Abstract
Brazil currently accounts for the majority of dengue cases reported in the Americas, with co-circulation of DENV 1, 2 and 3. Striking variation in the epidemiological pattern of infection within cities has been observed. Therefore, investigation of dengue transmission in small areas is important to formulate control strategies. A population-based household survey was performed in three diverse socio-economic and environmental areas of Recife, a large urban center of Brazil, between 2005 and 2006. Dengue serostatus and individual- and household-level risk factors for infection were collected in residents aged between 5 and 64 years. A total of 2,833 individuals were examined, and their residences were geo-referenced. Anti-dengue IgG antibodies were measured using commercial ELISA. The dengue seroprevalence and the force of infection were estimated in each area. Individual and household variables associated with seropositivity were assessed by multilevel models for each area. A spatial analysis was conducted to identify risk gradients of dengue seropositivity using generalized additive models (GAM). The dengue seroprevalence was 91.1%, 87.4% 74.3%, respectively, in the deprived, intermediate and high socioeconomic areas, inversely related to their socio-economic status. In the deprived area, 59% of children had already been exposed to dengue virus by the age of 5 years and the estimated force of infection was three times higher than that in the privileged area. The risk of infection increased with age in the three areas. Working or studying outside the home area was a risk factor for seropositivity in the deprived area (OR=2.26; 95% CI: 1.18-4.30). Number of persons per room was a risk factor for seropositivity in the intermediate (OR=3.00; 95% CI: 3.21-7.37) and privileged areas (OR=1.81; 95% CI: 1.07-3.04). Living in a house, as opposed to an apartment, was a risk factor for seropositivity in the privileged area (OR=3.62; 95% CI: 2.43-5.41). The main difference between the privileged and other areas could be attributed to the much larger proportion of apartment dwellers. Intensive vector control, surveillance and community education should be considered in deprived urban areas where a high proportion of children are infected by an early age.
Keywords: Dengue, seroprevalence, risk factors, spatial analysis
1. Introduction
Dengue is a vector-borne disease considered a global public health threat in tropical and subtropical countries. Approximately 2.5 billion people – two fifths of the world population - are at risk of infection, mostly in urban regions given that the main vector (Aedes aegypti) is widespread and well adapted to such environments (Gubler, 1998, WHO, 2009). South-east Asia is one of the most affected regions, where dengue haemorrhagic fever (DHF) has been a leading cause of hospitalization and death among children (WHO, 2009). In the Americas, where all four serotypes (DENV 1, 2, 3, 4) circulate, dengue incidence has increased dramatically in the last two decades (PAHO, 2008, Torres and Castro, 2007). From 2001 to 2008, more than 5 million cases of dengue were reported, including approximately 100,000 cases of dengue hemorrhagic fever and 1,500 deaths (PAHO, 2008). Brazil currently accounts for the majority (~80%) of the cases reported in Latin American, with co-circulation of three serotypes (DENV 1, 2, 3) in most of the country, with sporadic epidemic waves in several urban areas (Teixeira et al., 2009, PAHO, 2008, Siqueira Jr et al., 2005).
Dengue has a broad spectrum of manifestations and the number of reported cases underestimates the number of infections, because most are asymptomatic and not all symptomatic ones are reported (Kyle and Harris, 2008). Hence, serosurveys and longitudinal studies are used to assess past and current dengue infection and transmission rates at the population level (Thai et al., 2005, Endy et al., Teixeira et al., 2002) .
The spatial distribution of dengue infection can vary greatly be heterogeneous between neighboring areas in urban settings (Vanwambeke et al., 2006, Reiter et al., 2003, Vallee et al., 2009, Almeida et al, 2007). Among possible individual and household risk factors, predictors of infection have included higher age (Yew et al., 2009), low socioeconomic status (Vasconcelos et al., 1998, Siqueira Jr et al., 2004), lower education level (Teixeira et al., 2002, da Silva-Nunes et al., 2008) and lack of household protective measures such as unscreened houses or absence of air-conditioning (Ko et al., 1992, Reiter et al., 2003) In turn, this shows the importance of understanding small-scale variation in dengue infection when formulating control strategies.
The aim of the current population-based household study, conducted in the city of Recife in the Northeast of Brazil, was to estimate the prevalence of dengue infection between privileged and non-privileged areas and identify individual and area-level risk factors for infection in three urban areas. In addition, we describe the spatial distribution of risk of dengue infection to explore intra-urban variations in dengue infection that could be valuable in suggesting alternative control strategies.
2. Materials and Methods
2.1 Study settings
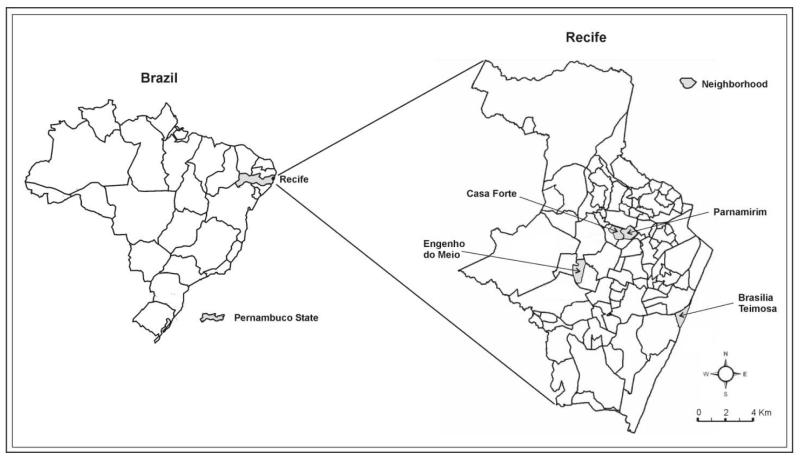
Recife (1.5 million inhabitants) lies on the Atlantic coastal plain (8° 03′ S 34° 52′ W) at a mean altitude of 5 meters above sea, level and is relatively flat. The city’s area of 217 km2 is divided in 94 neighborhoods (IBGE, 2009) as shown in Figure 1. Its climate is tropical humid, with average temperature of 25°C and rainfall of around 2000 mm per year, with no major variation between neighborhoods (ITEP, 2009). Dengue vector surveillance and control covers the whole city and relies on premise visits for elimination of breeding sites and use of biological larvicides (Bacillus sphaericus and Bacillus thuringiensis israelensis) (Regis et al., 2008).
Figure 1. Geographic location of selected study areas in Recife (Brazil).
Three non-contiguous areas were selected to represent deprived, intermediate and high socio-economic areas. Another criterion for selecting these areas was the feasibility of conducting field work there, due to the established willingness of the community to participate (Regis et al., 2008). The deprived area, Brasília Teimosa neighbourhood, is located on the coast in the southern region, largely composed of lower income families and lacking sanitation and waste collection (Figure 1, area 4.41 km2; population 19,155; with 2 minimum wages as the average income of the head of the family). The intermediate area, Engenho do Meio neighbourhood, is situated in the western zone of the city and is composed mostly of middle class families (area: 4.58 km2; population 10,560; with 5 minimum wages as the average income of the head of the family). The high socio-economic area is composed of the Casa Forte and Parnamirim neighborhoods in the northern part of the city, whose residents mainly live in apartment blocks (area: 6.76 km2; population 9,838; with 26 minimum wages as the average income of the head of the family) (IBGE, 2000).
2.2 Subjects and assessment of variables
The field work was carried out between August 2005 and September 2006. Residents aged between 5 and 64 years were eligible for the survey. During the household visits, all participants or guardians (for children under 13 years) were interviewed by a trained team using a structured questionnaire. Individual-level variables were age (in years), sex, schooling (University/secondary and Basic/illiterate levels), and whether the participant commuted outside the area to study or work. Household-level variables were type of household (house or apartment), number of persons per room (up to 1 and more than 1), whether connected to public water supply, regular water supply, water containers in the residence, whether connected to the public sewer and garbage collection. All the residences of participants were georeferenced using Global Positioning System (GPS) machines (GeoXM; Trimble Navigation Ltd., Sunnycale, CA) that, according to the product catalog, have an accuracy of 1 to 3 meters.
2.3 Blood collection and serologic tests
After the interview, a blood sample (10 mL) was collected into Vacutainer® tube (Becton Dickinson, Franklin Lakes, NJ) for all volunteers during the household visit. Serum samples were separated within 12 hours and stored at –20°C for later use. Serum samples were screened for IgG antibodies against dengue virus by using an enzyme-linked immunoassay commercial kit (Dengue IgG-ELISA, PanBio, Ltd., Brisbane, Australia). Samples were processed in duplicate and tests were performed according to the manufacturer’s instructions. Results were calculated as “PanBio units” with results <9.0, 9.0–11.0, and > 11.0 defined as negative, equivocal and positive, respectively. Samples that initially returned an equivocal result were retested to confirm the result. This commercial kit does not discriminate between dengue serotypes.
2.4 Sample size calculation
The study population was stratified into two age groups (5-14 and 15-64 years). The sample size was calculated assuming the following parameters: seropositivity of 30% in the population <15 years and of 40% for the population ≥15 years, based on a previous study (Siqueira Jr et al., 2004); precision of 5%; and 95% confidence interval (CI). A design effect of 1.5 was applied to take into account the variation of positives in each household. Finally, a non-response rate of 20% was allowed for in the estimated sample size. Under these parameters, it was determined that approximately 560 participants aged 5-14 years and 640 participants aged 15-64 years should be enrolled in each area.
2.5 Sampling strategy
The sampling strategy was based on Census 2000 Demographic that provide the total population size, number of households and age distribution in the three areas (IBGE, 2000). All census tracts of each study area were included in the sample. The census tract is the smallest geographical unit of the Demographic Census with approximately 300 households (about 1000 inhabitants). A systematic age stratified sample was drawn up, with a sampling fraction for each area that was calculated from the proportion aged 5 to 14 years, and the number of households. The sample was then drawn in accordance with the sampling fraction, seeking the target number of 5 to 14 years-olds inhabitants, with a subsample to obtain the target number of 15 to 64 years-olds. A greater number of houses was required to achieve the sample size for the younger age group than for the older one. Therefore, in some houses, only people aged 5-14 years were assessed.
2.6 Data analysis
The force of dengue infection was estimated in each of the three areas by assuming that it was constant over the exposure history of people in the study, and that seroconversion is permanent. The force of infection was fitted from a generalized linear model with serostatus as the outcome variable, a binomial distribution family, complementary log-log link function, and the natural logarithm of age as an offset (Collett, 1991).
The analysis of potential risk factors was weighted to adjust for age distribution. The weights were calculated by comparing the proportions of the age groups in the sample to those in the national census. These weights were implemented in the STATA software via the ‘iweight’ option. The association between outcome (seropositivity) and individual and household variables was initially assessed by odds ratio (OR). This was done with the Generalized Linear and Latent Mixed models (GLLAMM) package in Stata 9.2, again using the ‘iweight’ option.
2.7 Spatial point analysis
For each area, a spatial analysis was conducted to identify risk gradients of dengue infection using Generalized Additive Models (GAM), which extend the generalized linear models to include non-parametric smoothing terms such as spline curves (Kelsall and Diggle, 1998). For the current study, the dependent variable was seropositivity and all explanatory variables mentioned in section 2.2 were considered in the model, as well as spatial smoothing via spline functions of latitude and longitude of the residence of each participant (Wood, 2003). The final model was selected using backward stepwise procedure and was used to produce a smoothed map of the odds ratio of being seropositive, adjusted for the covariates. These models were fitted using the ‘mgcv’ library of the R software (Wood, 2006). No adjustment for multiple comparisons was made in the spatial or non-spatial analysis.
2.8 Ethical considerations
Written consent to participate in the study was obtained from each person (or their guardian) after a full explanation of the study was provided. All data were handled confidentially and anonymously. This study was reviewed and approved by ethics committee of the CPqAM-Fiocruz / Brazilian Ministry of Health (N° 49/04).
3. Results
A total of 2,819 individuals, approximately half of them aged 5 to 14 years, were analyzed in the three areas. Participants living in the high and intermediate socioeconomic status areas were, on average, more highly educated and more likely to commute outside their home area (Table 1). Around 90% of people lived in houses in the deprived and intermediary areas, compared to more than 40% in apartment buildings in the high socioeconomic area. Around 25% of the dwellings in the high socioeconomic area were not connected to public water services, since many buildings have private wells. Data show irregularity of water supply in a daily basis in all three areas, with the poorer area having the lowest proportion of households with regular water supply (12.7%). More than 90% of the households in each area have containers for storage of water.
Table 1. Main individual, household and area level characteristics of study areas. Recife, Northeastern Brazil, 2005-2006.
| Characteristics |
Area
|
||
|---|---|---|---|
|
Brasilia Teimosa
(n=976) |
Engenho do
Meio (n=923) |
Casa Forte/
Parnamirim (n=920) |
|
| Individual | |||
| Proportion in 5-14 year age stratum (%) | 44.9 | 48.6 | 42.9 |
| Age (Mean ± SD) | 23.4 ± 16.2 | 25.2 ± 17.3 | 29.5 ± 17.6 |
| Male sex (%) | 46.5 | 45.1 | 37.9 |
| University degree (%) | 1.9 | 7.0 | 21.7 |
| Commutes outside study area for school or work (%) | 17.5 | 34.4 | 50.4 |
| Household | |||
| Number of households visited | 350 | 436 | 434 |
| Apartment (%) | 8.2 | 9.1 | 44.7 |
| More than 1 person per room (%) | 45.2 | 24.2 | 26.5 |
| Connected to public water supply (%) | 93.1 | 99.7 | 74.8 |
| Regular water supply (%) | 12.7 | 59.3 | 56.4 |
| No water containers in the residence (%) | 4.9 | 4.9 | 8.9 |
| Connected to public sewer system (%) | 52.5 | 91.2 | 80.1 |
| Garbage collection (%) | 87.9 | 100.0 | 97.4 |
| Dengue seropositivity (% and 95% IC) | 91.1 (89.1-92.8) | 87.4 (85.1-89.5) | 74.3 (71.4-77.1) |
The seroprevalences in the deprived (91.1%) and intermediate areas (87.4%) were statistically significantly higher than that (74.3%) in the rich area (Table 1). The overall refusal rate was 13.2%, ranging from 10.2% to 23.7% in the intermediate and high socio-economic area respectively.
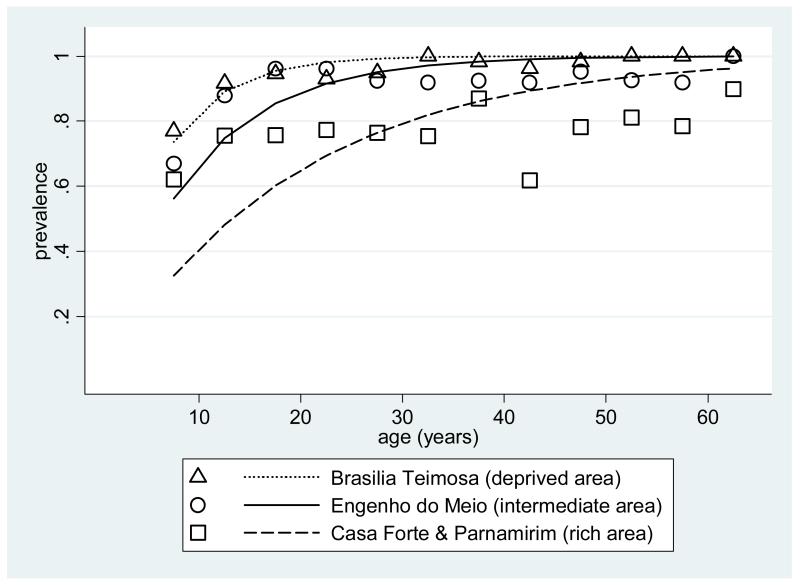
The estimated force of infection was 17.7, 11.0 and 5.3 per hundred person-years for the deprived, intermediate and privileged settings, respectively (Figure 2, see discussion for interpretation).
Figure 2. Age-specific dengue seropositivity in three areas of Recife, with lines showing the fitted values based on constant force of infection.
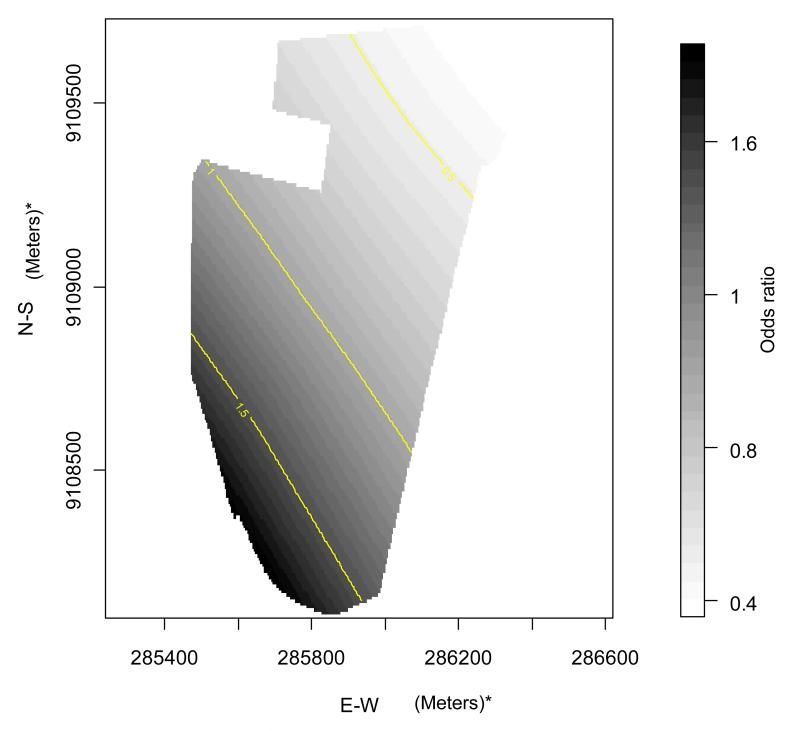
Table 2 shows the results of the univariate analysis of risk factors for dengue seropositivity for each area considering individual- and household-level characteristics. Older age was associated with higher risk in all three areas, while no association with sex was found. In the intermediate and high socio-economic status areas, living in a dwelling with more than one person per room was a risk factor for infection. In the high socio-economic area, living in a house instead of an apartment, was a risk factor, as were commuting for study or work, and irregular water supply. Low level of schooling was negatively associated with infection in the deprived and intermediate areas. In addition to age, which was uniformly significant in all areas, the following risk factors were generally maintained in the multivariable analysis (GAM model, Table 3). Not commuting away from the area was a risk factor for seropositivity in the deprived area. Higher number of persons per room remained a risk factor for seropositivity in the intermediate socio-economic area, whereas living in a house and higher number of persons per room were risk factors for seropositivity in the high socio-economic area. The smoothing terms were associated with dengue seropositivity in the intermediate area (Engenho do Meio neighborhood) suggesting spatial variation in risk. No such associations were found in the deprived (p=0.35) and high socio-economic areas (p=0.75) (Table 3). For this reason, we present the surface risk map only for the intermediate area: Figure 3 shows a gradient of increasing risk of dengue infection from the north to the south of the area.
Table 2. Individual and household risk factors for dengue infection (IgG) in three areas of Recife, Northeastern Brazil, 2005-2006.
| Variables | Area |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brasilia Teimosa | Engenho do Meio | Casa Forte/ Parnamirim | |||||||||||||
|
| |||||||||||||||
| Totala | Positives | ORb | 95% CI | Totala | Positives | ORb | 95% CI | Totala | Positives | ORb | 95% CI | ||||
| N | % | N | % | N | % | ||||||||||
| Individual characteristics | |||||||||||||||
| Age (years) | |||||||||||||||
| 5-14 | 438 | 368 | 84.0 | 1.0 | - | 400 | 315 | 78.8 | 1.0 | - | 395 | 276 | 69.9 | 1.0 | - |
| ≥15 | 538 | 521 | 96.8 | 6.99 | 3.6 – 13.6 | 523 | 492 | 94.1 | 6.30 | 3.3 – 12.1 | 525 | 408 | 77.7 | 2.19 | 1.3 – 3.8 |
| Sex | |||||||||||||||
| Female | 522 | 473 | 90.6 | 1.0 | - | 508 | 444 | 87.4 | 1.0 | - | 571 | 422 | 73.9 | 1.0 | - |
| Male | 455 | 417 | 91.6 | 1.2 | 0.7 – 1.9 | 415 | 363 | 87.5 | 0.9 | 0.6 – 1.5 | 350 | 262 | 74.9 | 1.0 | 0.6 – 1.6 |
| Schooling levels | |||||||||||||||
| University/ Secondary | 257 | 247 | 96.1 | 1.0 | - | 347 | 323 | 93.1 | 1.0 | - | 374 | 277 | 74.1 | 1.0 | - |
| Basic/ Illiterate | 715 | 639 | 89.4 | 0.3 | 0.2 – 0.7 | 575 | 483 | 84.0 | 0.3 | 0.2 – 0.6 | 545 | 406 | 74.5 | 0.9 | 0.5 – 1.6 |
| Commutes outside study area for school or work |
|||||||||||||||
| Yes | 171 | 156 | 91.2 | 1.0 | - | 316 | 279 | 88.3 | 1.0 | - | 463 | 318 | 68.7 | 1.0 | - |
| No | 805 | 734 | 91.2 | 0.9 | 0.5 – 1.9 | 607 | 528 | 87.0 | 0.9 | 0.5 – 1.5 | 457 | 366 | 80.1 | 1.8 | 1.1 – 3.0 |
| Household characteristics | |||||||||||||||
| Type of household | |||||||||||||||
| Apartment | 80 | 68 | 85.0 | 1.0 | - | 85 | 67 | 78.8 | 1.0 | - | 412 | 255 | 61.9 | 1.0 | - |
| House | 897 | 822 | 91.6 | 1.8 | 0.8 – 4.2 | 838 | 740 | 88.3 | 2.5 | 0.9 – 6.6 | 509 | 430 | 84.5 | 6.4 | 3.5 – 11.8 |
| Persons per room | |||||||||||||||
| Up to 1 | 532 | 485 | 91.2 | 1.0 | - | 698 | 597 | 85.5 | 1.0 | - | 676 | 475 | 70.3 | 1.0 | - |
| > 1 | 437 | 398 | 91.1 | 1.1 | 0.6 – 1.9 | 225 | 210 | 93.3 | 3.0 | 1.2 – 7.4 | 245 | 209 | 85.3 | 3.0 | 1.4 – 6.6 |
| Connected to public water supply | |||||||||||||||
| Yes | 909 | 827 | 91.0 | 1.0 | - | 920 | 804 | 87.4 | 1.0 | - | 689 | 525 | 76.2 | 1.0 | - |
| No | 66 | 62 | 93.9 | 1.3 | 0.4 – 4.2 | 3 | 3 | 100.0 | - | - | 232 | 159 | 68.5 | 0.5 | 0.3 – 1.0 |
| Regular water supply | |||||||||||||||
| Yes | 123 | 108 | 87.8 | 1.0 | - | 549 | 480 | 87.4 | 1.0 | - | 520 | 358 | 68.8 | 1.0 | - |
| No | 853 | 782 | 91.7 | 1.7 | 0.8 – 3.6 | 374 | 327 | 87.4 | 0.9 | 0.5 – 1.8 | 400 | 326 | 81.5 | 2.8 | 1.5 – 5.3 |
| Water containers at residence | |||||||||||||||
| None | 49 | 45 | 91.8 | 1.0 | - | 46 | 41 | 89.1 | 1.0 | - | 82 | 70 | 85.4 | 1.0 | - |
| Covered containers only | 746 | 675 | 90.5 | 0.8 | 0.2 – 2.6 | 754 | 653 | 86.6 | 0.8 | 0.2 – 3.1 | 758 | 549 | 72.4 | 0.3 | 0.1 – 1.00 |
| Uncovered containers | 182 | 170 | 93.4 | 1.2 | 0.3 – 4.8 | 123 | 113 | 91.9 | 1.5 | 0.3 – 9.0 | 80 | 65 | 81.3 | 0.7 | 0.2 – 3.2 |
| Sewage disposal | |||||||||||||||
| Yes | 512 | 462 | 90.2 | 1.0 | - | 844 | 733 | 86.8 | 1.0 | - | 738 | 542 | 73.4 | 1.0 | - |
| No | 465 | 428 | 92.0 | 1.3 | 0.7 – 2.2 | 79 | 74 | 93.7 | 2.7 | 0.8 – 9.0 | 183 | 143 | 78.1 | 1.5 | 0.7 – 3.2 |
| Garbage collection | |||||||||||||||
| Yes | 859 | 782 | 91.0 | 1.0 | - | 923 | 807 | 87.4 | 1.0 | - | 891 | 659 | 74.0 | 1.0 | - |
| No | 118 | 108 | 91.5 | 1.3 | 0.5 – 3.6 | 0 | 0 | 0.0 | - | - | 24 | 21 | 87.5 | 4.3 | 0.1 – 127.8 |
Percentages were calculated excluding missing values
corrected by design effect and weight sampling
Table 3.
Generalized Additive Models results for the association of past dengue infection with individual and household risk factors in three settings of Recife, Northeast of Brazil.
| Characteristics | Brasília Teimosa1 | Engenho do Meio2 | Casa Forte/ Parnamirim3 |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| OR4 | 95% CI | OR4 | 95% CI | OR4 | 95% CI | |
| Age group (years) | ||||||
| 5-14 | 1.00 | - | 1.00 | - | 1.00 | - |
| ≥ 15 | 8.15 | 4.48 – 14.84 | 4.86 | 3.21 – 7.37 | 2.51 | 1.72 – 3.66 |
| Commutes outside study area for school or work |
||||||
| Yes | 1.00 | - | ||||
| No | 2.26 | 1.18 – 4.30 | ||||
| Type of residence | ||||||
| Apartment | 1.00 | - | ||||
| House | 3.62 | 2.43 – 5.41 | ||||
| Persons per room | ||||||
| Up to 1 | 1.00 | - | 1.00 | - | ||
| > 1 | 3.00 | 3.21 – 7.37 | 1.81 | 1.07 – 3.04 | ||
Spatial smoothing term: p=0.349
Spatial smoothing term: p<0.001
Spatial smoothing term: p=0.748
odds ratio
Figure 3. Adjusted odds ratio for seropositivity in Engenho do Meio (intermediate socioeconomic area). Recife, Northeastern Brazil, 2005-2006.
(*)The Universal Transverse Mercator (UTM) System
4. Discussion
This household survey of a Northeastern Brazilian city found dengue seroprevalence ranging from 74% to 91%, with the risk being inversely associated with socio-economic status. This variation in prevalence is unlikely to be explained by climate, because local temperature, rainfall, humidity and altitude are similar among these three areas of the city. Further, entomological research found that infestation rates of Aedes aegypti are similarly high in the three study areas (Regis et al., 2008).
In the deprived area, 59% of children had already been exposed to dengue virus by the age of 5 years and the estimated force of infection was three times higher than that in the privileged area. In the intermediate and privileged areas, the age-prevalence curves reached plateaus by the age of about 20 years, suggesting that, rather than a constant force of infection, they experienced spikes in incidence sometime in the previous 20 years. The inverse association between dengue infection and wealth is in agreement with previous studies and reinforces the role of socio-economic factors in dengue transmission (Gomez-Dantes and Willoquet, 2009, Teixeira et al., 2002, Reiter et al., 2003).
As expected, given the cumulative nature of the serostatus outcome, older age was a risk factor in all three areas. Men and women had been similarly exposed to viral infection, as previously reported elsewhere (Siqueira Jr et al., 2004; Teixeira et al., 2002; Yew, 2009; Van Benthem et al., 2005) The negative association between schooling level and dengue seropositivity found in the univariate analysis for the intermediate and poor areas disappeared after adjustment in the multivariate model, suggesting possible confounding with age.
Of note, the risk factors identified differed between the three study areas. In particular, living in a house, as opposed to an apartment, was a risk factor for seropositivity in the rich area. The high proportion of apartment-dwellers in this area may explain the much lower seroprevalence, despite high entomological indices. The likely explanation for apartments being a protective factor, in this and other studies (Koh et al., 2008; Yew, 2009, 1971), is that some characteristics of these dwellings, such as the distance from potential breeding sites, may reduce man-vector contact. Another factor that is possibly involved is the fact that most apartment buildings in the city have private water wells that reduce the need to store water in containers indoors. The irregularity in water supply by the public system inner city favors water storage which is a potential breeding site. Nevertheless, no direct association with water storage containers was found in the current study.
In the intermediate and rich areas, a higher number of residents per room was identified as a risk factor for dengue seropositivity, as found in other studies (de Mattos Almeida et al., 2007) (Lian et al., 2006, Barrera et al., 2000). Greater density of people per household room has been considered to facilitate household dengue transmission possibly because gives greater feeding opportunities to the vector (Kuno, 1995). Although our study design does not permit establishing the place of infection, in the deprived area the risk for infection was associated with not commuting to study or work which suggests dengue transmission within the neighborhood. This finding is in agreement with a prospective study in Thai schoolchildren which concluded that most viral transmission occurred within the community (Endy et al., 2002).
In our study, the lack of demonstrable spatial variation within the privileged and low income areas could be explained by more homogeneous characteristics in terms of living conditions and environmental risk factors. There is no evidence of associations between dengue infection and several potential predictors, such as public sewerage or type and regularity of water supply. Only the intermediate area showed evidence of spatial heterogeneity in the distribution of seropositive individuals, with a northeast to southwest gradient. This spatial risk distribution is consistent with pattern of urban occupation of this area, in which low standard residences and junkyards are concentrated in the southwest region (data not shown).
In Central Brazil, two household surveys conducted in consecutive years showed spatial temporal variation in dengue infection, suggesting that herd immunity played a role in the pattern of inner city dengue distribution over time (Siqueira et al., 2008).
One of the limitations of the present study is that, given its large population screened, it was not feasible to use a test to distinguish between dengue serotypes such as Plaque Reduction and Neutralization Test (PRNT) (WHO, 2007). Moreover, children in the first four years of life were not surveyed, since it was not considered culturally feasible to collect blood at home from asymptomatic children of this age. On the other hand, we did include a large representative sample of older children and adolescents in each area, showing high prevalence of past dengue infection in early years of life in these settings. The simplest way to characterize this experience is in terms of a constant force of infection, corresponding to smooth increase in seroprevalence with age. This seems to capture the age profile in the deprived area. However, in the intermediate and rich areas, prevalence reached plateaus by an age of about 20 years, so the fitted curves underestimate prevalence at low ages and over-estimate it at high ages. Such patterns could have resulted from people being more exposed when younger, independently of calendar time. However, it seems more likely they resulted from past outbreaks, i.e. exposure varying over time, as found in other urban settings (Almeida et al., 2008; Egger, 2008).
This household survey estimated population levels of dengue seropositivity, and hence infection history, in multiple areas of a single city, showing the diversity of urban epidemiological scenarios. The main difference between the privileged and other areas can be attributed to the much larger proportion of apartment dwellers in the area. Intensive vector control, surveillance and community education should be considered in deprived urban areas where a high proportion of children are infected by an early age. Conversely, surveillance of this young group, in which seroprevalence has not yet saturated with age, should be useful in identifying areas of highest risk, to which control measures should be targeted.
Acknowledgments
The authors acknowledge the support of the National Institute of Allergy and Infectious Diseases (NIAID/NIH), Grant U19 AI56541 and PDTIS RVR09-FIOCRUZ. Martelli CMT, Albuquerque MFP and Souza WV were partially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico -CNPq (scholarships 307963/2004-7, 305947/2006-0, respectively)
References
- Almeida MC, Assuncao RM, Proietti FA, Caiaffa WT. [Intra-urban dynamics of dengue epidemics in Belo Horizonte, Minas Gerais State, Brazil, 1996-2002] Cad Saude Publica. 2008;24:2385–2395. doi: 10.1590/s0102-311x2008001000019. [DOI] [PubMed] [Google Scholar]
- de Mattos Almeida MCM et al. Spatial vulnerability to dengue in a Brazilian urban area duing a 7-year surveillance. Journal of Urban Health. 2007;84:334–345. doi: 10.1007/s11524-006-9154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Delgado N, Jimenez M, Villalobos I, Romero I. [Stratification of a hyperendemic city in hemorrhagic dengue] Rev Panam Salud Publica. 2000;8:225–233. doi: 10.1590/s1020-49892000000900001. [DOI] [PubMed] [Google Scholar]
- Collett D. Modelling binary data, Text in Statistical Science. Chapman & Hall Press; Boca Raton, FL: 2002. [Google Scholar]
- Silva-Nunes M, de Souza VA, Pannuti CS, Speranca MA, Terzian AC, Nogueira ML, Yamamura AM, Freire MS, da Silva NS, Malafronte RS, Muniz PT, Vasconcelos HB, da Silva EV, Vasconcelos PF, Ferreira MU. Risk factors for dengue virus infection in rural Amazonia: population-based cross-sectional surveys. Am J Trop Med Hyg. 2008;79:485–494. [PubMed] [Google Scholar]
- Mattos Almeida MC, Caiaffa WT, Assuncao RM, Proietti FA. Spatial vulnerability to dengue in a Brazilian urban area during a 7-year surveillance. J Urban Health. 2007;84:334–345. doi: 10.1007/s11524-006-9154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger JR, Ooi EE, Kelly DW, Woolhouse ME, Davies CR, Coleman PG. Reconstructing historical changes in the force of infection of dengue fever in Singapore: implications for surveillance and control. Bull World Health Organ. 2008;86:187–196. doi: 10.2471/BLT.07.040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endy TP, Nisalak A, Chunsuttiwat S, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Spatial and temporal circulation of dengue virus serotypes: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:52–59. doi: 10.1093/aje/kwf006. [DOI] [PubMed] [Google Scholar]
- Endy TP, Yoon IK, Mammen MP. Prospective cohort studies of dengue viral transmission and severity of disease. Curr Top Microbiol Immunol. 338:1–13. doi: 10.1007/978-3-642-02215-9_1. [DOI] [PubMed] [Google Scholar]
- Gomez-Dantes H, Willoquet JR. Dengue in the Americas: challenges for prevention and control. Cad Saude Publica. 2009;25(Suppl 1):S19–31. doi: 10.1590/s0102-311x2009001300003. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsall JE, Diggle P. Spatial variation in risk: a nonparametric binary regression approach. Applied Statistics. 1998;47:559–573. [Google Scholar]
- Ko YC, Chen MJ, Yeh SM. The predisposing and protective factors against dengue virus transmission by mosquito vector. Am J Epidemiol. 1992;136:214–220. doi: 10.1093/oxfordjournals.aje.a116487. [DOI] [PubMed] [Google Scholar]
- Koh B, Ng L, Kita Y, Tang C, Ang L, Wong K, James L, Goh K. The 2005 dengue epidemic in Singapore: epidemiology, prevention and control. Ann Acad Med Singapore. 2008;37:538–545. [PubMed] [Google Scholar]
- Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17:321–335. doi: 10.1093/oxfordjournals.epirev.a036196. [DOI] [PubMed] [Google Scholar]
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- Lian CW, Seng CM, Chai WY. Spatial, environmental and entomological risk factors analysis on a rural dengue outbreak in Ludun District in Sarawak, Malaysia. Tropical Biomedicine. 2006;23:85–96. [PubMed] [Google Scholar]
- PAHO . EID Updates: Emerging and Reemerging Infectious Diseases, Region of the Americas. Pan American Health Organization; Washington, DC: 2008. Number of Reported Cases of Dengue and Dengue Hemorrhagic Fever (DHF), Region of the Americas (by country and subregion) [Google Scholar]
- Regis L, Monteiro AM, Melo-Santos MA, Silveira JC, Jr, Furtado AF, Acioli RV, Santos GM, Nakazawa M, Carvalho MS, Ribeiro PJ, Jr, Souza WV. Developing new approaches for detecting and preventing Aedes aegypti population outbreaks: basis for surveillance, alert and control system. Mem Inst Oswaldo Cruz. 2008;103:50–59. doi: 10.1590/s0074-02762008000100008. [DOI] [PubMed] [Google Scholar]
- Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, Baber L, Amador M, Thirion J, Hayes J, Seca C, Mendez J, Ramirez B, Robinson J, Rawlings J, Vorndam V, Waterman S, Gubler D, Clark G, Hayes E. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis. 2003;9:86–89. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira-Junior JB, Maciel IJ, Barcellos C, Souza WV, Carvalho MS, Nascimento NE, Oliveira RM, Morais OLN, Martelli CM. Spatial point analysis based on dengue surveys at household level in central Brazil. BMC Public Health. 2008;8:361. doi: 10.1186/1471-2458-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira JB, Jr, Martelli CM, Maciel IJ, Oliveira RM, Ribeiro MG, Amorim FP, Moreira BC, Cardoso DD, Souza WV, Andrade AL. Household survey of dengue infection in central Brazil: spatial point pattern analysis and risk factors assessment. Am J Trop Med Hyg. 2004;71:646–651. [PubMed] [Google Scholar]
- Siqueira JB, Jr, Martelli CMT, Coelho GE, Simplício ACR, Hatch D. Dengue and dengue hemorrhagic fever, Brazil, 1981-2002. Emerging Infectious Diseases. 2005;11:48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technology Institute of Pernambuco (ITEP) [Accessed September 2009];Climatologia. 2009 Available from : http://www.itep.br/LAMEPE.asp.
- Teixeira MG, Barreto ML, Costa Mda C, Ferreira LD, Vasconcelos PF, Cairncross S. Dynamics of dengue virus circulation: a silent epidemic in a complex urban area. Trop Med Int Health. 2002;7:757–762. doi: 10.1046/j.1365-3156.2002.00930.x. [DOI] [PubMed] [Google Scholar]
- Teixeira MG, Costa Mda C, Barreto F, Barreto ML. Dengue: twenty-five years since reemergence in Brazil. Cad Saude Publica. 2009;25(Suppl 1):7–18. doi: 10.1590/s0102-311x2009001300002. [DOI] [PubMed] [Google Scholar]
- Thai KT, Binh TQ, Giao PT, Phuong HL, Hung le Q, Van Nam N, Nga TT, Groen J, Nagelkerke N, de Vries PJ. Seroprevalence of dengue antibodies, annual incidence and risk factors among children in southern Vietnam. Trop Med Int Health. 2005;10:379–386. doi: 10.1111/j.1365-3156.2005.01388.x. [DOI] [PubMed] [Google Scholar]
- IBGE . Demographic Census 2000: characteristics of the population and households, results from the population universe. The Brazilian Institute of Geography and Statistics (IBGE), Rio de Janeiro; Brazil: 2000. [Google Scholar]
- IBGE IBGE-cities@. [Accessed 11 September 2009];2009 Available from: www.ibge.gov.br/cidadesat/default.php.
- Torres JR, Castro J. The health and economic impact of dengue in Latin America. Cad Saude Publica. 2007;23(Suppl 1):S23–31. doi: 10.1590/s0102-311x2007001300004. [DOI] [PubMed] [Google Scholar]
- Vallee J, Dubot-Peres A, Ounaphom P, Sayavong C, Bryant JE, Gonzalez JP. Spatial distribution and risk factors of dengue and Japanese encephalitis virus infection in urban settings: the case of Vientiane, Lao PDR. Trop Med Int Health. 2009;14:1134–1142. doi: 10.1111/j.1365-3156.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- Van Benthem BH, Vanwambeke SO, Khantikul N, Burghoorn-Maas C, Panart K, Oskam L, Lambin EF, Somboon P. Spatial patterns of and risk factors for seropositivity for dengue infection. Am J Trop Med Hyg. 2005;72:201–208. [PubMed] [Google Scholar]
- Vanwambeke SO, van Benthem BH, Khantikul N, Burghoorn-Maas C, Panart K, Oskam L, Lambin EF, Somboon P. Multi-level analyses of spatial and temporal determinants for dengue infection. Int J Health Geogr. 2006;5:5. doi: 10.1186/1476-072X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos PF, Lima JW, da Rosa AP, Timbo MJ, da Rosa ES, Lima HR, Rodrigues SG, da Rosa JF. Dengue epidemic in Fortaleza, Ceara: randomized seroepidemiologic survey. Rev Saude Publica. 1998;32:447–454. doi: 10.1590/s0034-89101998000500007. [DOI] [PubMed] [Google Scholar]
- Wood SN. Thin Plate Regression Splines. Journal of Royal Statistical Society. 2003;65:95–114. [Google Scholar]
- Wood SN. Generalized Additive Models: An Introduction with R. Chapman & Hall/CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- WHO . Guidelines for plaque reduction neutralization testing of humans antibodies to dengue viruses, Immunization, vaccines and biologicals. World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- WHO [Accessed 9 September 2009];Dengue and dengue haemorragic fever. 2009 Fact sheet no 117. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/
- Yew YW, Ye T, Ang LW, Ng LC, Yap G, James L, Chew SK, Goh KT. Seroepidemiology of dengue virus infection among adults in Singapore. Ann Acad Med Singapore. 2009;38:667–675. [PubMed] [Google Scholar]