Abstract
The peroxisome proliferator-activated receptor γ (PPARγ) mediates the activity of the insulin-sensitizing thiazolidinediones and plays an important role in adipocyte differentiation and fat accretion. The analysis of PPARγ functions in mature adipocytes is precluded by lethality of PPARγ–/– fetuses and tetraploid-rescued pups. Therefore we have selectively ablated PPARγ in adipocytes of adult mice by using the tamoxifen-dependent Cre-ERT2 recombination system. We show that mature PPARγ-null white and brown adipocytes die within a few days and are replaced by newly formed PPARγ-positive adipocytes, demonstrating that PPARγ is essential for the in vivo survival of mature adipocytes, in addition to its well established requirement for their differentiation. Our data suggest that potent PPARγ antagonists could be used to acutely reduce obesity.
Keywords: conditional somatic mutagenesis, tamoxifen-dependent, adipocyte maintenance, obesity, nuclear receptor
The nuclear receptor PPARγ is a ligand-dependent transcriptional regulator that heterodimerizes with retinoid X receptors (RXRs) and is activated by natural ligands, such as arachidonic acid metabolites and fatty acid-derived components, and by the insulin-sensitizing drugs thiazolidinediones. Activation of PPARγ by thiazolidinediones in white and brown preadipocyte cell lines results in robust differentiation into adipocytes, and administration of thiazolidinediones to rodents increases accumulation of white (WAT) and brown (BAT) adipose tissue deposits. Moreover, overexpression of PPARγ in fibroblasts induces adipogenesis, and PPARγ-null embryonic stem cells and fibroblastic cells from PPARγ-deficient embryos cannot differentiate into adipocytes in vitro. PPARγ is also known to be indispensable for adipose tissue formation in vivo, because mice chimeric for WT and PPARγ-null cells show little or no contribution of null cells to adipose tissue, and PPARγ-deficient pups, derived by tetraploid rescue that bypasses placental defects, lack BAT and WAT (for reviews, see refs. 1–4). To study PPARγ functions in mature adipocytes, we ablated PPARγ in adipocytes of adult mice through Tamoxifen (Tam) treatment of transgenic mice bearing a LoxP site-containing (floxed) PPARγ gene and expressing the ligand-dependent fusion protein between the Cre recombinase and a mutated ligand-binding domain of the human estrogen receptor α (Cre-ERT2) recombinase selectively in brown and white adipocytes. We show here that PPARγ-deficient adipocytes die within a few days, which triggers an inflammatory reaction in BAT and WAT, and are replaced by newly differentiated PPARγ-expressing adipocytes, which most probably derive from fibroblast-like preadipocyte cells.
Materials and Methods
Transgenic Mice. The aP2-Cre-ERT2 transgenic mice were described (5).
Genotyping. Genomic DNA was isolated from various tissues and cell types as described (5). Macrophages were collected from the i.p. cavity. The aP2-Cre-ERT2 transgene was genotyped on tail DNA (5). To identify the various PPARγ alleles on DNA extracted from tissues and cell types, genomic PCR was performed with the following primers (see Fig. 1 A): P1 (5′-CAGAAACATCTCTAGTGAAG-3′) and P2 (5′-ATGGGAGCATAGAAGCTTTGA-3′), 241 bp PPARγ + allele; P1 and P3 (5′-AAGTTATGCTAGCAAGCTTTGA-3′), 239 bp PPARγ L2 allele; P4 (5′-AAGAGAAGAGAAGGATATGGAG-3′) and P5 (5′-ATATTAATATGCTTAATATTACAGC-3′), 234 bp PPARγ L– allele; and P1 and P6 (5′-TGACATAGTAATTTTTAGTTCCC-3′), 226 bp control (CT) allele. The 32P-end-labeled oligonucleotide O7 (5′-TATACTATACACTGTGCAGCC-3′) was used as a probe to reveal the PCR products by Southern blotting. Purification of adipocytes by collagenase II treatment of adipose tissue and centrifugation was described (5).
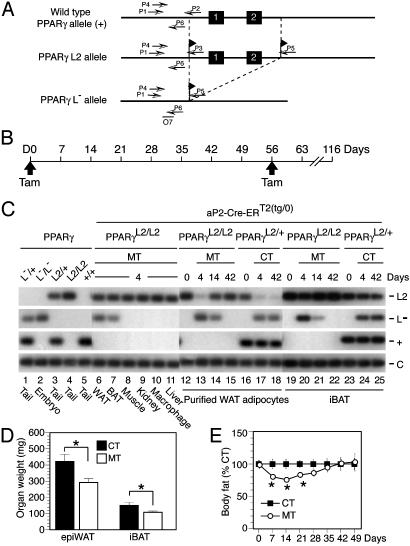
Fig. 1.
Selective Tam-induced PPARγ gene disruption in adipocytes of WAT and BAT of adult mice. (A) Diagram of the WT PPARγ genomic locus (+), the floxed PPARγ L2 allele, and the PPARγ L–-null allele obtained after Cre-mediated excision of exons 1 and 2. Black boxes and arrows indicate exons and PCR primers, respectively. The probe O7 location is shown. (B) Timing of Tam administration and phenotypic analysis. (C) Adipocyte-selective Tam-induced generation of PPARγ L– alleles. PCR analysis of DNA from the indicated cell types and tissues of 8-week-old aP2-Cre-ERT2(tg/0)/PPARγL2/L2 mice at D4 (lanes 6–11), from purified epididymal adipocytes and iBAT isolated from (i) aP2-Cre-ERT2(tg/0)/PPARγL2/L2 mice at D0, D4, D14, and D42 (lanes 12–15 and lanes 19–22, respectively) and (ii) control aP2-Cre-ERT2(tg/0)/PPARγL2/+ mice at D0, D4, and D42 (lanes 16–18 and lanes 23–25, respectively). Control PCR on genomic DNA from of PPARγL–/+, PPARγL2/+, PPARγL2/L2, and PPARγ+/+ mice and from 9.5 days postconception PPARγL–/L– embryos are presented in lanes 1–5; PCR fragments corresponding to the PPARγ L2, L–, and + alleles are displayed. Macrophage: i.p. cells containing ≈30% macrophages. (D) Epididymal fat pad (epiWAT) and iBAT weight of CT and of MT mice at D7. Values are expressed as the mean ± SEM (n = 5). *, P < 0.05. (E) Body fat content evaluated by DEXA scanning. CT and PPARγad–/– premutant mice (MT) were analyzed before and for 7 weeks after Tam treatment. The percentage of fat content in mutant mice relative to CT mice for each time point is shown. Values (black squares for CT and open circles for MT) are expressed as the mean ± SEM (n = 7). The body fat content of CT mice for each time point was set to 100. *, P < 0.05.
Animal Treatments and Analyses. Mice were housed on a 12 h light/12 h dark cycle and fed a standard laboratory chow [2,800 kcal/kg (1 cal = 4.184 J); Usine d'Alimentation Rationnelle, Villemoisson-sur-Orge, France]. Tam (1 mg in 100 μl of sunflower oil) was i.p. injected as described (6). Body fat content was evaluated by dual energy x-ray absorptiometry (PIXIMUS, GE Medical Systems, Buc, France) (7).
Histological and Electron Microscopic Analysis. For 5-μm sections, samples were fixed in Bouin's fixative, embedded in paraffin, and stained with hematoxylin and eosin or trichrome. Electron microscopy (EM) was performed as described (8).
RNA Analysis. RNA was isolated and Pref-1 expression analyzed by RT-PCR (5). Other transcripts levels were determined by real-time PCR with a Roche light cycler (Roche Diagnostics) and the Cyber Green kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. Hypoxanthine phosphoribosyltransferase was used as an invariant control. The following oligonucleotides were used: PGC1α, 5′-GGCTCTTGGTGACAGTGTGT-3′ and 5′-TGTGTCTTCATGGAAATGCTG-3′; ANC, 5′-CGTTCTCAGAGGCATGGGT-3′ and 5′-GATGAATATTATTGCTTCCCAC-3′; αATPase, 5′-ACGACTTCATGTTGAGTTCCA-3′ and 5′-CCGAATTTCATAGTGGACAG-3′; βATPase, 5′-CTTCCATGCAGGCCACACA-3′ and 5′-CCACCACTGTGAGCTCAATT-3′; δATPase, 5′-CAGCAGTGCTCCAGTTGCT-3′ and 5′-CCTGGTGTTTAATGGAGACAG-3′; cytochrome c, 5′-GTTGATCTGCAAATTAAAATGCT-3′ and 5′-CACGATCTGTGGTTGTTTTAAT-3′; COX II, 5′-CCCCTCCCTAGGACTTAAAA-3′ and 5′-TGGGCATAAAGCTATGGTTAG-3′; and COX IV, 5′-ACTTTCGATCGTGACTGGGT-3′ and 5′-AACTGGTCTTTTATTAGCATGG-3′.
Statistical Analysis. Values are reported as mean ± SEM. Statistical significance (P < 0.05) was determined by unpaired Student's t test (statview, Abacus Concepts, Berkeley, CA).
Results
To determine the function of PPARγ in mature adipocytes, PPARγ was selectively ablated in adipocytes of adult mice by using mice bearing LoxP-flanked PPARγ L2 alleles (Fig. 1A and Supporting Text, which is published as supporting information on the PNAS web site) and aP2-Cre-ERT2 transgenic mice that express, under the control of the adipocyte-selective aP2 promoter, the conditional Cre-ERT2 Cre recombinase whose activity depends on Tam administration (5). Eight-week-old aP2-Cre-ERT2(tg/0)/PPARγL2/L2 mice (hemizygous for the aP2-Cre-ERT2 transgene and homozygous for the PPARγ L2 allele, hereafter named PPARγad–/– premutant mice) and control littermates (aP2-Cre-ERT2(tg/0)/PPARγL2/+, aP2-Cre-ERT2(0/0)/PPARγL2/+, aP2-Cre-ERT2(tg/0)/PPARγ+/+, aP2-Cre-ERT2(0/0)/PPARγ+/+, and aP2-Cre-ERT2(0/0)/PPARγL2/L2 mice), were i.p. injected with 1 mg of Tam [day 0 (D0), Fig. 1B] to produce PPARγad–/– mutant (MT) mice, selectively bearing a PPARγ-null mutation (see Supporting Text) in adipocytes, and CT mice that were indistinguishable from WT mice. At day 4 (D4), 30–40% and 20–30% of the PPARγ L2 alleles from epididymal WAT and interscapular brown adipose tissue (iBAT) of PPARγad–/– premutant mice, respectively, were converted into PPARγ L– alleles, whereas no Cre-mediated recombination was observed in other cell types or tissues, such as peritoneal macrophages, muscle, kidney, and liver (Fig. 1C, lanes 6–11, and data not shown). Similar levels of PPARγ ablation were observed after Tam treatment for 5 consecutive days (data not shown). Purification of adipocytes from epididymal WAT of MT mice revealed that PPARγ was ablated in >90% of D4 adipocytes, whereas no DNA excision was observed at D0 (Fig. 1C, lanes 12 and 13, and data not shown). Thus, temporally controlled PPARγ ablation in mature white and brown adipocytes was efficiently induced by Tam treatment of premutant mice. Epididymal fat pad and iBAT weights were ≈30% lower in MT than in CT mice at D7 (Fig. 1D). Moreover, dual-energy x-ray adsorptiometry (DEXA) scanning revealed 17–25% reductions of the relative body fat content in MT animals from D7 to D21, whereas at D42 and D49 the relative body fat content was similar in MT and CT mice (Fig. 1E).
To estimate the fraction of PPARγ-deficient adipocytes over time, PPARγ alleles were characterized in purified epididymal adipocytes and iBAT from Tam-treated PPARγad–/– premutant (MT) and aP2-Cre-ERT2(tg/0)/PPARγL2/+ CT mice at various times after Tam administration. In WAT adipocytes from CT mice, most if not all PPARγ L2 alleles were converted into L– alleles at D4, and similar amounts of L– alleles were found for at least 6 weeks (Fig. 1C, lanes 16–18). In marked contrast, whereas PPARγ L2 alleles were efficiently converted into PPARγ L– alleles at D4 in WAT adipocytes from MT mice, only half of these PPARγ L– alleles were left by D14, and none were present at D42 (Fig. 1C, lanes 12–15). A similar disappearance of PPARγ L– alleles between D4 and D42 also occurred in BAT of MT, but not of CT mice (Fig. 1C, compare lanes 19–22 and 23–25). Because the aP2 promoter is known to be active only at late stages of adipocyte differentiation (2), we concluded from these data that mature white and brown adipocytes most probably died within 2–3 weeks after PPARγ ablation, to be progressively replaced by new adipocytes generated from PPARγL2/L2 preadipocytes. This possibility was first investigated at the histological level.
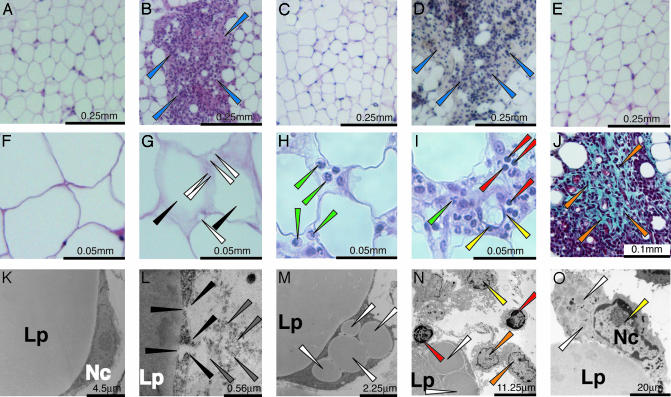
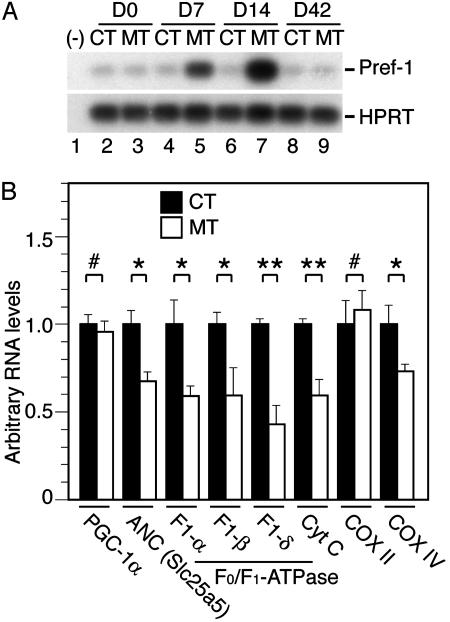
Before Tam treatment of PPARγad–/– premutant and CT mice and after Tam treatment of CT mice, epididymal fat pad contained large cells with a single, central large fat droplet and a peripheral nucleus surrounded by a thin rim of cytoplasm (Fig. 2 A and F, and data not shown). At D1 and D2, few (<5%) epididymal adipocytes of MT mice displayed irregular outlines, and about half of them contained supernumerary small lipid droplets (Fig. 2, compare G and F, and data not shown). Neutrophil infiltration was occasionally seen in the vicinity of adipocytes with irregular outlines (data not shown). With time, the number of abnormal adipocytes increased, and by D4 to D7 ≈30% of the adipocytes were affected and fat droplets were laying free in the connective tissue. Lymphocytes (B and T cells) and macrophages were infiltrating the adipose tissue, forming inflammatory foci in some regions (Fig. 2, compare A and B, see H and I, and data not shown). EM performed at D4 and D7 revealed the presence of adipocytes with a disrupted cell membrane (e.g., Fig. 2L). Because only some of these necrotic adipocytes contained groups of small lipid droplets adjacent to the large lipid droplet (Fig. 2 M and N and data not shown), it is unlikely that adipocyte death was caused by defects in lipid droplet formation or holding. Lymphocytes, macrophages, and fibroblasts were present in the vicinity of necrotic adipocytes and lipid droplets (Fig. 2 N and O; see below). Trichrome staining of histological sections at D7, D14, and D21 revealed abundant collagen deposits in inflammatory regions (Fig. 2 J and data not shown). Similar defects were observed in s.c. fat (data not shown). In marked contrast, at D42–D56 epididymal and s.c. fat pads of mutant mice were similar to those of CT mice (Fig. 2C and data not shown), thus suggesting that WAT had regenerated through differentiation of preadipocytes into mature adipocytes. This possibility was further supported by the expression of the Pref-1 gene which is known to occur in preadipocytes but not in mature adipocytes (9). Pref-1 transcripts were strongly increased at D7 and D14 in epididymal fat pad of MT mice, whereas their levels remained unchanged in CT mice (Fig. 3A). In agreement with the histological data, Pref-1 RNA levels were similar in D42 MT and CT mice (see Fig. 3A and data not shown). However, when Tam was readministered to MT mice at D56, abnormalities similar to those described above were observed over a 4-week period, but had totally disappeared by D116 (Fig. 2 D and E, respectively, and data not shown).
Fig. 2.
Histological analysis of WAT of Tam-treated PPARγad–/– premutant mice. Histological sections from paraffin-embedded epididymal WAT from CT (aP2-Cre-ERT2(0/0)/PPARγL2/L2) mice at D4 (A) and D2 (F) and MT mice at D2 (G), D4 (H and I), D7 (B), D14 (J), and D42 (C) (Tam injection at D0), and D67 (D) and D116 (E), after a second Tam injection at D56. (A–I) Hematoxylin/eosin staining. (J) Trichrome staining. Ultrastructure of adipose tissue of CT (aP2-Cre-ERT2(0/0)/PPARγL2/L2) at D4 (K) and MT mice at D 4 (L) and D7 (M–O). Blue arrows point to clusters of infiltrating cells (B and D); black arrows point to adipocytes with abnormal profiles (G); white arrows point to small lipid droplets in adipocytes (G, M, and N) and to phagocytosed lipid droplets (O); green arrows point to neutrophils (H and I); red arrows point to lymphocytes (I and N); yellow arrows point to macrophages (I, N, and O); orange arrows point to fibroblast-like cells (J and N); and black and gray arrows point to disrupted adipocyte cell membrane and cell debris (L). Lp, lipid droplet; Nc, nucleus. (Scales are indicated in each image.)
Fig. 3.
Expression of Pref-1 and genes involved in respiratory chain function in Tam-treated PPARγad–/– premutant mice. (A) Pref-1 expression was analyzed by RT-PCR performed on RNA extracted from epididymal WAT of CT (lanes 2, 4, 6, and 8) and MT (lanes 3, 5, 7, and 9) mice at D0, D7, D14, and D42. Hypoxanthine phosphoribosyltransferase was used as an internal control. (B) Transcript levels of the indicated genes analyzed by quantitative RT-PCR on RNA isolated from BAT of CT (filled bars) and MT (open bars) mice at D4. Values are expressed as mean ± SEM (relative to CT). n = 5. *, P < 0.05; **, P < 0.005; #, no statistically significant difference.
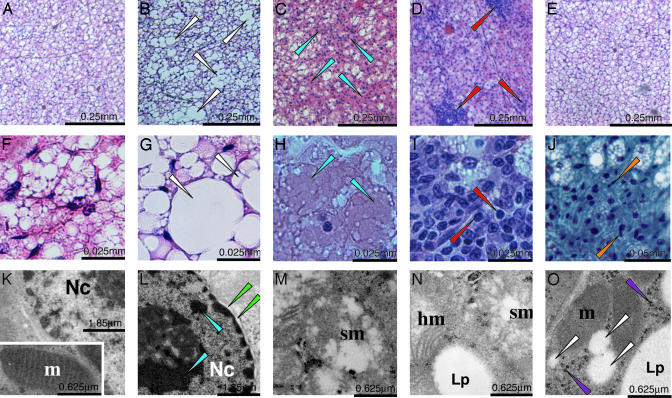
Histological examination of BAT sections at D1 and D2 did not reveal any obvious difference between CT and MT animals (data not shown). However, at D4 ≈30% of brown adipocytes from MT mice contained much larger lipid droplets than those from CT mice (Fig. 4, compare B and G with A and F, respectively). This increase was not caused by hypertriglyceridemia, as seen in ob/ob or fatless mice for example, because a 30% reduction of triglyceride plasma levels occurred in MT mice 1–4 weeks after Tam treatment (data not shown). EM analysis revealed the presence of numerous brown adipocytes with clumping of nuclear chromatin in MT mice (Fig. 4 compare K and L). Moreover, hypertrophic mitochondria, with normal or increased cristae density and swollen mitochondria, characterized by rare or peripherally displaced cristae, were seen in 5–10% and 60–70% of adipocytes, respectively (Fig. 4 M and N and data not shown). Impaired mitochondrial function was supported by a 25–50% reduction in transcripts of nuclear genes involved in respiratory chain function, such as the ATP synthase F1 α, β, and δ subunits, the adenine nucleotide translocator, the cytochrome c and the cytochrome c-oxidase subunit COX IV at D4, whereas those of the cold-inducible coactivator of nuclear receptors PGC-1α, and of the mitochondrially encoded COX II were not altered (Fig. 3B). At D7 and D14, 30–40% of the BAT section areas contained necrotic adipocytes (Fig. 4 C and H), markedly infiltrated by lymphocytes (Fig. 4 D and I). Moreover, foci of fibrosis were much larger than at D4 (Fig. 4J and data not shown). EM analysis at D14 confirmed the presence of numerous necrotic brown adipocytes with swollen and hypertrophic mitochondria and fibroblasts and collagen fibers (data not shown). However, ≈30% of cells with a high number of mitochondria contained numerous small lipid droplets (diameter, <0.3 μm), abundant ribosomes and polysomes, and small clusters of glycogen (Fig. 4O and data not shown), features reported in newborn adipoblasts (see ref. 10). Taken together with the progressive loss of PPARγ L– alleles and the concomitant increase of PPARγ L2 alleles (Fig. 1C), it appears that PPARγ-null brown adipocytes were progressively replaced by newly differentiated brown adipocytes. At D42, all BAT abnormalities had disappeared (Fig. 4E and data not shown).
Fig. 4.
Histological analysis of BAT of Tam-treated PPARγad–/– premutant mice. Histological sections from paraffin-embedded interscapular BAT from CT (aP2-Cre-ERT2(0/0)/PPARγL2/+) mice at D4 (A and F) and MT mice (B–E and G–J) at D4 (B and G), D7 (C and H), D14 (D, I, and J), and D42 (E). (A–I) Hematoxylin and eosin staining. (J) Trichrome staining. (K–O) EM. (K) CT (aP2-Cre-ERT2(0/0)/PPARγL2/+) at D4. (Inset) Typical mitochondria. (L–N) MT at D4. (O) mutant at D14. White arrows point to fused lipid droplets (B and G) and small lipid droplets (O); blue arrows point to necrotic area (C and H) and clumped chromatin (L); green arrows point to detached nuclear membrane (L); red arrows point to lymphocytes (D and I); orange arrows point to fibroblast-like cells (J); purple arrows point to glycogen (O). Lp, lipid droplet; Nc, nucleus; m, mitochondria; hm and sm, hypertrophic and swollen mitochondria, respectively. (Scales are indicated in each image.)
Discussion
The present study, in which PPARγ is selectively ablated in white and brown adipocytes of adult mice, shows that PPARγ-null adipocytes die within a few days, thus demonstrating that this nuclear receptor is essential for the survival of mature adipocytes. Surprisingly, since the completion of our study, it was reported that mice in which PPARγ was ablated in adipocytes by the Cre recombinase expressed under the control of the aP2 promoter became lipodystrophic only after several months (11). Because PPARγ is most probably ablated in these mice during adipocyte differentiation rather than in mature adipocytes, compensatory mechanisms might substitute for PPARγ functions during adipocyte differentiation. Alternatively or concomitantly, in young mutant animals, PPARγ null adipocytes might die and be efficiently replaced by newly differentiated adipocytes, but with age, progenitor cells might be exhausted, thus leading to lipodystrophy. The high number of small adipocytelike cells in aged mutant mice (11) might correspond to PPARγ-null preadipocytes that cannot further differentiate, whereas the hypertrophic adipocytes might represent, at least in part, PPARγ-expressing adipocytes, because PPARγ was only ablated in 90–95% of adipocytes.
Even though no adipocyte death was noticed in either RXRβ- or RXRγ-null mice (refs. 12 and 13 and unpublished results) or in mice selectively lacking RXRα in adipocytes [RXRαad–/– mice (5)], PPARγ most probably exerts its adipocyte vital functions as a heterodimer with RXR. Indeed, ablation of RXRα in adipocytes of RXRγ-null mice (but not of RXRβ-null mice) also results in similar mature adipocyte death, thus revealing a functional redundancy between RXRα and RXRγ, which could result from a compensatory enhanced expression of RXRγ in adipocytes of RXRαad–/– mice (5). No such redundancy occurs between RXRα and RXRγ for the function(s) exerted by PPARγ/RXR heterodimers when hypertrophic adipocytes are formed during a high-fat diet treatment, because mice selectively lacking RXRα in their adipocytes are resistant to high-fat diet-induced obesity (5). It appears, therefore, that the vital and lipogenic functions exerted by PPARγ in mature adipocytes have different requirements for RXR heterodimeric partners: the vital function can be mediated by either PPARγ/RXRα or PPARγ/RXRγ heterodimers, whereas the lipogenic function requires PPARγ/RXRα heterodimers. Because much more RXRα than RXRγ occurs in adipocytes, the threshold level of PPARγ/RXR heterodimers required to ensure the survival of adipocytes is therefore much lower than that required to trigger lipogenesis. These differential requirements offer an explanation as to why heterozygous PPARγ-deficient mice (14, 15) and mice treated with low-affinity PPARγ or RXR antagonists (15, 16) are resistant to high-fat diet-induced obesity, whereas administration for several weeks of such low-affinity RXR or PPARγ antagonists to heterozygous PPARγ-deficient mice is necessary to reach a lipoatrophic state with disappearance of visible WAT (16). It has been proposed (15, 16) that partial antagonists of PPARγ/RXR activity could be used to prevent obesity and related diseases such as type 2 diabetes. Our present data further suggest that high-affinity full antagonists of PPARγ/RXRα activity could possibly be used in treatment strategies aimed at acutely reducing obesity.
Finally, our study shows that, after the death of PPARγ-ablated white and brown adipocytes, newly differentiated adipocytes expressing PPARγ appear within a few weeks. They most probably derive from fibroblast-like cells, whose number was strongly increased after ablation of PPARγ. This adipocyte regeneration is in agreement with previous findings showing that adipose progenitor cells, the origin of which remains controversial, are widely distributed in connective tissues and can proliferate and differentiate into adipocytes in adult tissues (17). The present possibility to massively stimulate this process in adult mice might allow further characterization of the origin and nature of these precursor cells in vivo.
Supplementary Material
Acknowledgments
We thank R. Lorenz, N. Chartoire, M. F. Champy (Institut Clinique de la Souris), A. Dierich, and the animal facility staff for excellent technical assistance, and the secretariat for typing the manuscript. R.T. was supported by a fellowship from the Fondation de la Recherche Médicale, and E.D was supported by a fellowship from the Ministère de la Jeunesse, de l'Education Nationale et de la Recherche. This work was supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Collège de France, the Hôpital Universitaire de Strasbourg, the Association pour la Recherche sur le Cancer, the Fondation pour la Recherche Médicale, the Human Frontier Science Program, the Ministère de l'Education Nationale de la Recherche et de la Technologie, the Swiss National Science Foundation, the Etat de Vaud, and the European Community.
Abbreviations: BAT, brown adipose tissue; Cre-ERT2, fusion protein between the Cre recombinase and a mutated ligand-binding domain of the human estrogen receptor α; CT, control; EM, electron microscopy; iBAT, interscapular BAT; MT, mutant; PPAR, peroxisome proliferator-activated receptor; PPARγad–/–, adipocyte-selective PPARγ-deficient; RXR, retinoid X receptor; RXRαad–/–, adipocyte-selective RXRα-deficient; Tam, tamoxifen; WAT, white adipose tissue; Dn, day n.
References
- 1.Desvergne, B. & Wahli, W. (1999) Endocr. Rev. 20, 649–688. [DOI] [PubMed] [Google Scholar]
- 2.Rosen, E. D., Walkey, C. J., Puigserver, P. & Spiegelman, B. M. (2000) Genes Dev. 14, 1293–1307. [PubMed] [Google Scholar]
- 3.Kadowaki, T. (2000) J. Clin. Invest. 106, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, C. H., Olson, P. & Evans, R. M. (2003) Endocrinology 144, 2201–2207. [DOI] [PubMed] [Google Scholar]
- 5.Imai, T., Jiang, M., Chambon, P. & Metzger, D. (2001) Proc. Natl. Acad. Sci. USA 98, 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metzger, D. & Chambon, P. (2001) Methods 24, 71–80. [DOI] [PubMed] [Google Scholar]
- 7.Picard, F., Gehin, M., Annicotte, J., Rocchi, S., Champy, M. F., O'Malley, B. W., Chambon, P. & Auwerx, J. (2002) Cell 111, 931–941. [DOI] [PubMed] [Google Scholar]
- 8.Li, M., Chiba, H., Warot, X., Messaddeq, N., Gérard, C., Chambon, P. & Metzger, D. (2001) Development (Cambridge, U.K.) 128, 675–688. [DOI] [PubMed] [Google Scholar]
- 9.Gregoire, F. M., Smas, C. M. & Sul, H. S. (1998) Physiol. Rev. 78, 783–809. [DOI] [PubMed] [Google Scholar]
- 10.Cinti, S. (1999) The Adipose Organ (Editrice Kurtis, Milano, Italy).
- 11.He, W., Barak, Y., Hevener, A., Olson, P., Liao, D., Le, J., Nelson, M., Ong, E., Olefsky, J. M. & Evans, R. M. (2003) Proc. Natl. Acad. Sci. USA. 100, 15712–15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastner, P., Mark, M., Leid, M., Gansmuller, A., Chin, W., Grondona, J. M., Decimo, D., Krezel, W., Dierich, A. & Chambon, P. (1996) Genes Dev. 10, 80–92. [DOI] [PubMed] [Google Scholar]
- 13.Krezel, W., Dupe, V., Mark, M., Dierich, A., Kastner, P. & Chambon, P. (1996) Proc. Natl. Acad. Sci. USA 93, 9010–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota, N., Terauchi, Y., Miki, H., Tamemoto, H., Yamauchi, T., Komeda, K., Satoh, S., Nakano, R., Ishii, C., Sugiyama, T., et al. (1999) Mol. Cell 4, 597–609. [DOI] [PubMed] [Google Scholar]
- 15.Rieusset, J., Touri, F., Michalik, L., Escher, P., Desvergne, B., Niesor, E. & Wahli, W. (2002) Mol. Endocrinol. 16, 2628–2644. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi, T., Waki, H., Kamon, J., Murakami, K., Motojima, K., Komeda, K., Miki, H., Kubota, N., Terauchi, Y., Tsuchida, A., et al. (2001) J. Clin. Invest. 108, 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi, N., Toriyama, K., Nicodemou-Lena, E., Inou, K., Torii, S. & Kitagawa, Y. (1998) Proc. Natl. Acad. Sci. USA 95, 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.