Abstract
Background
Adolescent marijuana use is associated with increased risk for schizophrenia. We previously reported that marijuana misuse in conjunction with specific cannabinoid receptor 1 (CNR1) genetic variants (rs12720071-Gallele carriers) contributed to white-matter (WM) brain volume deficits in schizophrenia patients. In this study, we assessed the influence of another cannabinoid-related gene, mitogen-activated protein kinase 14 (MAPK14), and potential MAPK14–CNR1 gene–gene interactions in conferring brain volume abnormalities among schizophrenia patients with marijuana abuse/dependence. MAPK14 encodes a member of the MAPK family involved in diverse cellular processes, including CNR1-induced apoptosis.
Method
We genotyped 235 schizophrenia patients on nine MAPK14 tag single nucleotide polymorphisms (tSNPs). Approximately one quarter of the sample had marijuana abuse or dependence. Differential effects of MAPK14 tSNPs on brain volumes across patients with versus without marijuana abuse/dependence were examined using ANCOVA.
Results
Of the MAPK14 tSNPs, only rs12199654 had significant genotype effects and genotype × marijuana misuse interaction effects on WM volumes. rs12199654-A homozygotes with marijuana abuse/dependence had significantly smaller total cerebral and lobar WM volumes. The effects of MAPK14 rs12199654 on WM volume deficits remained significant even after controlling for the CNR1 rs12720071 genotype. There were significant main effects of the MAPK14 CNR1 diplotype and diplotype × marijuana interaction on WM brain volumes, with both genetic variants having additive contributions to WM volume deficits only in patients with marijuana misuse.
Conclusions
Given that CNR1-induced apoptosis is preceded by increased MAPK phosphorylation, our study suggests that potential MAPK14–CNR1 gene–gene interactions may mediate brain morphometric features in schizophrenia patients with heavy marijuana use.
Keywords: Cannabis, epistasis, gene–environment interaction, MRI, white matter
Introduction
Marijuana is the most commonly abused illicit drug in many countries including the USA (WHO, 1997; NSDUH, 2005). It is often the first illicit drug to be used, with the majority of users starting during adolescence (Pacula et al. 2000; Gfroerer et al. 2002). Adolescent marijuana use is associated with a twofold increased risk for schizophrenia (Andreasson et al. 1987; Zammit et al. 2002; Henquet & van Os, 2008). Although this link between marijuana misuse and schizophrenia has already been well replicated in large prospective epidemiologic studies (van Os et al. 2002; Stefanis et al. 2004; Henquet et al. 2005), whether adolescent marijuana use is causally related to subsequent schizophrenia remains uncertain (Degenhardt et al. 2003; Kumra, 2007; Murray et al. 2007; DeLisi, 2008; Henquet & van Os, 2008; D’Souza et al. 2009; Hickman et al. 2009; Sewell et al. 2009).
Animal studies suggest that adolescence is a sensitive time period during which the effects of marijuana on the developing brain may be most deleterious (Schneider & Koch, 2003; Murray et al. 2007). Tetrahydrocannabinol (THC), the psychoactive component in marijuana, activates brain cannabinoid receptors (cannabinoid receptor type 1, CB1 or CNR1) (Wilson & Nicoll, 2002). Chronic THC administration in adolescent rats, but not adult or pre-pubescent THC exposure, leads to enduring cognitive deficits in adulthood, including learning and memory deficits and prepulse inhibition abnormalities commonly observed in schizophrenia patients (O’Shea et al. 2004, 2006; Schneider & Koch, 2007). THC-related cognitive deficits are associated with changes in Fos protein expression within brain regions rich in CNR1, including the hippocampus, cerebellum and basal ganglia (Wegener & Koch, 2009). CNR1 activation by THC and other cannabinoids has also been shown to induce apoptosis through a complex cascade of kinases and caspases (Chan et al. 1998; Downer et al. 2003). CNR1-induced apoptosis is preceded by phosphorylation of p38 (Derkinderen et al. 2001; Powles et al. 2005), a member of the mitogen-activated protein kinases (MAPKs).
Despite clear evidence from animal studies that THC induces neural cell death, human studies have been less certain regarding the harmful effect of marijuana on brain structure (Quickfall & Crockford, 2006; Lorenzetti et al. 2010; Martin-Santos et al. 2010) or on cognitive function (Fried et al. 2005; Jockers-Scherubl et al. 2007; Rodriguez-Sanchez et al. 2010; Fernández-Serrano et al. 2011; Rabin et al. 2011; Yücel et al. 2012). The first published literature review of in vivo neuroimaging studies concluded that ‘ (structural brain) abnormalities generally have not been identified with chronic (marijuana) use’ (Quickfall & Crockford, 2006). However, two subsequent reviews of additional studies indicate that marijuana use is associated with medial temporal lobe volume decrement (Lorenzetti et al. 2010; Martin-Santos et al. 2010). Studies published after 2008 provide strong support that marijuana use is associated with brain volume deficits (Ashtari et al. 2009, 2011; Medina et al. 2009, 2010; Mata et al. 2010; Lopez-Larson et al. 2011; McQueeny et al. 2011; Solowij et al. 2011). For example, marijuana users have reduced frontal and lingual cortical thickness (Lopez-Larson et al. 2011), smaller hippocampal volumes (Ashtari et al. 2011), and cerebellar vermis abnormalities correlate with poor cognitive function (Medina et al. 2010). In schizophrenia patients, some (Szeszko et al. 2007; Bangalore et al. 2008; Rais et al. 2008, 2010; Peters et al. 2009; Dekker et al. 2010; Ho et al. 2011b; James et al. 2011) but not all studies (Wobrock et al. 2009; Cohen et al. 2011) find that, compared to patients who are non-users, patients with co-morbid marijuana use have greater frontotemporal and cerebellar deficits. Szeszko et al. (2007) reported that schizophrenia patients with marijuana misuse had smaller anterior cingulate gray matter (GM) volumes. Schizophrenia patients who continued to use marijuana have greater GM volume loss than non-users (Rais et al. 2010). In a recent study, our group reported that schizophrenia patients with marijuana misuse had smaller frontotemporal white-matter (WM) volumes than patients without heavy marijuana use (Ho et al. 2011b). We also found that heavy marijuana use in conjunction with specific CNR1 gene variants (rs12720071-G-allele carriers) contributed to greater WM brain volume deficits and cognitive impairment among schizophrenia patients (Ho et al. 2011b).
In the current study, we evaluated the effects of another cannabinoid-related gene, MAPK14, on magnetic resonance imaging (MRI) brain morphometry in schizophrenia patients. Schizophrenia has been linked to a pathophysiological failure to mount an effective response to an apoptotic insult (Jarskog 2006). There is also supporting evidence that apoptosis is down-regulated in schizophrenia (Benes 2006). Because CNR1-induced apoptosis is preceded by p38 MAPK phosphorylation (Derkinderen et al. 2001; Powles et al. 2005), we wanted to see how genetic variations within genes encoding both mediators of CNR1-induced apoptosis may influence brain morphology in the presence of marijuana misuse among schizophrenia patients. Our hypothesis was that patients with specific MAPK14 genotypes are more vulnerable to the effects of heavy marijuana misuse and would show greater brain volume deficits than patients without marijuana misuse.
Method
Subject selection
The study sample consists of 235 patients with schizophrenia-spectrum disorders who were recruited through the University of Iowa Mental Health Clinical Research Center (MHCRC). Our subjects participated in various MHCRC research studies approved by the University of Iowa human subjects research review board. All the subjects gave written informed consent to undergo research assessments, which included a morphometric MR brain scan and blood sampling for DNA analyses. These subjects have been included in a previous report (Ho et al. 2011b).
Demographic, clinical and genetic characteristics of the sample are summarized in Table 1. Most of the subjects (94%, n = 221) met DSM-IV criteria for schizophrenia; 6.0% (n = 14) had schizo-affective disorder. The subjects were of Caucasian ancestry and were predominantly male (74.5%). They were relatively young, with a mean age of 27.9 years (S.D. = 9.44), and had become psychiatrically ill recently at the time of study enrollment. The mean age at illness onset was 24.9 years (S.D. = 8.4) and the mean duration of illness was 3.2 years (S.D. = 5.7).
Table 1.
Demographic, clinical and genetic characteristics of the study population by marijuana status
| Marijuana abuse/dependence (MJ+) | No marijuana abuse/dependence (MJ−) | t or χ2 (p) | |
|---|---|---|---|
| n | 52 | 183 | |
| Age (years), mean (S.D.) | 24.0 (6.5) | 29.0 (9.9) | 4.29 (<0.001) |
| Male gender, n (%) | 48 (92.3) | 127 (69.4) | 11.2 (0.001) |
| Mean illness duration (years) | 2.5 (4.5) | 3.3 (6.0) | 1.04 (0.30) |
| Other substance usea, n (%) | 31 (59.6) | 32 (17.5) | 36.60 (<0.001) |
| Antipsychotic naïve, n (%) | 8 (15.4) | 25 (13.7) | 0.10 (0.75) |
| Ever needed clozapine, n (%) | 4 (7.7) | 20 (10.9) | 0.46 (0.50) |
| Minor allele frequency (%) MJ+ subjects versus MJ− subjects | |||
| rs3804454 (C) | 26.9 | 20.5 | 2.34 (0.31) |
| rs2237094 (G) | 10.6 | 5.5 | 5.93 (0.052) |
| rs12199654 (G) | 4.8 | 6.8 | 0.55 (0.46) |
| rs851007 (T) | 42.3 | 49.2 | 2.11 (0.35) |
| rs851006 (A) | 29.8 | 27.3 | 0.79 (0.67) |
| rs3804452 (T) | 12.5 | 12.0 | 0.01 (0.90) |
| rs8510 (T) | 13.5 | 11.8 | 0.22 (0.64) |
| rs7757672 (G) | 26.9 | 29.0 | 0.16 (0.92) |
| rs916346 (A) | 18.3 | 18.6 | 0.01 (0.94) |
Lifetime alcohol and/or non-marijuana illicit drug abuse/dependence.
Substance use
Subjects were assessed for substance use (including alcohol and illicit drugs) using the semi-structured interview instrument, the Comprehensive Assessment of Symptoms and History (CASH; Andreasen et al. 1992). Information on substance use history from multiple sources was available (including the subject, family members and medical records) and used to determine lifetime substance abuse or dependence diagnoses meeting DSM-IV criteria (Ho et al. 2004). The CASH evaluates eight drug categories : alcohol, barbiturates/hypnotics, opioids, cocaine, amphetamines/stimulants, phencyclidine, hallucinogens and marijuana. For a given drug category, the subjects are asked if they have ever used the drug, pattern of use, period of heaviest use, and associated impairment relating to DSM abuse and dependence diagnostic criteria. We have good inter-rater reliability in our CASH alcohol/illicit drug ratings (mean intra-class r = 0.75, S.D. = 0.16).
We contrasted patients with marijuana abuse or dependence [MJ+, n = 52 (i.e. 33 patients with marijuana abuse and 19 patients with marijuana dependence)] against 183 patients who never met DSM criteria for marijuana abuse or dependence (MJ−). MJ+ patients were significantly younger, more likely to be male and to have co-morbid alcohol and/or non-marijuana illicit substance misuse (Table 1, p≤0.001). Otherwise, the two groups were comparable with respect to other sociodemographic measures, illness characteristics and antipsychotic treatment (p≥0.30).
Selection of tag single nucleotide polymorphisms (tSNPs) and genotyping
In this study we investigated tSNPs so as to maximally represent common genetic variants in the population. Nine MAPK14 tSNPs were selected using Haploview (Barrett et al. 2005) (aggressive tagging 2-marker haplotype r2 ≥0.8) and the HapMap CEU population SNP database (www.hapmap.org, Release 22/Phase II). These tSNPs (all of which are synonymous) span approximately 81 kb at chromosome 6p21.3-p21.2. To genotype the study participants, DNA was prepared by high-salt extraction from whole blood (Lahiri & Nurnberger, 1991) and assayed using Infininium II assay BeadChips (Illumina, USA). Genotype call rates were 100% for each of the nine MAPK14 tSNPs. Illumina makes use of their proprietary software to ascertain genotyping quality. A 10% GenCall score (i.e. the 10th percentile rank for all GenCall scores of the study samples at a given locus) ≥0.7 constitutes high-quality genotype data. The mean 10% GenCall score for the nine MAPK14 tSNPs was 0.83 (S.D. = 0.15). We selected the CNR1 rs12720071 SNP because this variant has been previously associated with reduced WM brain volumes and heavy marijuana use (Ho et al. 2011b). The genotype call rate for CNR1 rs12720071 was also 100% (10% GenCall score = 0.84).
MRI acquisition and image processing
High-resolution morphometric brain MR data were collected using one of two imaging protocols. For subjects enrolled into the study before the year 2000, MRI brain scans were acquired on a 1.5-T GE (General Electric Medical Systems, USA) Signa MR scanner. In this imaging protocol (termed ‘MR5’), three-dimensional (3D) T1-weighted images were obtained in the coronal plane using a spoiled Gradient Recalled Acquisition in the Steady State (GRASS) sequence (SPGR) [parameters: echo time (TE) = 5 ms, repetition time (TR) = 24 ms, numbers of excitations (NEX) = 2, nutation angle = 45°, field of view (FOV) = 26×24×18.8 cm, matrix = 256×192×124]. Two-dimensional (2D) proton density (PD) and T2 sequences were acquired as follows: 3.0-or 4.0-mm-thick coronal slices, TR = 3000 ms, TE = 36 or 96 ms (PD/T2), NEX = 1, FOV = 26×26 cm, matrix = 256×192. For subjects recruited in 2000 or later, we used a 1.5-T Siemens Avanto scanner (Siemens AG, Germany). In this more recent imaging protocol (termed ‘MR6’), the T1 sequence was obtained in the coronal plane as a 3D volume using SPGR (parameters: TE = 6 ms, TR = 20 ms, flip angle = 30°, FOV = 16×16×19 cm, matrix = 256× 256×124, NEX = 2). The MR6 T2-weighted images were acquired in the coronal plane using a 2D fast spin–echo sequence (parameters: TE = 85 ms, TR = 4800 ms, slice thickness/gap = 1.8/0.0 mm, FOV = 16×16 cm, matrix = 256×256, NEX = 3, number of echoes = 8, 124 slices).
MR images were processed using our locally developed BRAINS2 (Brain Research: Analysis of Images, Networks, and Systems, version 2) software package (Magnotta et al. 2002). Detailed descriptions of the image analysis methods have been provided elsewhere (Andreasen et al. 1993, 1994, 1996; Harris et al. 1999). In brief, the T1-weighted images were spatially normalized and resampled so that the anterior–posterior axis of the brain was realigned parallel to the anterior–posterior commissure line, and the interhemispheric fissure was aligned on the other two axes. The T2-weighted images were aligned to the spatially normalized T1-weighted image using an automated image registration program (Woods et al. 1992). These images were then subjected to a linear transformation into standardized stereotaxic Talairach atlas space (Talairach & Tournoux, 1988) to generate automated measurements of frontal, temporal, parietal and occipital lobes (Andreasen et al. 1996). To further classify tissue volumes into GM, WM and cerebrospinal fluid (CSF), we used a discriminant analysis method of tissue segmentation based on automated training class selection that used data from the T1 and T2 sequences (Harris et al. 1999). In this study, we examined total and lobar (Talairach atlas-based frontal, temporal and parietal subdivisions) GM and WM brain volumes and lateral ventricles.
To enhance MR5 and MR6 data compatibility, MR6 scans were resampled into the same resolution and image size as the MR5 scans so as to simulate similar amounts of partial volume effects in voxels that bordered two tissue types. To verify our ability to combine data from the two MR protocols, we have acquired both MR5 and MR6 scans on 60 patients (Ho et al. 2011a). Brain volume differences between the two imaging sequences were small (median difference = 0.19%). Intra-class correlations (ICCs) were high across the regions of interest (median ICC = 0.97). Hence, MR5 and MR6 data are compatible for combined statistical analyses.
Statistical analyses
Analyses were performed using Haploview (Barrett et al. 2005) and SAS version 9.2 (SAS Institute, USA). Inter-correlations between the nine MAPK14 tSNPs were analyzed with pair-wise linkage disequilibrium (LD) statistics within Haploview. Because only a minority of the sample had heavy marijuana misuse, we grouped patients with marijuana abuse and patients with marijuana dependence together (n = 52) for statistical analyses. Furthermore, as there were no significant group differences in sociodemographics, illness characteristics, MRI brain volumes or MAPK14 tSNP allele frequencies between patients without prior marijuana exposure (n = 106) and patients whose marijuana use had not met DSM criteria for marijuana abuse or dependence (n = 77) (data not shown but available upon request), these patients were grouped together (n = 183) for comparison with patients with marijuana abuse or dependence. Group differences on categorical variables were tested using the χ2 test and continuous variables the independent group t test or ANCOVA.
Statistical analyses were conducted in stages to reduce Type I errors, which may arise from multiple comparisons. To assess brain volume–MAPK14 relationships, we first tested the effects of each MAPK14 genotype (minor allele carriers versus major allele homozygotes) on total cerebral GM or WM volumes using the adaptive false discovery rate (FDR) procedure (Benjamini & Hochberg, 2000). For each general linear model, total cerebral brain volume was entered as the dependent measure and genotype as the independent variable. On MAPK14 genotypes in which the total cerebral brain volume test was statistically significant (FDR-adjusted p≤0.05), follow-up analyses were carried out to further assess brain volume–MAPK14 relationships between patients with versus patients without marijuana abuse/dependence. In each follow-up ANCOVA, the dependent variable was frontal, temporal or parietal lobar brain volume. Genotype, marijuana misuse (presence versus absence of lifetime marijuana abuse or dependence) and genotype × marijuana misuse interaction terms were the independent measures. Covariates included in all ANCOVAs were intracranial volume, age, gender, imaging protocol, antipsychotic treatment (lifetime antipsychotic exposure) and alcohol/non-cannabis illicit substance abuse/dependence. Intracranial volume adjusts for cranial size differences among subjects. Age, gender, antipsychotic exposure and alcohol/other illicit substance use (presence versus absence of lifetime alcohol abuse/dependence or non-marijuana illicit substance abuse/dependence) have previously been shown to affect brain volumes, and may potentially confound brain volume–MAPK14 relationships. We included imaging protocol (i.e. MR5 versus MR6 scanning protocol) as a covariate in the statistical models even though we have previously shown that these two scanning sequences provide comparable neuroimaging data (Ho et al. 2011a).
Results
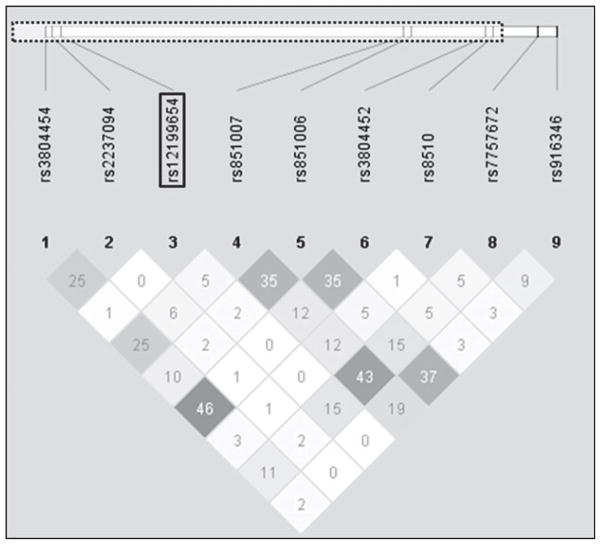
Genotype distributions of the nine MAPK14 tSNPs were in Hardy–Weinberg equilibrium (p≥0.08). These MAPK14 tSNPs were not in LD with one another (Fig. 1; pair-wise r2 ≤0.46). Allele frequency distributions for MAPK14 tSNPs did not differ significantly between MJ+ and MJ− subjects (see Table 1).
Fig. 1.
Pair-wise linkage disequilibrium (R2) between nine mitogen-activated protein kinase 14 (MAPK14) tag single nucleotide polymorphisms (tSNPs) and their relative genomic positions to the p38α MAPK14 gene (dotted line box).
Relationships between MAPK14 tSNPs, brain volumes and marijuana misuse
Table 2 summarizes the effects of MAPK14 tSNPs and total cerebral brain volumes. After accounting for multiple testing, only rs12199654 was significantly associated with total cerebral WM volumes (F = 9.41, FDR-adjusted p = 0.02). The effects of rs12199654 on total cerebral GM volume were not statistically significant (F = 5.17, FDR-adjusted p = 0.22, uncorrected p = 0.02). None of the remaining eight MAPK14 tSNPs were significantly associated with total cerebral GM volumes (F≤2.60, FDR-adjusted p≥0.11) or with total cerebral WM volumes (F≤3.18, FDR-adjusted p≥0.34).
Table 2.
Relationshipsa between nine MAPK14 tSNPs and total cerebral brain volumes
| Genotype | Total cerebral GM (p)
|
Total cerebral WM (p)
|
||
|---|---|---|---|---|
| Uncorrected | FDR adjusted | Uncorrected | FDR adjusted | |
| rs3804454 | 0.11 | 0.33 | 0.99 | 0.99 |
| rs2237094 | 0.11 | 0.33 | 0.85 | 0.95 |
| rs12199654 | 0.02 | 0.22 | 0.002 | 0.02 |
| rs851007 | 0.62 | 0.90 | 0.28 | 0.63 |
| rs851006 | 0.88 | 0.90 | 0.52 | 0.87 |
| rs3804452 | 0.90 | 0.90 | 0.62 | 0.87 |
| rs8510 | 0.20 | 0.45 | 0.13 | 0.39 |
| rs7757672 | 0.69 | 0.90 | 0.08 | 0.34 |
| rs916346 | 0.72 | 0.90 | 0.68 | 0.87 |
MAPK, Mitogen-activated protein kinase; tSNP, tag single nucleotide polymorphism; GM, gray matter; WM, white matter; FDR, false discovery rate.
ANCOVA (uncorrected and FDR-adjusted p values) assessing the main effects of the MAPK14 genotype on total cerebral GM or WM volumes (covariates : intracranial volume, age, sex, imaging protocol, antipsychotic treatment and alcohol/non-cannabis drug abuse/dependence).
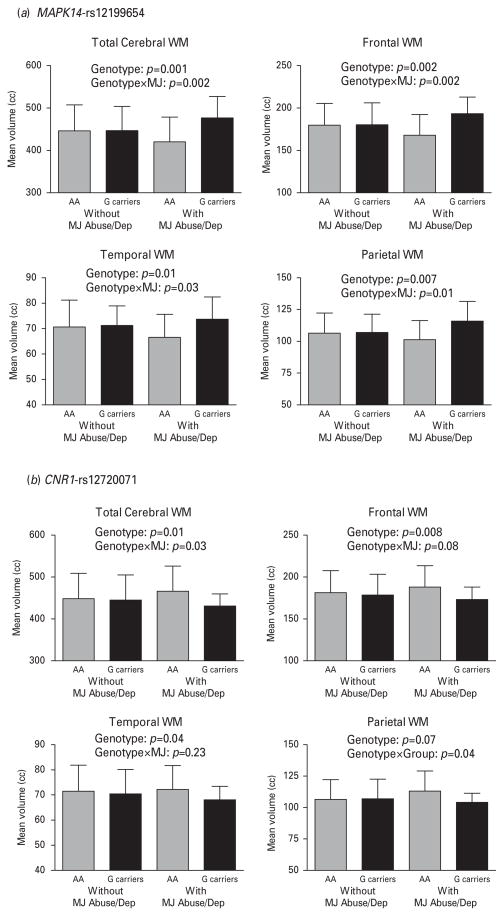
Next, we examined the effect of rs12199654 on total cerebral and lobarWMvolumes in patients with versus without marijuana misuse (Table 3). There were significant main effects for the rs12119654 genotype and genotype×MJ interaction on total cerebral, frontal, temporal and parietal WM volumes. Among patients with marijuana misuse, rs12199654-A homozygotes had significantly smaller WM volumes than G-allele carriers (F≥4.91, df = 1,51, p≤0.03). By contrast, WM volumes did not differ significantly among MJ− patients across the rs12199654 genotype groupings (F≤0.22, df = 1,182, p≥0.64).
Table 3.
ANCOVA main effects of the MAPK14 rs12199654 genotype (alonea and in conjunction with CNR1 rs12720071b) on white-matter (WM) brain volumes among schizophrenia patients with or without marijuana misuse
| Cerebral WM
|
Frontal WM
|
Temporal WM
|
Parietal WM
|
|||||
|---|---|---|---|---|---|---|---|---|
| G | G×MJ | G | G×MJ | G | G×MJ | G | G×MJ | |
| MAPK14 rs12199654a | 0.002 | 0.002 | 0.003 | 0.003 | 0.01 | 0.03 | 0.009 | 0.01 |
| MAPK14 rs12199654b | 0.001 | 0.002 | 0.002 | 0.002 | 0.01 | 0.03 | 0.007 | 0.01 |
| CNR1 rs12720071b | 0.01 | 0.03 | 0.008 | 0.03 | 0.04 | 0.23 | 0.07 | 0.04 |
G, Genotype; G×MJ, genotype interaction with marijuana misuse.
With both genotypes included in the ANCOVA (covariates : intracranial volume, age, sex, imaging protocol, antipsychotic treatment and alcohol/non-cannabis drug abuse/dependence).
Independent effects of MAPK14 rs12199654 and CNR1 rs12720071 on WM brain volumes in association with marijuana misuse
When the CNR1 rs12720071 genotype was included in the ANCOVA general linear models, the main effects of the MAPK14 rs12199654 genotype and genotype×marijuana misuse interaction on WM volumes did not change substantially and remained statistically significant (Table 3 and Fig. 2a ; F≥6.66, df = 1,234, p≤0.01). After controlling for the effects of MAPK14 rs12199654, there were significant main effects of CNR1 rs12720071 on total cerebral, frontal and temporal WM volumes (Table 3 and Fig. 2b; F≥6.76, df = 1,234, p≤0.01). The effects of CNR1 rs12720071 on parietal WM volumes approached but did not achieve statistical significance (p = 0.07). There were also significant CNR1 rs12720071 genotype×MJ interaction effects on total cerebral, frontal and parietal WM volumes (F≥4.72, df = 1,234, p≤0.03), such that rs12720071-G-allele carriers with heavy marijuana use had significantly smaller WM volumes than their A homozygote counterparts (Fig. 2b, F≥4.74, df = 1,51, p≤0.03). However, among MJ− patients, WM volumes did not differ significantly across CNR1 rs12720071 genotype groupings (F≤0.07, df = 1,182, p≥0.79). There were no significant CNR1 rs12720071 genotype×marijuana misuse interactions on temporal WM volumes.
Fig. 2.
Mean (error bars show standard deviation) white-matter (WM) brain volumes of patient subgroups and ANCOVAs showing independent effects of genotype [(a) mitogen-activated protein kinase 14 (MAPK14) rs12199654 or (b) cannabinoid receptor 1 (CNR1) rs12720071] and genotype×marijuana misuse interaction (genotype×MJ) on WM brain volumes. Subgroup samples subdivided based on genotype and presence/absence of lifetime marijuana abuse or dependence (MJ Abuse/Dep).
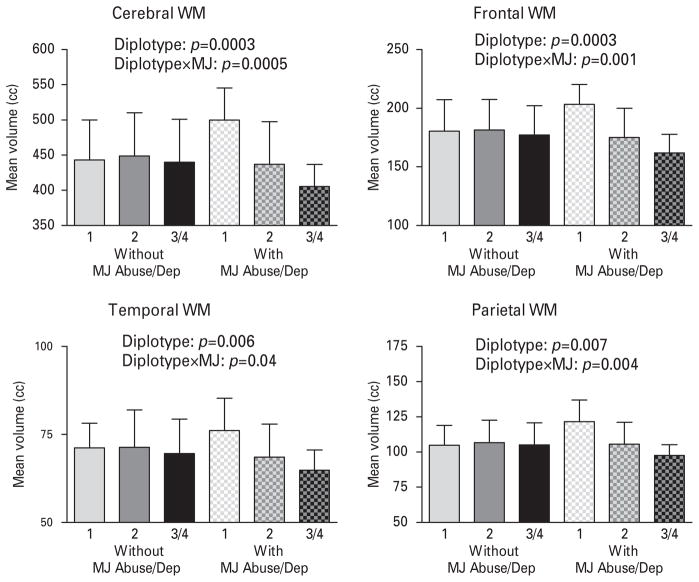
To further illustrate the additive effects of these two genes known to mediate a common biological pathway, we categorized subjects into three distinct diplotypes based on the number of ‘ risk ’ alleles within MAPK14 rs12199654(A) and within CNR1 rs12720071(G) associated with smaller WM volumes (Table 4). Patients with the MAPK14 rs12199654-AG and CNR1 rs12720071-AA diplotype had one ‘ risk ’ allele. Patients with the MAPK14 rs12199654-AA and CNR1 rs12720071-AA diplotype or the MAPK14 rs12199654-AG and CNR1 rs12720071-AG diplotype had two ‘ risk ’ alleles. Patients with the MAPK14 rs12199654-AA and CNR1 rs12720071-AG diplotype or the MAPK14 rs12199654-AA and CNR1 rs12720071-GG diplotype had three or four ‘ risk ’ alleles. There were significant main effects of diplotype grouping (p≤0.007) and diplotype×marijuana misuse interaction (p≤0.04) on WM brain volumes (Fig. 3). A greater number of MAPK14-CNR1 ‘ risk ’ alleles was associated with a smaller WM volume only among subjects with marijuana misuse.
Table 4.
Study sample distribution (n) of MAPK14 rs12199654 and CNR1 rs12720071 diplotype groupingsa subdivided by patients with (MJ+) or without (MJ−) marijuana abuse/dependence
| MAPK14 rs12199654 |
CNR1 rs12720071
|
|||||
|---|---|---|---|---|---|---|
| MJ− (n)
|
MJ+ (n)
|
|||||
| AA | AG | GG | AA | AG | GG | |
| AA | 137 | 20 | 1 | 40 | 7 | 0 |
| AG | 19 | 6 | 0 | 4 | 1 | 0 |
Based on the number of ‘ risk ’ alleles (i.e. MAPK14 rs12199654-A allele or CNR1 rs12720071-G allele) associated with smaller white matter (WM) brain volumes: one ‘ risk ’ allele : patients with MAPK14 rs12199654-AG and CNR1 rs12720071-AA diplotype ; two ‘ risk ’ alleles : patients with MAPK14 rs12199654-AA and CNR1 rs12720071-AA diplotype or MAPK14 rs12199654-AG and CNR1 rs12720071-AG diplotype ; three or four ‘ risk ’ alleles : patients with MAPK14 rs12199654-AA and CNR1 rs12720071-AG diplotype or MAPK14 rs12199654-AA and CNR1 rs12720071-GG diplotype.
Fig. 3.
Mean (error bars show standard deviation) white-matter (WM) brain volumes of mitogen-activated protein kinase 14 (MAPK14) rs12199654 and cannabinoid receptor 1 (CNR1) rs12720071 diplotype groupings (see Table 4 footnote) subdivided by patients with or without marijuana abuse/dependence and independent effects of diplotype and diplotype×marijuana misuse interaction (diplotype×MJ) on WM brain volumes.
Discussion
In the present study, we investigated the relationships between MAPK14 and CNR1 genetic variants and brain volumes of schizophrenia patients stratified by severity of marijuana misuse. These two genes were examined because CNR1 and p38α MAPK have been implicated in THC-induced apoptosis. We found that, in the case of heavy marijuana use, specific allelic combinations of these two cannabinoid-related genes were associated with smaller WM brain volumes. The MAPK14 rs12199654-A-allele and the CNR1 rs12720071-G-allele each had independent effects on diffuse WM volume decrement among schizophrenia patients with heavy marijuana use. Such marijuana misuse–MAPK14–CNR1 inter-relationships may mediate increased apoptosis, disrupt WM maturation, and heighten disease vulnerability within subgroups of schizophrenia patients.
CNR1 is a member of the superfamily of G-protein-coupled receptors. CNR1 transduction occurs through Gi/o proteins interacting with a wide variety of second messengers including phosphorylation of MAPK, inhibition of adenylyl cyclase and regulation of ion (calcium and potassium) channels (Howlett & Mukhopadhyay, 2000; Turu & Hunyady, 2010). CNR1 stimulation by THC and other CNR1 agonists is followed by p38 MAPK activation in various neural cell types (Derkinderen et al. 2001). Of the four known p38 MAPKs in mammals (α, β, γ and Δ), p38α (MAPK14) is the most well-characterized isoform (Mielke & Herdegen, 2000). These p38 MAPK family members are approximately 60% identical in their amino acid sequences, but are encoded by different genes and have different tissue expression patterns. p38α is widely expressed at significant levels in multiple cell types, including neural cells (Lee et al. 2000). MAPK14 is localized to chromosome 6p21.3-p21.2, a schizophrenia susceptibility locus (Vawter et al. 2001). There are several alternatively spliced variants of p38α itself. Each isoform has different but overlapping substrate specificities and mechanisms of activation (Yagasaki et al. 2004; Casar et al. 2007; Cuadrado & Nebreda, 2010). MAPKs have been implicated in numerous biological processes (Cuadrado & Nebreda, 2010). Besides CNR1-associated activation, the p38 MAPK pathway is also triggered in response to stress and inflammation (Kyriakis & Avruch, 2001). Furthermore, MAPKs play important roles in regulating developmental processes such as cell proliferation, differentiation and survival (Cuenda & Rousseau, 2007).
Previous studies suggest that MAPK14 may be associated with schizophrenia (Vawter et al. 2004; Olsen et al. 2008; Xu et al. 2010). There is reduced MAPK14 gene expression in the dorsolateral prefrontal cortex of subjects with schizophrenia (Vawter et al. 2004). Xu et al. (2010) reported the combined effects of two microRNA transcripts (i.e. mir-30e and mir-24) and their respective target gene sites (including mir-24-MAPK14 rs3804452 gene–gene interaction) were nominally associated with schizophrenia risk. In the current study we did not find any significant associations between the rs3804452 SNP on brain volumes, marijuana misuse or interaction effects. Olsen et al. (2008) reported that three MAPK14 SNPs (i.e. rs9470207, rs6908372 and rs9462156) were weakly associated with schizophrenia.
Given that CNR1 and p38α are both vital components within the cascade pathways mediating THC-induced apoptosis, our findings suggest that genetic variants within CNR1 and MAPK14 may contribute to WM brain volume deficits through the deleterious effects of heavy marijuana use. Among schizophrenia patients without heavy marijuana misuse, we observed no significant differences in brain volumes across CNR1 and MAPK14 genotype or diplotype groupings. The MAPK family of proteins plays an important role in the regulation of oligodendrocyte differentiation and Schwann cell myelination (Fragoso et al. 2007; Haines et al. 2008). CNR1 has been found in oligodendrocytes (Moldrich & Wenger, 2000; Rodriguez et al. 2001) and in subventricular oligodendrocyte progenitor cells. Cannabinoid-mediated cellular signaling has been shown to control post-natal subventricular zone oligodendrogenesis (Arevalo-Martin et al. 2007), and enhance oligodendrocyte lineage cell survival during neurodevelopment (Molina-Holgado et al. 2002). Thus, our findings of associations between MAPK14 and CNR1 genetic variations and WM brain volumes are consistent with the roles of MAPK and CNR1 in maintaining neural integrity. Alternatively, the effects of MAPK14 rs12199654 on WM brain volume deficits may be unrelated to THC-induced apoptosis. p38 MAPKs serve diverse functions, including determination of cell survival during neurodevelopment and in mediating stress and immune responses. Aberrant neurodevelopment (Murray & Lewis, 1987; Weinberger, 1987) and abnormalities in immunoreactivity (Meyer et al. 2009) have been implicated in the neurobiology of schizophrenia. Other limitations of the current study include our small sample size of patients with marijuana misuse, lobar brain volume measures, absence of healthy comparison groups and potential confounding effects from co-morbid substance misuse. Our findings should therefore be considered preliminary and require further replication. Future studies will also need to examine healthy controls and subjects without concurrent alcohol and non-marijuana substance use to establish the specificity of the effects of these genetic polymorphisms on brain structure.
In conclusion, the current study indicates that, in the case of heavy marijuana use, specific MAPK14 and CNR1 genotypic combinations may mediate brain morphometric differences in schizophrenia patients.
Acknowledgments
This research was supported in part by National Institute of Mental Health (NIMH) grants MH68380, MH31593, MH40856, MH80128 and MH43271, and by Ortho-McNeil Janssen Scientific Affairs. Drs Andreasen, Ho and Wassink have received grant support from Ortho-McNeil Janssen Scientific Affairs.
Footnotes
Parts of this research were presented at the 49th Annual Meeting of the American College of Neuropsychopharmacology, Miami Beach, FL, USA, 7 December 2010.
Declaration of Interest
None.
References
- Andreasen NC, Cizadlo T, Harris G, Swayze V, O’Leary DS, Cohen G, Ehrhardt J, Yuh WT. Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. Journal of Neuropsychiatry and Clinical Neurosciences. 1993;5:121–130. doi: 10.1176/jnp.5.2.121. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, 2nd, O’Leary DS, Ehrhardt JC, Yuh WT. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. Journal of the American Medical Association. 1994;272:1763–1769. [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Archives of General Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze V, 2nd, Flashman LA, O’Leary DS, Ehrhardt JC, Yuh WT. Automatic atlas-based volume estimation of human brain regions from MR images. Journal of Computer Assisted Tomography. 1996;20:98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Andreasson S, Allebeck P, Engstrom A, Rydberg U. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–1486. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Arevalo-Martin A, Garcia-Ovejero D, Rubio-Araiz A, Gomez O, Molina-Holgado F, Molina-Holgado E. Cannabinoids modulate Olig2 and polysialylated neural cell adhesion molecule expression in the subventricular zone of post-natal rats through cannabinoid receptor 1 and cannabinoid receptor 2. European Journal of Neuroscience. 2007;26:1548–1559. doi: 10.1111/j.1460-9568.2007.05782.x. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, Gee J, Sevy S, Kumra S. Medial temporal structures and memory functions in adolescents with heavy cannabis use. Journal of Psychiatric Research. 2011;45:1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research. 2009;43:189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore SS, Prasad KM, Montrose DM, Goradia DD, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia – a region of interest, voxel based morphometric study. Schizophrenia Research. 2008;99:1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benes FM. Strategies for improving sensitivity of gene expression profiling : regulation of apoptosis in the limbic lobe of schizophrenics and bipolars. Progress in Brain Research. 2006;158:153–172. doi: 10.1016/S0079-6123(06)58008-2. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. Journal of Educational and Behavioral Statistics. 2000;25:60–83. [Google Scholar]
- Casar B, Sanz-Moreno V, Yazicioglu MN, Rodriguez J, Berciano MT, Lafarga M, Cobb MH, Crespo P. Mxi2 promotes stimulus-independent ERK nuclear translocation. EMBO Journal. 2007;26:635–646. doi: 10.1038/sj.emboj.7601523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GC, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol. Journal of Neuroscience. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Rasser PE, Peck G, Carr VJ, Ward PB, Thompson PM, Johnston P, Baker A, Schall U. Cerebellar grey-matter deficits, cannabis use and first-episode schizophrenia in adolescents and young adults. International Journal of Neuropsychopharmacology. 2011 doi: 10.1017/S146114571100068X. Published online : 4 May 2011. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochemical Journal. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochimica et Biophysica Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Testing hypotheses about the relationship between cannabis use and psychosis. Drug and Alcohol Dependence. 2003;71:37–48. doi: 10.1016/s0376-8716(03)00064-4. [DOI] [PubMed] [Google Scholar]
- Dekker N, Schmitz N, Peters BD, van Amelsvoort TA, Linszen DH, de Haan L. Cannabis use and callosal white matter structure and integrity in recent-onset schizophrenia. Psychiatry Research : Neuroimaging. 2010;181:51–56. doi: 10.1016/j.pscychresns.2009.06.003. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. The effect of cannabis on the brain : can it cause brain anomalies that lead to increased risk for schizophrenia ? Current Opinion in Psychiatry. 2008;21:140–150. doi: 10.1097/YCO.0b013e3282f51266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkinderen P, Ledent C, Parmentier M, Girault JA. Cannabinoids activate p38 mitogen-activated protein kinases through CB1 receptors in hippocampus. Journal of Neurochemistry. 2001;77:957–960. doi: 10.1046/j.1471-4159.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- Downer EJ, Fogarty MP, Campbell VA. Tetrahydrocannabinol-induced neurotoxicity depends on CB1 receptor-mediated c-Jun N-terminal kinase activation in cultured cortical neurons. British Journal of Pharmacology. 2003;140:547–557. doi: 10.1038/sj.bjp.0705464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Sewell RA, Ranganathan M. Cannabis and psychosis/schizophrenia : human studies. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:413–431. doi: 10.1007/s00406-009-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Pérez-García M, Verdejo-García A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neuroscience and Biobehavioral Reviews. 2011;35:377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Fragoso G, Haines JD, Roberston J, Pedraza L, Mushynski WE, Almazan G. p38 mitogen-activated protein kinase is required for central nervous system myelination. Glia. 2007;55:1531–1541. doi: 10.1002/glia.20567. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana – a comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gfroerer JC, Wu L-T, Penne MA. Initiation of Marijuana Use: Trends, Patterns, and Implications. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2002. Analytic Series : A-17, DHHS Publication No. SMA 02–3711. [Google Scholar]
- Haines JD, Fragoso G, Hossain S, Mushynski WE, Almazan G. p38 Mitogen-activated protein kinase regulates myelination. Journal of Molecular Neuroscience. 2008;35:23–33. doi: 10.1007/s12031-007-9011-0. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. Journal of Computer Assisted Tomography. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen H-U, van Os J. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. British Medical Journal. 2005;330:11. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, van Os J. Letter to the Editor : The coherence of the evidence linking cannabis with psychosis. Psychological Medicine. 2008;38:461–464. doi: 10.1017/S0033291707002279. [DOI] [PubMed] [Google Scholar]
- Hickman M, Vickerman P, Macleod J, Lewis G, Zammit S, Kirkbride J, Jones P. If cannabis caused schizophrenia – how many cannabis users may need to be prevented in order to prevent one case of schizophrenia ? England and Wales calculations. Addiction. 2009;104:1856–1861. doi: 10.1111/j.1360-0443.2009.02736.x. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Archives of General Psychiatry. 2011a;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Flaum M, Hubbard W, Arndt S, Andreasen NC. Validity of symptom assessment in psychotic disorders : information variance across different sources of history. Schizophrenia Research. 2004;68:299–307. doi: 10.1016/j.schres.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, Ziebell S, Andreasen NC. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophrenia Research. 2011b;128:66–75. doi: 10.1016/j.schres.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Mukhopadhyay S. Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chemistry and Physics of Lipids. 2000;108:53–70. doi: 10.1016/s0009-3084(00)00187-0. [DOI] [PubMed] [Google Scholar]
- James A, Hough M, James S, Winmill L, Burge L, Nijhawan S, Matthews PM, Zarei M. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS) Schizophrenia Research. 2011;128:91–97. doi: 10.1016/j.schres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Jarskog LF. Apoptosis in schizophrenia : pathophysiologic and therapeutic considerations. Current Opinion in Psychiatry. 2006;19:307–312. doi: 10.1097/01.yco.0000218603.25346.8f. [DOI] [PubMed] [Google Scholar]
- Jockers-Scherubl MC, Wolf T, Radzei N, Schlattmann P, Rentzsch J, Gomez-Carrillo de Castro A, Kuhl KP. Cannabis induces different cognitive changes in schizophrenic patients and in healthy controls. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31:1054–1063. doi: 10.1016/j.pnpbp.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Kumra S. Schizophrenia and cannabis use. Minnesota Medicine. 2007;90:36–38. [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological Reviews. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Research. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Park J, Che Y, Han P-L, Lee J-K. Constitutive activity and differential localization of p38α and p38β MAPKs in adult mouse brain. Journal of Neuroscience Research. 2000;60:623–631. doi: 10.1002/(SICI)1097-4547(20000601)60:5<623::AID-JNR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behavioural Brain Research. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yucel M. Structural MRI findings in long-term cannabis users : what do we know? Substance Use and Misuse. 2010;45:1787–1808. doi: 10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WTC, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Martin-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, Fusar-Poli P, Borgwardt S, Seal M, Busatto GF, McGuire P. Neuroimaging in cannabis use : a systematic review of the literature. Psychological Medicine. 2010;40:383–398. doi: 10.1017/S0033291709990729. [DOI] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, Vazquez-Barquero JL, Crespo-Facorro B. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Research. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behavioural Brain Research. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users : subtle gender effects. Addiction Biology. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophrenia Bulletin. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke K, Herdegen T. JNK and p38 stress kinases – degenerative effectors of signal-transduction-cascades in the nervous system. Progress in Neurobiology. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival : involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. Journal of Neuroscience. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder ? British Medical Journal (Clinical Research Edition) 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society : the hash realities. Nature Reviews Neuroscience. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- NSDUH. Results from the 2004 National Survey on Drug Use and Health : National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2005. [Google Scholar]
- Olsen L, Hansen T, Jakobsen K, Djurovic S, Melle I, Agartz I, Hall H, Ullum H, Timm S, Wang A, Jonsson E, Andreassen O, Werge T. The estrogen hypothesis of schizophrenia implicates glucose metabolism: association study in three independent samples. BMC Medical Genetics. 2008;9:39. doi: 10.1186/1471-2350-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. Journal of Psychopharmacology. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. Journal of Psychopharmacology. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Pacula RL, Grossman M, Chaloupka FJ, O’Malley P, Johnston LD, Farrelly MC. Marijuana and Youth. National Bureau of Economic Research; Cambridge, MA: 2000. [Accessed 14 October 2011]. NBER Working Paper No. 7703. www.nber.org/papers/w7703. [Google Scholar]
- Peters BD, de Haan L, Vlieger EJ, Majoie CB, den Heeten GJ, Linszen DH. Recent-onset schizophrenia and adolescent cannabis use : MRI evidence for structural hyperconnectivity ? Psychopharmacology Bulletin. 2009;42:75–88. [PubMed] [Google Scholar]
- Powles T, te Poele R, Shamash J, Chaplin T, Propper D, Joel S, Oliver T, Liu WM. Cannabis-induced cytotoxicity in leukemic cell lines : the role of the cannabinoid receptors and the MAPK pathway. Blood. 2005;105:1214–1221. doi: 10.1182/blood-2004-03-1182. [DOI] [PubMed] [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use : a review. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:318–332. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia : a meta-analysis. Schizophrenia Research. 2011;128:111–116. doi: 10.1016/j.schres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Rais M, Cahn W, Van Haren N, Schnack H, Caspers E, Hulshoff Pol H, Kahn R. Excessive brain volume loss over time in cannabis-using first-episode schizophrenia patients. American Journal of Psychiatry. 2008;165:490–496. doi: 10.1176/appi.ajp.2007.07071110. [DOI] [PubMed] [Google Scholar]
- Rais M, van Haren NE, Cahn W, Schnack HG, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. European Neuropsychopharmacology. 2010;20:855–865. doi: 10.1016/j.euroneuro.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sanchez JM, Ayesa-Arriola R, Mata I, Moreno-Calle T, Perez-Iglesias R, Gonzalez-Blanch C, Perianez JA, Vazquez-Barquero JL, Crespo-Facorro B. Cannabis use and cognitive functioning in first-episode schizophrenia patients. Schizophrenia Research. 2010;124:142–151. doi: 10.1016/j.schres.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat caudate putamen nucleus. Journal of Neuroscience. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. The effect of chronic peripubertal cannabinoid treatment on deficient object recognition memory in rats after neonatal mPFC lesion. European Neuropsychopharmacology. 2007;17:180–186. doi: 10.1016/j.euroneuro.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Sewell RA, Ranganathan M, D’Souza DC. Cannabinoids and psychosis. International Review of Psychiatry. 2009;21:152–162. doi: 10.1080/09540260902782802. [DOI] [PubMed] [Google Scholar]
- Solowij N, Yucel M, Respondek C, Whittle S, Lindsay E, Pantelis C, Lubman DI. Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychological Medicine. 2011;41:2349–2359. doi: 10.1017/S003329171100050X. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99:1333–1341. doi: 10.1111/j.1360-0443.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Sevy S, Kumra S, Rupp CI, Betensky JD, Lencz T, Ashtari M, Kane JM, Malhotra AK, Gunduz-Bruce H, Napolitano B, Bilder RM. Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. British Journal of Psychiatry. 2007;190:230–236. doi: 10.1192/bjp.bp.106.024521. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Turu G, Hunyady L. Signal transduction of the CB1 cannabinoid receptor. Journal of Molecular Endocrinology. 2010;44:75–85. doi: 10.1677/JME-08-0190. [DOI] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis : a longitudinal population-based study. American Journal of Epidemiology. 2002;156:319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Barrett T, Cheadle C, Sokolov BP, Wood WH, 3rd, Donovan DM, Webster M, Freed WJ, Becker KG. Application of cDNA microarrays to examine gene expression differences in schizophrenia. Brain Research Bulletin. 2001;55:641–650. doi: 10.1016/s0361-9230(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Ferran E, Galke B, Cooper K, Bunney WE, Byerley W. Microarray screening of lymphocyte gene expression differences in a multiplex schizophrenia pedigree. Schizophrenia Research. 2004;67:41–52. doi: 10.1016/s0920-9964(03)00151-8. [DOI] [PubMed] [Google Scholar]
- Wegener N, Koch M. Behavioural disturbances and altered Fos protein expression in adult rats after chronic pubertal cannabinoid treatment. Brain Research. 2009;1253:81–91. doi: 10.1016/j.brainres.2008.11.081. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- WHO. Cannabis : A Health Perspective and Research Agenda. World Health Organization; Geneva: 1997. [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Sittinger H, Behrendt B, D’Amelio R, Falkai P. Comorbid substance abuse and brain morphology in recent-onset psychosis. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:28–36. doi: 10.1007/s00406-008-0831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li F, Zhang B, Zhang K, Zhang F, Huang X, Sun N, Ren Y, Sui M, Liu P. MicroRNAs and target site screening reveals a pre-microRNA-30e variant associated with schizophrenia. Schizophrenia Research. 2010;119:219–227. doi: 10.1016/j.schres.2010.02.1070. [DOI] [PubMed] [Google Scholar]
- Yagasaki Y, Sudo T, Osada H. Exip, a splicing variant of p38alpha, participates in interleukin-1 receptor proximal complex and downregulates NF-kappaB pathway. FEBS Letters. 2004;575:136–140. doi: 10.1016/j.febslet.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Yücel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, Conus P, Takagi MJ, Fornito A, Wood SJ, McGorry PD, Pantelis C. The impact of cannabis use on cognitive functioning in patients with schizophrenia : a meta-analysis of existing findings and new data in a first-episode sample. Schizophrenia Bulletin. 2012;38:316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. British Medical Journal. 2002;325:1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]