMC free article] [PubMed] [Google Scholar]
- 9.Stata Statistical Software. StataCorp LP; College Station, TX, USA: 2011. (release 12). [Google Scholar]
- 10.Altman D.G., Bland J.M. Interaction revisited: The difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker N., Fischer-Walker C., Bryce J., Bahl R., Cousens S. Standards for CHERG reviews of intervention effects on child survival. Int. J. Epidemiol. 2010;39:i21–i31. doi: 10.1093/ije/dyq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faruque A.S., Mahalanabis D., Haque S.S., Fuchs G.J., Habte D. Double-blind, randomized, controlled trial of zinc or vitamin A supplementation in young children with acute diarrhoea. Acta Paediatr. 1999;88:154–160. doi: 10.1111/j.1651-2227.1999.tb01074.x. [DOI] [PubMed] [Google Scholar]
- 13.Roy S.K., Tomkins A.M., Haider R., Behren R.H., Akramuzzaman S.M., Mahalanabis D., Fuchs G.J. Impact of zinc supplementation on subsequent growth and morbidity in Bangladeshi children with acute diarrhoea. Eur. J. Clin. Nutr. 1999;53:529–534. doi: 10.1038/sj.ejcn.1600734. [DOI] [PubMed] [Google Scholar]
- 14.Strand T.A., Chandyo R.K., Bahl R., Sharma P.R., Adhikari R.K., Bhandari N., Ulvik R.J., Molbak K., Bhan M.K., Sommerfelt H. Effectiveness and efficacy of zinc for the treatment of acute diarrhea in young children. Pediatrics. 2002;109:898–903. doi: 10.1542/peds.109.5.898. [DOI] [PubMed] [Google Scholar]
- 15.Polat T.B., Uysalol M., Cetinkaya F. Efficacy of zinc supplementation on the severity and duration of diarrhea in malnourished Turkish children. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2003;45:555–559. doi: 10.1046/j.1442-200X.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- 16.Brooks W.A., Santosham M., Roy S.K., Faruque A.S., Wahed M.A., Nahar K., Khan A.I., Khan A.F., Fuchs G.J., Black R.E. Efficacy of zinc in young infants with acute watery diarrhea. Am. J. Clin. Nutr. 2005;82:605–610. doi: 10.1093/ajcn.82.3.605. [DOI] [PubMed] [Google Scholar]
- 17.Al-Sonboli N., Gurgel R.Q., Shenkin A., Hart C.A., Cuevas L.E. Zinc supplementation in Brazilian children with acute diarrhoea. Ann. Trop. Paediatr. 2003;23:3–8. doi: 10.1179/000349803125002797. [DOI] [PubMed] [Google Scholar]
- 18.Larson C.P., Hoque A.B., Khan A.M., Saha U.R. Initiation of zinc treatment for acute childhood diarrhoea and risk for vomiting or regurgitation: A randomized, double-blind, placebo-controlled trial. J. Health Popul. Nutr. 2005;23:311–319. [PubMed] [Google Scholar]
- 19.Fischer Walker C.L., Bhutta Z.A., Bhandari N., Teka T., Shahid F., Taneja S., Black R.E. Zinc supplementation for the treatment of diarrhea in infants in Pakistan, India and Ethiopia. J. Pediatr. Gastroenterol. Nutr. 2006;43:357–363. doi: 10.1097/01.mpg.0000232018.40907.00. [DOI] [PubMed] [Google Scholar]
- 20.Patel A., Dibley M.J., Mamtani M., Badhoniya N., Kulkarni H. Zinc and copper supplementation in acute diarrhea in children: A double-blind randomized controlled trial. BMC Med. 2009;7:22. doi: 10.1186/1741-7015-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elnemr M.A.M., Abdullah A.K. Effect of zinc supplementation on morbidity due to acute diarrhoea in infants and children in Sanaa, Yemen: A randomized controlled double blind clinical trial. Sultan Qaboos Univ. Med. J. 2007;7:219–225. [PMC free article] [PubMed] [Google Scholar]
- 22.Patro B., Szymanski H., Szajewska H. Oral zinc for the treatment of acute gastroenteritis in Polish children: A randomized, double-blind, placebo-controlled trial. J. Pediatr. 2010;157:984–988. e1. doi: 10.1016/j.jpeds.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 23.Dutta P., Mitra U., Dutta S., Naik T.N., Rajendran K., Chatterjee M.K. Zinc, vitamin A, and micronutrient supplementation in children with diarrhea: A randomized controlled clinical trial of combination therapy versus monotherapy. J. Pediatr. 2011;159:633–637. doi: 10.1016/j.jpeds.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L., Li X., Sun W. 40 Cases of oral licorzinc asist treatment of diarrhea. Chin. J. Esthet. Med. 2011;20:455. (in Chinese) [Google Scholar]
- 25.Zhang L. The effect of supplement zinc orally assist treatment on autumal diarrhea. Mod. Hosp. 2009;9:54–56. (in Chinese) [Google Scholar]
- 26.Lin C., Li Q., Zhang M. Observation ofthe therapeutic efficiacy of oral zinc supplement with infantile rotaviral enteritis. Contemp. Med. 2010;16:8–10. (in Chinese) [Google Scholar]
- 27.Zhou X., Sun Y., Ouyang J. Analysis on the therapeutic and preventative effects of zinc supplement on rotavirus enteritis in children. J. Xianning Univ. 2010;24:401–403. (in Chinese) [Google Scholar]
- 28.Yang H. Clinical analysis of oral zinc adjuvant treatment for infants with acute diarrhea. China Mod. Med. 2011;18:7. (in Chinese) [Google Scholar]
- 29.Liu G. Clinical observation on the therapeutic efficacy of oral zinc supplement in infants with acute diarrhea. J. Qiaihar Med. Coll. 2010;31:1416. (in Chinese) [Google Scholar]
- 30.Chen L., Bao Y., Gao S. The comparison of the therapeutic effects on children diarrhea treated with smecta and oral zinc. J. Pediatr. Pharm. 2006;12:26–27. (in Chinese) [Google Scholar]
- 31.Liu H., Nie X. Chinical observation on the therapeutic efficacy of oral zinc supplement in infants with diarrhea. Pract. Clin. Med. 2011;12:86–88. (in Chinese) [Google Scholar]
- 32.Liu X., Lan X. Therapeutic effect of oral Zinc preparation on infantile diarrhea. Int. Med. Health Guid. News. 2009;15:74–75. (in Chinese) [Google Scholar]
- 33.Fu Y., Zhang W. Chinical analysis on the therapeutic efficacy of oral zinc supplement in infants with rotavirus enteritis. Guide China Med. 2010;8:260–262. (in Chinese) [Google Scholar]
- 34.Zhou G. Clinical efficacy of licorzinc in treatment of infantile acute diarrhea. Youjiang Med. J. 2008;36:573–574. (in Chinese) [Google Scholar]
- 35.Chen J. Clinical efficacy of licorzinc in treatment of infantile autumal diarrhea. J. Huaihai Med. 2008;26:442. (in Chinese) [Google Scholar]
- 36.Guan Y., Yingwei Y., Yu J., Zhou Y. Clinical efficacy of licorzinc in treatment of infantile rotavirus enteritis. Stud. Trace Elem. Health. 2012;29:21–22. (in Chinese) [Google Scholar]
- 37.Wu W., Li F. 46 Cases of licorzinc in treatment of infantile rotavirus enteritis. Shaanxi J. Tradit. Chin. Med. 2010;31:296–297. (in Chinese) [Google Scholar]
- 38.Zhou Y. Clinical observation of licorzinc treatment for children with diarrhea. Contemp. Med. 2010;16:20–21. (in Chinese) [Google Scholar]
- 39.Luo L.E.A. Clinical observations on curative effects of licorzinc particles in adjuvant treating rotavirus enteritis. Clin. J. Med. Off. 2009;37:862–863. (in Chinese) [Google Scholar]
- 40.Zhang S., Wei M. Observation of therapeutic effect of licorzinc granules adjuvant treatment for children with autumn diarrhea. Gems Health. 2010;5:127–128. (in Chinese) [Google Scholar]
- 41.Ju Z., Zhang Y., Teng L. Observation of therapeutic effect of licorzinc granules adjuvant treatment for children with autumn diarrhea. Shanxi Med. J. 2007;36:661. (in Chinese) [Google Scholar]
- 42.Wang L. Observation of therapeutic effect of licorzinc granules treatment for 30 cases of children with autumn diarrhea. Chin. Pediatr. Integr. Tradit. West. Med. 2012;4:168–169. (in Chinese) [Google Scholar]
- 43.Hong Y., Wang Z., Shen C. Clinical analysis of zinc sulfate adjuvant treatment for children with rotavirus enteritis. Zhejiang Clin. Med. J. 2009;11:845–846. (in Chinese) [Google Scholar]
- 44.Lin G., Wang J., Zhou T. Observation of therapeutic effect of zinc sulfate treatment for 46 cases of autumn diarrhea. Stud. Trace Elem. Health. 1994;11:61–62. (in Chinese) [Google Scholar]
- 45.Yan M., Liu Y. Clinical analysis on efficacy of zinc sulfate in adjuvant treating 120 children with diarrhea. J. Taishan Med. Coll. 2011;32:303. (in Chinese) [Google Scholar]
- 46.He S., Quan Y., Li S. Determination of zinc in hair from infant patients with diarrhea and observation of curative effect with a supply of zinc. Guangdong Trace Elem. Sci. 1997;4:42–43. (in Chinese) [Google Scholar]
- 47.Wei S. Observation of therapeutic effect of zinc gluconate treatment for children with acute diarrhea. Youjiang Med. J. 2011;39:50–52. (in Chinese) [Google Scholar]
- 48.Yang C., Xu L., Huang Z. Observation of therapeutic effect of zinc gluconate adjuvant treatment for children with acute diarrhea. Chin. Community. 2012;14:186. (in Chinese) [Google Scholar]
- 49.Pu Z. Observation of therapeutic effect of zinc gluconate adjuvant treatment for children with autumn diarrhea. J. Clin. Exp. Med. 2010;9:1016–1017. (in Chinese) [Google Scholar]
- 50.Zhang W. Analysis of therapeutic effect of zinc gluconate adjuvant treatment for children with rotavirus enteritis. China Mod. 2011;49:123–124. (in Chinese) [Google Scholar]
- 51.Sun Y., Sun H., Bian X. Rotavirus enteritis suooprtive treated by glucoside zinc. J. Appl. Clin. Pediatr. 2008;23:1532–1533. (in Chinese) [Google Scholar]
- 52.Zhang Y., He J. Observation of therapeutic effect of zinc gluconate adjuvant treatment for children with rotavirus enteritis. J. Med. Theory Pract. 2011;24:2319–2320. (in Chinese) [Google Scholar]
- 53.Lin T., Deng J. Effect of zinc gluconate tablets on serum zinc and diarrhea of children with rotariras enteritis. J. Hainan Med. Univ. 2010;16:491–493. (in Chinese) [Google Scholar]
- 54.Liu D. Clinical observation of zinc gluconate’s effect on acute diarrhea of children. Sichuan Med. J. 2009;30:696–697. (in Chinese) [Google Scholar]
- 55.Qiao J., Wu B. Observation of therapeutic effect of zinc gluconate treatment for children with acute diarrhea. Shanghai J. Prev. Med. 2011;23:581–582. (in Chinese) [Google Scholar]
- 56.Zhang P., Wu J. Observation of therapeutic effect of zinc gluconate treatment for children with autumn diarrhea. Chin. Pract. J. Rural. 2007;14:29–30. (in Chinese) [Google Scholar]
- 57.Zhao S., Gao Z. Observation of therapeutic effect of zinc gluconate treatment for children with autumn diarrhea. J. Clin. Res. 2012;25:463–464. (in Chinese) [Google Scholar]
- 58.Cai A. The effect of zinc gluconate on infants with acutie diarrhea. Med. J. Qilu. 2011;26:255–256. (in Chinese) [Google Scholar]
- 59.Zhang J. Observation of curative effect of zinc gluconate in treatment of children rotavirus enteritis. Guide China Med. 2012;10:218–219. (in Chinese) [Google Scholar]
- 60.Qiao R. Observation of therapeutic effect of zinc gluconate treatment for children with autumn diarrhea. Shanxi Med. J. 2012;41:380–381. (in Chinese) [Google Scholar]
- 61.Zhong Z. Therapeutic effect of zinc gluconate on infantile rotavirus enteritis. Contemp. Med. 2012;18:130–131. (in Chinese) [Google Scholar]
- 62.Wang Z. Observation of clinical efficacy of zinc gluconate on infantile rotavirus enteritis. China Med. Pharm. 2011;1:94–95. (in Chinese) [Google Scholar]
- 63.Ying Y., Mei Q. Clinical observation of therapeutic effect of zinc gluconate on infantile rotavirus diarrhea. Chongqing Med. 2008;37:2442–2443. (in Chinese) [Google Scholar]
- 64.Zhao Y. Clinical observation of therapeutic effect of zinc gluconate on infantile rotavirus diarrhea. China Foreign Med. Treat. 2012;13:113. (in Chinese) [Google Scholar]
- 65.Ma Z. Validity and security of zinc gluconate on infantile rotavirus diarrhea. China J. Pharm. Econ. 2012;2:273–274. (in Chinese) [Google Scholar]
- 66.Chen Q., Su H. Efficacy and safety analysis of zinc gluconate in treatment of children with rotavirus diarrhea. Contemp. Med. 2012;18:144–145. (in Chinese) [Google Scholar]
- 67.Hu X., Zhang X., Chen A. Observation of clinical efficacy of zinc gluconate on 60 cases with infantile acute duarrhea. Pract. Clin. Med. 2009;10:85. (in Chinese) [Google Scholar]
- 68.Yuan C., Guan J. Therapeutic effect of zinc gluconate adjuvant treatment on infantile acute diarrhea. Pract. Clin. Med. 2011;12:76–77. (in Chinese) [Google Scholar]
- 69.Tan Z. Observation of therapeutic effect of zinc gluconate adjuvant treatment for children with autumn diarrhea. Anhui Med. J. 2011;32:802–803. (in Chinese) [Google Scholar]
- 70.Liu J. Clinical treatment and analysis in 69 Children with chronic diarrhea. Chin. Manip. Rehabil. Med. 2012;3:245–246. (in Chinese) [Google Scholar]
- 71.Hu Y., Deng H. Observation of therapeutic effect of zinc gluconate granules for children with acute diarrhea. Chin. Foreign Women Health. 2011;19:205. (in Chinese) [Google Scholar]
- 72.Li X., Wang H., Feng G. Observation of therapeutic effect of zinc gluconate granules for children with autumn diarrhea. China Pharm. 2008;11:218–219. (in Chinese) [Google Scholar]
- 73.Gao R. Clinical observation of supplemental zinc to treat 74 children with acute diarrhea. Asia Pac. Tradit. Med. 2012;8:58–59. (in Chinese) [Google Scholar]
- 74.Wu H. The effect of zinc supplementation on children with diarrhea. Strait Pharm. J. 2011;23:156–158. (in Chinese) [Google Scholar]
- 75.Liu J. Observation of therapeutic effects of zinc supplementation in treatment of children with acute diarrhea. Chin. J. Mod. Drug Appl. 2011;5:27–28. (in Chinese) [Google Scholar]
- 76.Chen L., Wang P. Clinical analysis of zinc supplementation to treat infant diarrhea. Chin. J. Misdiagn. 2010;10:7582. (in Chinese) [Google Scholar]
- 77.Ma K. Clinical observation of zinc supplementation to treat children with acute diarrhea. Chin. Foreign Med. Res. 2012;10:32–33. (in Chinese) [Google Scholar]
- 78.Lu J. Clinical observation of zinc supplementation in treatment of autumn diarrhea. Med. Recapitul. 2012;18:1101–1102. (in Chinese) [Google Scholar]
- 79.Ma M., Yang H. Clinical analysis of zinc preparation adjuvant treatment for children with acute diarrhea. Matern. Child Health Care China. 2012;27:3847–3848. (in Chinese) [Google Scholar]
- 80.Ao Z., Wang J., Lin L. Formulation adjuvant therapy efficacy in children with diarrhea. Hebei Med. 2012;18:1091–1093. (in Chinese) [Google Scholar]
- 81.Gu Z., Shen H., Zhao P. Effect of zinc gluconate on acute diarrhea in children. J. Clin. Pediatr. 2011;29:249–251. (in Chinese) [Google Scholar]
- 82.Wen Y., Wang W., Yang C. Zinc supplement in adjuvant treatment of children acute diarrhea. Chin. J. Prim. Med. Pharm. 2006;13:1208. (in Chinese) [Google Scholar]
- 83.Wang G., Wu X. Observation of therapeutic effect of zinc adjuvant treatment for infantile autumn diarrhea. China Med. Pharm. 2011;1:66–98. (in Chinese) [Google Scholar]
- 84.Liu R. Zinc therapy in the treatment of children with rotavirus enteritis. Jilin Med. J. 2012;33:5648. (in Chinese) [Google Scholar]
- 85.Liu G. Observation of therapeutic effects of zinc supplementation in treatment of children with acute diarrhea. J. Med. Theory Pract. 2012;25:2287–2288. (in Chinese) [Google Scholar]
- 86.Tong W. Observation of therapeutic effects of zinc supplementation in treatment of children with diarrhea. China Health Ind. 2011;8:84. (in Chinese) [Google Scholar]
- 87.Qiu Y. Serum zinc level of children with rotaviral diarrhea before and after zinc supplementation and clinical efficacy of the therapy. Chin. J. Woman Child Health Res. 2010;21:616–617. (in Chinese) [Google Scholar]
- 88.Kong X. Cinical analysis of combination of lysine hydrochloride and zinc gluconate granules with ribavirin for children with diarrhea in autumn and winter. Inn. Mong. J. Tradit. Chin. Med. 2011;30:8. (in Chinese) [Google Scholar]
- 89.He Y. Observation of therapeutic effects of oral zinc supplementation adjuvant treatment for 60 cases of rotavirus enteritis. Zhejiang J. Clin. Med. 2007;9:1635. (in Chinese) [Google Scholar]
- 90.Kang C. Serum zinc levels and zinc supplementation treatment of young children with rotaviral enteritis. J. Kunming Med. Univ. 2010;31:109–113. (in Chinese) [Google Scholar]
- 91.Su M. Observation of effect of serum zinc levels and zinc supplementation treatment of young children with rotaviral enteritis. China Health Ind. 2012;6:76–77. (in Chinese) [Google Scholar]
- 92.Huang Z. Observation of therapeutic effects of zinc adjuvant treatment for 200 cases of infantile rotavirus enteritis. Chin. J. Ethnomed. Ethnopharm. 2010;19:80. (in Chinese) [Google Scholar]
- 93.Zhang J. Observation of therapeutic effect of licorzinc granules with smectite powder for infantile diarrhea. Jiangxi Med. J. 2006;41:500–501. (in Chinese) [Google Scholar]
- 94.Wang D. Observation of therapeutic effect of zinc treatment for infantile diarrhea. China Health Care Nutr. 2012;5:249. (in Chinese) [Google Scholar]
- 95.Lin T. Observation of therapeutic effect of zinc gluconate adjuvant treatment for 60 cases of infantile acute diarrhea. Chin. Community. 2008;24:29. (in Chinese) [Google Scholar]
- 96.Yan P. Observation of therapeutic effect of zinc gluconate adjuvant treatment for children with acute diarrhea. China Foreign Med. Treat. 2011;30:118–119. [Google Scholar]
- 97.Yu J. Clinical observation of therapeutic effect of zinc gluconate adjuvant treatment for 80 cases of children with diarrhea. Jilin Med. J. 2012;26:5644. (in Chinese) [Google Scholar]
- 98.Zhang X. Effect of zinc gluconate among the treatment for infantile rotavirus diarrhea. Chin. J. Midiagn. 2011;11:7848. (in Chinese) [Google Scholar]
- 99.Xu X. Observation of therapeutic effect of zinc gluconate treatment for children with rotavirus enteritis. Jiangsu Med. J. 2010;36:2327–2328. (in Chinese) [Google Scholar]
- 100.Tan J. Observation of therapeutic effect of zinc gluconate treatment for children with autumn diarrhea. Med. J. Chin. People Health. 2010;22:1122. (in Chinese) [Google Scholar]
- 101.Shen D. Clinical observation of therapeutic Effect of zinc gluconate on infantile rotavirus enteritis. Guide China Med. 2012;10:46–47. (in Chinese) [Google Scholar]
- 102.Wang J. Study of effect of zinc gluconate combined with Saccharomyces boulardii Sachets in treatment for children with autumn diarrhea. Contemp. Med. 2010;16:141–142. (in Chinese) [Google Scholar]
- 103.Chen L. Clinical observation of therapeutic effect of Lysine and zinc gluconate adjuvant treatment for infantile acute diarrhea. Chin. J. Mod. Drug Appl. 2011;5:85–86. (in Chinese) [Google Scholar]
- 104.Meng F. Observation of therapeutic effect of Lysine and zinc gluconate treatment for infantile autumn and winter diarrhea. Chin. Community. 2012;14:152. (in Chinese) [Google Scholar]
- 105.Zhong L. Observation of therapeutic effect of Lysine and zinc gluconateadjuvant treatment for infantile acute diarrhea. Chin. J. Aesthet. Med. 2010;19:258. (in Chinese) [Google Scholar]
- 106.Xie J. Serum zinc level of rotaviral enteritis in children and the significance of zinc treatment. J. Pediatr. Pharm. 2010;16:18–20. (in Chinese) [Google Scholar]
- 107.Fan J. Application of zinc preparation in treatment for infantile diarrhea. Chin. J. Misdiagn. 2012;12:1306. (in Chinese) [Google Scholar]
- 108.Zhou X. Therapeutic effect of zinc in the prevention and treatment of children rotvirus enteritis. Chin. Gen. Pract. 2012;15:1393–1394. (in Chinese) [Google Scholar]
- 109.Zhao Y. Observation of effect of zinc preparation adjuvant treatment for infantile acute diarrhea. Zhejiang J. Prev. Med. 2008;20:44. (in Chinese) [Google Scholar]
- 110.Wan H. Observation of therapeutic effect of Treasured zinc and selenium in adjuvant treatment for infantile diarrhea. Shandong Med. J. 2006;46:65. (in Chinese) [Google Scholar]
- 111.Yang S. Observation of therapeutic effect of zinc preparation in adjuvant treatment for infantile autumn diarrhea. Health World. 2012;2:244. (in Chinese) [Google Scholar]
- 112.Luo Y. Observation of therapeutic effect of zinc adjuvant treatment for 168 cases of infantile acute diarrhea. Public Med. Forum Mag. 2012;16:3338. (in Chinese) [Google Scholar]
- 113.Patel A.B., Dibley M.J., Mamtani M., Badhoniya N., Kulkarni H. Influence of zinc supplementation in acute diarrhea differs by the isolated organism. Int. J. Pediatr. 2010;2010 doi: 10.1155/2010/671587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fischer Walker C.L., Rudan I., Liu L., Nair H., Theodoratou E., Bhutta Z.A., O’Brien K.L., Campbell H., Black R.E. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baqui A.H., Black R.E., El Arifeen S., Yunus M., Chakraborty J., Ahmed S., Vaughan J.P. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: Community randomised trial. BMJ. 2002;325:1059. doi: 10.1136/bmj.325.7372.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roy S.K., Tomkins A.M., Mahalanabis D., Akramuzzaman S.M., Haider R., Behrens R.H., Fuchs G. Impact of zinc supplementation on persistent diarrhoea in malnourished Bangladeshi children. Acta Paediatr. 1998;87:1235–1239. doi: 10.1080/080352598750030898. [DOI] [PubMed] [Google Scholar]
- 117.Fontaine O. Effect of zinc supplementation on clinical course of acute diarrhoea. J. Health Popul. Nutr. 2001;19:339–346. [PubMed] [Google Scholar]