Abstract
During meiosis, homologous chromosomes recombine and become closely apposed along their lengths within the synaptonemal complex (SC). In part because Spo11 is required both to make the double-strand breaks (DSBs) that initiate recombination and to promote normal SC formation in many organisms, it is clear that these two processes are intimately coupled. The molecular nature of this linkage is not well understood, but it has been proposed that SC formation initiates locally at the sites of ongoing recombination and in particular at the subset of sites that will eventually give rise to crossovers. To test this hypothesis, we examined further the relationship between DSBs and SC formation in Saccharomyces cerevisiae. SCs were monitored in a series of spo11 missense mutants with varying DSB frequencies. Alleles that blocked DSB formation gave SC phenotypes indistinguishable from a deletion mutant, and partial loss-of-function mutations with progressively more severe DSB defects caused corresponding defects in SC formation. These results strongly correlate SC formation with Spo11 catalytic activity per se. Numbers of Zip3 complexes on chromosomes, thought to represent the sites of SC initiation, also declined when Spo11 activity decreased, but in a markedly nonlinear fashion: hypomorphic spo11 alleles caused larger defects in DSB formation than in Zip3 complex formation. This nonlinear response of Zip3 closely paralleled the response of crossover recombination products. The quantitative relationship between Zip3 foci, SC formation, and crossing over strongly implicates crossover-designated recombination intermediates as the sites of SC initiation.
Two prominent features of meiotic prophase are homologous recombination and formation of the synaptonemal complex (SC). Recombination establishes physical connections between homologous chromosomes in the form of crossovers, which help orient chromosomes properly on the meiosis I spindle. The widely conserved SC connects homologous chromosomes along their lengths during a portion of meiotic prophase and consists of two lateral elements, each formed along the axis connecting a pair of sister chromatids, and a central element linking the lateral elements together (reviewed in refs. 1 and 2).
Chromosome synapsis is tightly coupled to homologous recombination in many organisms. The DNA double-strand breaks (DSBs) that initiate recombination occur before synapsis begins in budding yeast (3), and cytological features (e.g., histone H2AX phosphorylation and formation of strand exchange protein complexes) indicate that the same is true in other fungi, higher plants, and mammals (4–8). DSBs are created by the Spo11 protein (9, 10), and spo11 null mutants in many organisms are defective for SC formation (11–16). In some cases, exogenous DSBs can at least partially substitute for Spo11 in SC formation (15–17). Moreover, mutations that block processing of DSBs once they are formed (e.g., rad50S, dmc1) also cause defects in SC formation (18–20). These results place Spo11 action upstream of SC formation, both temporally and functionally. This coupling is not universal, however; in Drosophila melanogaster and Caenorhabditis elegans, normal SC is formed in mutants defective for recombination initiation (21, 22), and in yet other organisms (e.g., females of the silk moth Bombyx mori), SC formation in the absence of recombination is the norm (23).
How is the linkage achieved when recombination and SC formation are tied together? At least a part of this coupling may come through nucleation of SC formation at sites where meiotic recombination is occurring and in particular at the subset of sites that will give rise to crossovers. There is a one-to-one correspondence between synapsis and crossing over for certain unusual chromosome configurations in maize (24–26) and other organisms (27, 28). Based on such findings, Maguire (24–26) proposed a causal link between crossing over and synapsis, although the correlative nature of the relationship could not establish which was cause and which was effect. In the fungus Sordaria macrospora, SC initiation is spatially and functionally coordinated with crossover-designated sites: during zygotene, short segments of SC surround crossover-related recombination nodules (although not all synaptic initiation sites have an associated nodule), and mutants with fewer crossovers have fewer synaptic initiation events (29). Spatial correlation of crossover-related nodules and SC segments was also reported for Neurospora (30). Finally, in budding yeast, proteins involved in initiating SC formation play roles in generating crossovers. SC assembly requires Zip2 and Zip3, which form discrete Spo11-dependent complexes that colocalize with the earliest central element (Zip1) structures and which are thus thought to nucleate central element polymerization (31, 32). Zip2/Zip3 complexes localize to a subset of recombination sites (31, 32), and the Zip proteins are required for processing crossover-designated recombination intermediates (33, 51).
Taken together, these studies draw strong connections between SC initiation and crossover formation. However, several issues arise. The correlative nature of many of these results does not prove a cause-and-effect relationship. Moreover, most of the studies relied for technical reasons on mutants in which the chromosome structure is grossly perturbed, through either chromosome rearrangements or removal of one or more structural protein components, raising the possibility that the observed correlations were indirect. Finally, some of the SC–recombination connection rests on the SC defect in spo11 nulls, but such studies cannot distinguish whether the synaptic defect stems from the absence of Spo11 catalytic activity (i.e., the inability to make DSBs) or from the absence of a DSB-independent Spo11 function. Recent studies indicate that Spo11 does indeed play roles in meiosis independent of its ability to form DSBs. Specifically, mutation of a tyrosine residue (Tyr-135) responsible for cleaving DNA abolishes DSB formation (9) but does not affect the ability of Spo11 to support normal S phase kinetics or homologous chromosome pairing (ref. 34, but see also ref. 35). Moreover, Spo11 is required to recruit Rec102 and Rec104 to meiotic chromosomes independent of DSB formation (52).
We addressed these issues by examining SC and Zip3 complex formation in a series of yeast strains that differed with respect to DSB frequency but were otherwise wild type; i.e., they expressed all of the recombination and chromosome structure proteins. To do this, we tested a series of spo11 alleles that reduce or abolish DSB formation without altering steady-state protein levels. Our results reveal that Spo11 promotes SC formation by virtue of its catalytic activity per se, arguing strongly that recombination directly promotes SC formation. Furthermore, the quantitative relationship between SC formation and crossing over in this study strongly supports the model that SC formation initiates locally at the subset of recombination intermediates that are destined to give rise to crossovers.
Materials and Methods
Meiotic cultures were prepared as described (3, 18). Yeast strains are derivatives of SK1 and are listed in Table 2, which is published as supporting information on the PNAS web site. Tagged spo11-HA3His6 and the spo11 missense mutants were described (36, 37). For simplicity, the tag will hereafter be referred to as “HA.” The spo11-HA allele complements a null spo11 mutation at 30°C, although it confers a mild cold-sensitive phenotype that is recessive to wild type (36). ZIP3-GFP::URA was provided by G. S. Roeder, Yale University, New Haven, CT (32).
Details of the subcellular fractionation assay will be provided elsewhere (52). Western blotting, and measurements of DSBs and intragenic recombination frequencies were performed as described (36, 37). For SC analysis, chromosomes were surface spread and stained as described (38, 39). Affinity-purified rabbit anti-Zip1 antibody (generously provided by G. S. Roeder) was used at 1:100 dilution and goat anti-rabbit-Alexa 488 at 1:1,000 (Molecular Probes). For Zip3-GFP analysis, spreads were prepared according to ref. 11, except that cells were treated with zymolyase for 10 min at 30°C, and the spheroplast pellet was resuspended in a 2:1:1 mixture of 4% paraformaldehyde, 1% Lipsol, and MEM. Zip3-GFP was detected by using affinity-purified goat anti-GFP at 1:200 (Rockland, Gilbertsville, PA) and donkey anti-goat-Alexa 488 at 1:1,000 (Molecular Probes). Zip1 was detected by using affinity-purified rabbit anti-Zip1 at 1:100 and donkey anti-rabbit-rhodamine at 1:200 (Molecular Probes). Electron microscopy was performed as described (11) by using a JEOL 1200EX microscope.
Results
Wild-Type and Mutant Spo11 Proteins Are Associated with Chromatin. Given its role in promoting DSB formation, Spo11 should be associated with chromatin. To confirm this and to determine whether DSB-defective mutant Spo11 proteins are still capable of localizing to chromatin, we used a differential extraction assay to follow Spo11 subcellular distribution (diagrammed in Fig. 6, which is published as supporting information on the PNAS web site). Meiotic cells were spheroplasted, hypotonically lysed, and then separated by centrifugation into a soluble cytoplasmic fraction and a pellet containing nuclei and other insoluble material. The pellet was then sequentially extracted with nonionic detergent and with DNase I digestion. We define a protein as being chromatin-associated if it is enriched in the pellet containing the chromosomes (P2) and then solubilized by DNase I treatment (S3). Tubulin is in the first soluble extract (S1) and serves as a control for the efficiency of spheroplast formation and lysis. At 4 h in meiosis, this analysis revealed that a significant fraction of cellular Spo11 protein is associated with meiotic chromatin (Fig. 6B).
We chose several spo11 mutants for the work presented here. In one, DSB formation was abrogated by substituting phenylalanine for the catalytic residue Tyr-135 (9). Two other mutants came from a structure/function analysis of yeast Spo11 (37). Mutation of Asp-288 (thought to directly coordinate a magnesium ion) to asparagine compromised DSB formation as severely as a deletion mutant. Substituting alanine for Asp-290 yielded a partially DSB-defective mutant. These mutant proteins accumulated to similar steady-state levels as wild type (37), and they behaved similarly to wild type in the differential extraction assay (Fig. 6C), indicating that they are also chromatin-associated.
spo11 Missense Mutants That Do Not Form DSBs Do Not Form SC. To determine SC phenotypes, meiotic chromosome spreads were immunostained for Zip1, a component of the SC central element (40). Spread nuclei were assigned to classes according to the Zip1 pattern: no staining or faint diffuse Zip1 staining; bright Zip1 foci; partial SC (at least one linear stretch of Zip1 staining; i.e., zygotene); or extensive SC (late zygotene/pachytene). Examples of nuclei with partial or extensive SC are shown in Fig. 1A i and ii. A typical time course in a spo11-HA strain is shown in Fig. 1B. The kinetics and frequency of SC formation in this strain were indistinguishable from an isogenic wild-type strain (data not shown; see also below) and are consistent with earlier studies in this strain background (3).
Fig. 1.
(A) Zip1 staining patterns observed in wild-type and/or spo11 mutants. Meiotic chromosome spreads were analyzed by indirect immunofluorescence with anti-Zip1 antibodies. Examples are shown for (i) partial SC, (ii) extensive SC, (iii) polycomplex, (iv) partial synapsis plus polycomplex, and (v) extensive synapsis plus polycomplex. (Bar, 1 μm.) (B) Kinetics of SC formation in spo11-HA and spo11Δ strains. At least 100 nuclei were scored per time point.
Consistent with earlier electron microscopic studies (12, 13), a spo11Δ mutant showed severe SC defects. Nuclei with partial or extensive SC formation were entirely absent (Fig. 1B). Somewhat surprisingly, nuclei containing bright Zip1 foci appeared at near-normal frequency and with normal kinetics, indicating that a significant fraction of such foci probably do not represent sites of synaptic initiation and may instead be Zip1 aggregates bound nonspecifically to chromatin. Two missense mutants completely defective for DSB formation, spo11-Y135F-HA and spo11-D288N-HA, also showed severe SC defects (Fig. 2Av and vi). Similar results for spo11-Y135F were reported earlier (B. Weiner and N. Kleckner, unpublished results; see ref. 34). These findings support the idea that SC formation is closely tied to DSB formation per se.
Fig. 2.
Synaptic defects in spo11 mutants with reduced DSB levels. Chromosome spreads from the indicated strains were immunostained for Zip1. At least 50 nuclei were scored at each time point. Approximate relative DSB activities (from Table 1) are provided for convenience. (A) Extent of SC formation. (B) Occurrence of nuclei containing polycomplexes (PC), with or without partial or extensive synaptonemal complex.
spo11 Mutants with Partial DSB Defects Show Partial Synaptic Defects. We next examined an isogenic set of strains with progressively more severe DSB defects. One strain was homozygous for spo11-D290A-HA, which reduces DSB activity in vivo to ≈4–12% of wild type, as estimated from direct DSB measurements and from frequencies of intragenic recombination at the his4::LEU2 hot spot (Fig. 3 and Table 1). Two other strains took advantage of dominant negative effects of spo11-Y135F (37). The extent of the DSB defect in a spo11-Y135F/+ heterozygote depends on the precise combination of epitope tags on the two alleles. If both alleles are tagged, the Y135F mutation is semidominant and the strain shows 17–23% of wild-type recombination initiation (Fig. 3 and Table 1). However, if the wild-type allele is tagged and the point mutant untagged (spo11-Y135F/spo11-HA), the mutation is almost completely dominant and the strain shows <5% of normal recombination initiation (Fig. 3 and Table 1). The reason for these effects is not known, but for purposes of this study, we can view these allelic combinations simply as a way to titrate Spo11 activity in vivo. Pulse-field gel analysis revealed that these mutations affect DSB frequencies to similar extents across several regions of the genome (R. Diaz and S.K., unpublished observations), so it is likely that measurements at a single recombination hot spot provide a reasonable estimate of relative DSB levels in the genome as a whole.
Fig. 3.
DSBs in a SPO11 allelic series. Genomic DNA was prepared at the indicated times from rad50S strains differing in their genotype at the SPO11 locus. DSBs at the his4::LEU2 locus were analyzed by Southern blotting of PstI-digested DNA. Note that the spo11-D290A mutation affects both the level and distribution of DSBs within the hot spot, as described (37).
Table 1. Relative recombination frequencies for various spo11 mutants.
| SPO11 genotype | Frequency His+, ×103 (percent of wild type)* | rad50S DSBs, percent of total DNA (percent of wild type)† |
|---|---|---|
| SPO11 / SPO11 | 18.9 ± 3.6 (≡ 100)‡ | 9.8 ± 1.8 (≡ 100) |
| spo11-HA/spo11-HA | 11.8 ± 5.8 (62)‡ | 2.6 ± 0.40 (27) |
| spo11-Y135F-HA/spo11-HA | 4.4 ± 2.2 (23)‡ | 1.7 ± 0.34 (17) |
| spo11-D290A-HA/spo11-D290A-HA | 0.84 ± 0.35 (4.4) | 1.2 ± 0.27 (12) |
| spo11-Y135F/spo11-HA | 0.12 ± 0.046 (0.63)‡ | 0.42 ± 0.08 (4.3) |
Intragenic recombination at his4X::LEU2/his4B::LEU2, measured as the frequency of His + prototrophs per viable cell after 8 h in sporulation medium (mean ± SD of at least three determinations).
DSB frequencies were measured at his4LEU2 site I in rad50S derivatives of the strains used for return-to-growth measurements (mean ± SD of at least two determinations).
Data are from ref. 37.
The reduction in DSB frequencies in a spo11-HA strain (to ≈30–60% of wild type) caused no detectable defect in SC formation, but further reductions in DSB levels correlated with reduced SC formation. The semidominant negative mutant (spo11-Y135F-HA/spo11-HA) formed SC at a reduced frequency (Fig. 2 Aii); spo11-D290A-HA gave no cells with extensive SC formation, although 5–10% of cells eventually made at least short SC stretches (Fig. 2 Aiii); and the configuration with very low recombination (spo11-Y135F/spo11-HA) showed a profound SC defect that was only marginally better than completely DSB-defective mutants (Fig. 2 Aiv). Thus, partial DSB defects cause partial synaptic defects but only after a threshold in DSB levels is crossed.
Polycomplex Formation. Polycomplexes are aggregates of SC components that form at increased frequency in strains unable to form proper SC (13, 18, 19, 31, 41–43). Because they can be easily visualized by Zip1 immunofluorescence (Fig. 1 A iii–v), polycomplexes are useful indirect indicators of synaptic defects. Whereas polycomplexes were rare in wild-type or spo11-HA cells (Fig. 2Bi and data not shown), polycomplexes were prevalent in spo11Δ cells (data not shown), in agreement with earlier studies (13). Similarly, DSB-null missense mutants (spo11-Y135F-HA and spo11-D288N-HA) frequently yielded nuclei that contained only polycomplex with no other obvious Zip1 structures, and the dominant-negative configuration (spo11-Y135F/spo11-HA) was again essentially indistinguishable from these (Fig. 2B iv–vi). Strains with intermediate DSB frequencies (spo11-Y135F-HA/spo11-HA or spo11-D290A-HA) showed intermediate polycomplex formation that progressively worsened as the DSB defect worsened. Interestingly, these strains frequently showed polycomplex simultaneously with SC (Figs. 1Aiv and v and 2Bii and iii). Such nuclei were rare in wild type (Fig. 2Bi). The inverse correlation between DSB frequency and polycomplex formation further reinforces the connection between Spo11 catalytic activity and chromosome synapsis.
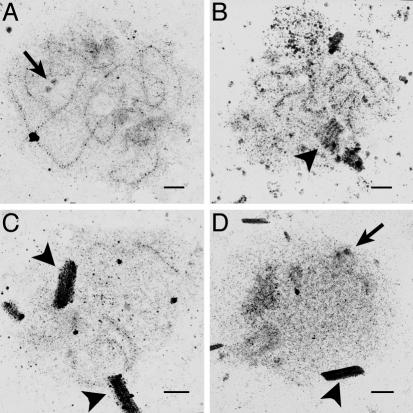
Confirmation of Aberrant Synaptic Structures by Electron Microscopy. To confirm the structures classified by Zip1 immunofluorescence, chromosome spreads were also analyzed by silver staining and electron microscopy. In agreement with Zip1 immunofluorescence, cells with a partial Spo11 defect often showed SC simultaneously with polycomplexes (Fig. 4 B and C). Of >100 spo11Δ nuclei examined, none showed SC, but many contained duplicated unseparated spindle pole bodies and polycomplex (Fig. 4D). None of the spo11Δ nuclei contained obvious structures resembling axial elements. Similar observations were made for the completely DSB-defective mutant spo11-Y135F-HA (data not shown).
Fig. 4.
Electron microscopic confirmation of normal and aberrant synaptic structures. Chromosome spreads prepared at 4 h in meiosis were stained with silver and analyzed by electron microscopy. (A) Extensive SC in a spo11-HA nucleus. (B and C) Extensive SC plus polycomplex (B) and partial SC plus polycomplex (C), both from a spo11-Y135F-HA/spo11-HA strain. (D) Polycomplex only, from a spo11Δ nucleus. Arrows, spindle pole bodies; arrowheads, polycomplexes. (Bar, 0.5 μm.)
Reducing DSB Formation Decreases the Number of Zip3 Complexes. As described in the introduction, Zip3 complexes are thought to mark the sites of SC initiation (31, 32). If the main contribution of Spo11 to SC formation is to provide these initiation sites, then the number of Zip3 complexes on chromosomes should decrease coordinately with decreasing Spo11 activity. Chromosome spreads were prepared from four isogenic strains varying in their SPO11 genotype, all expressing a Zip3-GFP fusion (32). The spreads were stained with anti-Zip1 and anti-GFP antibodies, then at least 50 nuclei were selected at random from each of five time points (2–6 h in meiosis), grouped according to the extent of SC formation, and the numbers of Zip3 foci were counted. Representative nuclei are shown in Fig. 5A, and the numbers of Zip3 foci are shown in Fig. 5B.
Fig. 5.
Decreased numbers of Zip3 foci form when DSB formation is reduced. (A) Representative nuclear spreads stained for Zip1 (red) and Zip3-GFP foci (green). (Bar, 1 μm.) (B) Zip3 focus counts. For each strain, nuclear spreads were prepared hourly from 2–6 h in meiosis. At least 50 nuclei were selected at random for each time point and grouped according to the extent of SC formation irrespective of the presence of polycomplex, and the Zip3 foci were counted. Bar graphs show the percent of nuclei that contained the indicated number of Zip3 foci (0–9 foci/nucleus in pale yellow, etc.). The number of nuclei in each Zip1 class (n) and the mean number of Zip3 foci (±SD) are given (Right). **, For spo11-D290A-HA, only one nucleus (with 28 Zip3 foci) had extensive SCs. (C) Nonlinear relationship between DSB frequency and the number of SC initiation sites. Relative numbers of Zip3 foci are plotted against relative DSB frequencies for wild-type, spo11-HA, spo11-Y135F-HA/spo11-HA, and spo11-D290A-HA (○). Zip3 numbers are the averages for all nuclei scored as having either partial or extensive SC (from B). The gray line marks the diagonal expected for a linear relationship.
In agreement with previous studies (32), Zip3 foci first appeared in wild type at or before the time when the first Zip1 staining structures appeared, and they increased in number as SC formation progressed (Fig. 5B, wild type). No significant differences in the number or timing of Zip3 complex formation were observed in spo11-HA compared to SPO11, consistent with the normal pattern of SC formation noted above for this strain. As Spo11 activity was decreased further, however, the number of Zip3 complexes declined. In spo11-Y135F-HA/spo11-HA, significantly fewer Zip3 foci relative to wild type were observed in nuclei containing partial (zygotene) or full (pachytene) SCs (Fig. 5B, P < 0.001, Wilcoxon rank sum test). In the more severely compromised strain (the spo11-D290A-HA homozygote), Zip3 focus counts were reduced even further relative to wild type (P = 0.006 for the nuclei with only focal Zip1 staining patterns; P < 0.001 for nuclei with partial SC). Zip3 foci were almost entirely absent in a spo11-Y135F-HA mutant, as for spo11Δ (data not shown). These results agree well with the SC phenotypes of these mutants, so it is likely that the synaptic defects can be directly attributed to decreases in the number of synaptic initiation sites. It is important to note, however, that the relationship between DSB frequencies and the number of Zip3 complexes is distinctly nonlinear (Fig. 5C). Implications of this observation are discussed below.
Discussion
To test whether recombination promotes SC formation, we used a panel of yeast spo11 missense mutants that show a range of DSB frequencies. The mutant proteins localized properly to meiotic chromosomes and supported at least some of the normal functions carried out by wild-type Spo11. Two different point mutations that abrogated DSB formation blocked SC formation as severely as a deletion mutation. Our results agree with other studies of the spo11-Y135F mutant in yeast (N. Kleckner and B. Weiner, personal communication and unpublished data; see ref. 34) and an equivalent mutant in S. macrospora (8). We also found that mutants with intermediate DSB defects gave intermediate SC defects. Because several different missense mutations and allele combinations were used, it seems unlikely that we managed fortuitously to covary two independent functions of Spo11. Thus, these findings strongly link the DNA-cleaving activity of Spo11 to its activity in promoting SC formation.
Crossover-Designated Recombination Sites Initiate Synapsis. The results presented here indicate that the major role for Spo11 in SC formation is to provide the sites where Zip3 loads onto chromosomes and nucleates Zip1 polymerization. More importantly, the results also support the idea that synapsis initiates at a defined subset of recombination intermediates with properties characteristic of crossovers. This conclusion is based on the finding that the effects of spo11 mutants on Zip3 focus numbers correlate better with effects on crossover frequencies than with effects on total DSB levels. In separate studies, crossover frequencies were analyzed in a similar SPO11 allelic series as was used here. Surprisingly, even as Spo11 activity decreased, crossover frequencies did not decrease in parallel (E. Martini, R. Diaz, N. Hunter, and S.K., unpublished observations). It thus appears that cells have a mechanism to maintain appropriate numbers of crossovers despite variation in the frequency of recombination initiation. We refer to this phenomenon as “crossover homeostasis.”
As the DSB frequency decreased, the number of Zip3 foci also decreased, but the two did not vary together in a linear fashion. Specifically, Zip3 foci showed a threshold effect (Fig. 5C), indicating that the number of Zip3 foci is buffered against changes in DSB frequency. This behavior is quantitatively indistinguishable from crossover homeostasis. While this paper was in preparation, a striking converse correlation was described: increases in crossing over caused by loss of Sgs1 function in yeast were accompanied by increases in numbers of synaptic initiation sites (Zip3 complexes) (44). The idea that SC formation initiates primarily, if not exclusively, at the subset of recombination events that are destined to become crossovers provides a simple way to explain the similar responses of Zip3 foci and crossover numbers in these two studies. Moreover, this explanation is consistent with the implication of Zip2 and Zip3 in controlling the processing of crossover-designated recombination intermediates (51) and with the observation that the number of Zip2/Zip3 foci per cell is sufficient to account for only a subset of the total DSBs (31, 32).
Our observations agree well with recent studies of the effects of hypomorphic ski8 alleles on SC formation in S. macrospora (17). These findings, along with earlier studies in S. macrospora, N. crassa, and maize (see Introduction), make it likely that preferred nucleation of central element formation at crossover-designated intermediates is a general phenomenon in many organisms. It is also clear that this is not the only mechanism, however. For example, plants with very long chromosomes have more SC initiation sites than crossovers (45, 46); SC initiation sites without associated recombination nodules are observed near telomeres in S. macrospora (29); SC formation can occur in spo11 mutants in mice, albeit between nonhomologous chromosomes (14, 15); and SC formation occurs normally in the absence of recombination in a number of organisms (see Introduction).
How Defective for SC Formation Are spo11 Mutants in Yeast? In some studies of spo11 and other DSB null mutants in budding yeast, no SC was detected (12, 18, 43, 47, 48). In others, at least limited SC formation could be detected, although it is not clear whether this was homologous or nonhomologous synapsis (refs. 13 and 49 and K. Schmekel, personal communication). Despite the differences, it seems fair to say that no study has led to the conclusion that SC formation is normal in these mutants, and almost all studies document profound defects. We suggest that the preferred pathway for SC formation in yeast is to install the central element first at the sites where crossovers will occur, but that limited Zip1 assembly can occur elsewhere under at least some conditions (variations in culture conditions, strain background, temperature, etc.). Given the rarity of recombination-independent synapsis under most conditions, it seems likely that this pathway contributes relatively to SC formation in normal meiosis.
The ideas presented here predict that SC should form at any DSB if it is being processed along an appropriately controlled crossover-designated pathway. DSBs induced by exogenous DNA damage can rescue SC formation in spo11 mutants in several organisms, so recombination need not be initiated by Spo11 to promote SC formation (15–17). An endonuclease-mediated DSB can be repaired to generate a crossover in yeast, with many of the hallmarks of Spo11-induced recombination (35, 50). Although a single such break could not induce substantial SC formation (50), these studies most likely would not have detected formation of a short stretch of SC, and formation of Zip2/Zip3 foci was not analyzed (J. Haber, personal communication). Thus, it will be interesting to determine whether a single DSB can promote local SC initiation.
Supplementary Material
Acknowledgments
We thank Doug Bishop (University of Chicago, Chicago), Mike Dresser (Oklahoma University, Oklahoma City, OK), and Shirleen Roeder (Yale University, New Haven, CT) for providing strains, antibodies, and/or technical advice; Nancy Kleckner for critical comments on the manuscript; Adam Olshen for help with statistical analysis; and Nina Lampen for help with electron microscopy. This research was supported in part by National Institutes of Health Grant GM58673.
Abbreviations: DSB, DNA double-strand break; SC, synaptonemal complex.
References
- 1.Zickler, D. & Kleckner, N. (1999) Annu. Rev. Genet. 33, 603–754. [DOI] [PubMed] [Google Scholar]
- 2.Roeder, G. S. (1997) Genes Dev. 11, 2600–2621. [DOI] [PubMed] [Google Scholar]
- 3.Padmore, R., Cao, L. & Kleckner, N. (1991) Cell 66, 1239–1256. [DOI] [PubMed] [Google Scholar]
- 4.Mahadevaiah, S. K., Turner, J. M. A., Baudat, F., Rogakou, E. P., de Boer, P., Blanco-Rodriguez, J., Jasin, M., Keeney, S., Bonner, W. M. & Burgoyne, P. S. (2001) Nat. Genet. 27, 271–276. [DOI] [PubMed] [Google Scholar]
- 5.Barlow, A. L., Benson, F. E., West, S. C. & Hulten, M. A. (1997) EMBO J. 16, 5207–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moens, P. B., Chen, D. J., Shen, Z., Kolas, N., Tarsounas, M., Heng, H. H. Q. & Spyropoulos, B. (1997) Chromosoma 106, 207–215. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, L. K., Offenberg, H. H., Verkuijlen, W. M. H. C. & Heyting, C. (1997) Proc. Natl. Acad. Sci. USA 94, 6868–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storlazzi, A., Tessé, S., Gargano, S., James, F., Kleckner, N. & Zickler, D. (2003) Genes Dev. 17, 2675–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergerat, A., de Massy, B., Gadelle, D., Varoutas, P. C., Nicolas, A. & Forterre, P. (1997) Nature 386, 414–417. [DOI] [PubMed] [Google Scholar]
- 10.Keeney, S., Giroux, C. N. & Kleckner, N. (1997) Cell 88, 375–384. [DOI] [PubMed] [Google Scholar]
- 11.Dresser, M. E. & Giroux, C. N. (1988) J. Cell Biol. 106, 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giroux, C. N., Dresser, M. E. & Tiano, H. F. (1989) Genome 31, 88–94. [DOI] [PubMed] [Google Scholar]
- 13.Loidl, J., Klein, F. & Scherthan, H. (1994) J. Cell Biol. 125, 1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baudat, F., Manova, K., Yuen, J. P., Jasin, M. & Keeney, S. (2000) Mol. Cell 6, 989–998. [DOI] [PubMed] [Google Scholar]
- 15.Romanienko, P. J. & Camerini-Otero, R. D. (2000) Mol. Cell 6, 975–987. [DOI] [PubMed] [Google Scholar]
- 16.Celerin, M., Merino, S. T., Stone, J. E., Menzie, A. M. & Zolan, M. E. (2000) EMBO J. 19, 2739–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tessé, S., Storlazzi, A., Kleckner, N., Gargano, S. & Zickler, D. (2003) Proc. Natl. Acad. Sci. USA 100, 12865–12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alani, E., Padmore, R. & Kleckner, N. (1990) Cell 61, 419–436. [DOI] [PubMed] [Google Scholar]
- 19.Bishop, D. K., Park, D., Xu, L. & Kleckner, N. (1992) Cell 69, 439–456. [DOI] [PubMed] [Google Scholar]
- 20.Pittman, D. L., Cobb, J., Schimenti, K. J., Wilson, L. A., Cooper, D. M., Brignull, E., Handel, M. A. & Schimenti, J. C. (1998) Mol. Cell 1, 697–705. [DOI] [PubMed] [Google Scholar]
- 21.Dernburg, A. F., McDonald, K., Moulder, G., Barstead, R., Dresser, M. & Villeneuve, A. M. (1998) Cell 94, 387–398. [DOI] [PubMed] [Google Scholar]
- 22.McKim, K. S., Green-Marroquin, B. L., Sekelsky, J. J., Chin, G., Steinberg, C., Khodosh, R. & Hawley, R. S. (1998) Science 279, 876–878. [DOI] [PubMed] [Google Scholar]
- 23.Rasmussen, S. W. (1976) Chromosoma 54, 245–293. [DOI] [PubMed] [Google Scholar]
- 24.Maguire, M. P. (1965) Genetics 51, 23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maguire, M. P. (1966) Genetics 53, 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire, M. P. (1972) Genetics 70, 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stack, S. & Soulliere, D. (1984) Chromosoma 90, 72–83. [Google Scholar]
- 28.Nur, U. (1968) Chromosoma 25, 198–214. [DOI] [PubMed] [Google Scholar]
- 29.Zickler, D., Moreau, P. J., Huynh, A. D. & Slezec, A. M. (1992) Genetics 132, 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bojko, M. (1989) Genome 32, 309–317. [DOI] [PubMed] [Google Scholar]
- 31.Chua, P. R. & Roeder, G. S. (1998) Cell 93, 349–359. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal, S. & Roeder, G. S. (2000) Cell 102, 245–255. [DOI] [PubMed] [Google Scholar]
- 33.Storlazzi, A., Xu, L., Schwacha, A. & Kleckner, N. (1996) Proc. Natl. Acad. Sci. USA 93, 9043–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha, R. S., Weiner, B. M., Keeney, S., Dekker, J. & Kleckner, N. (2000) Genes Dev. 14, 493–503. [PMC free article] [PubMed] [Google Scholar]
- 35.Neale, M. J., Ramachandran, M., Trelles-Sticken, E., Scherthan, H. & Goldman, A. S. H. (2002) Mol. Cell 9, 835–846. [DOI] [PubMed] [Google Scholar]
- 36.Kee, K. & Keeney, S. (2002) Genetics 160, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz, R. L., Alcid, A. D., Berger, J. M. & Keeney, S. (2002) Mol. Cell. Biol. 22, 1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loidl, J., Klein, F. & Engebrecht, J. (1998) Methods Cell Biol. 53, 257–285. [DOI] [PubMed] [Google Scholar]
- 39.Gasior, S. L., Wong, A. K., Kora, Y., Shinohara, A. & Bishop, D. K. (1998) Genes Dev. 12, 2208–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sym, M., Engebrecht, J. A. & Roeder, G. S. (1993) Cell 72, 365–378. [DOI] [PubMed] [Google Scholar]
- 41.Tung, K. S. & Roeder, G. S. (1998) Genetics 149, 817–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sym, M. & Roeder, G. S. (1995) J. Cell Biol. 128, 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhargava, J., Engebrecht, J. & Roeder, G. S. (1992) Genetics 130, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockmill, B., Fung, J. C., Branda, S. S. & Roeder, G. S. (2003) Curr. Biol. 13, 1954–1962. [DOI] [PubMed] [Google Scholar]
- 45.Gillies, C. B. (1985) Chromosoma 92, 165–175. [Google Scholar]
- 46.Hasenkampf, C. (1984) Chromosoma 90, 275–284. [Google Scholar]
- 47.Menees, T. M. & Roeder, G. S. (1989) Genetics 123, 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rockmill, B., Engebrecht, J. A., Scherthan, H., Loidl, J. & Roeder, G. S. (1995) Genetics 141, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klapholz, S., Waddell, C. S. & Esposito, R. E. (1985) Genetics 110, 187–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malkova, A., Klein, F., Leung, W. Y. & Haber, J. E. (2000) Proc. Natl. Acad. Sci. USA 97, 14500–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boerner, G. V., Kleckner, N. & Hunter, N. (2004) Cell, in press. [DOI] [PubMed]
- 52.Kee, K., Protacio, R. U., Arora, C. & Keeney, S. (2004) EMBO J., in press. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.