Abstract
The cellular composition of visceral adipose tissue (VAT) and release of cytokines by such cells within VAT has been implicated in regulating obesity and metabolic homeostasis. We show the importance of IL-25-responsive innate cells, that release the Th2 cytokine IL-13, in regulating weight and glucose homeostasis in mouse models of diet-induced obesity. Treating obese mice with IL-25 induces weight loss and improves glucose tolerance, and is associated with increased infiltration of ILC2s, type I and type II natural killer T (NKT) cells, eosinophils and alternatively activated macrophages (AAM) into the VAT. By depleting ILC2 in obese Rag1−/− mice we observe exacerbated weight gain and glucose intolerance. Conversely, transferring ILC2 or type I or type II NKT cells into obese mice induces transient weight loss and stabilizes glucose homeostasis. Our data identifies a mechanism whereby IL-25 eliciting IL-13 producing innate cells regulates inflammation in adipose tissue and prevents diet-induced obesity.
A hallmark of obesity is chronic low-grade inflammation that has a pivotal role in the progression to metabolic disorders, such as atherosclerosis and type 2-diabetes. The inflammatory cell composition of adipose tissue can profoundly influence the regulation of weight and the maintenance of metabolic homeostasis. While there are a range of resident populations of immune cells in visceral adipose tissue (VAT), such as eosinophils, effector and memory T cells, regulatory T cells (Treg), and natural killer T (NKT) cells, there is also a marked macrophage infiltration that is further increased in VAT of obese individuals (1). The polarization of macrophages within VAT to classically-activated (CAM) or alternatively-activated macrophages (AAM) influences metabolic hemostasis; in the VAT of lean individuals AAM predominate whereas obesity leads to a CAM dominated infiltration of the VAT (2). The cells in VAT that release cytokines that initiate the polarization of macrophages to a CAM (INFγ, IL-6 or TNFα) or AAM (IL-4 and IL-13) profile are not fully elucidated.
While studies into homeostatic glucose regulation have outlined the importance of AAM in promoting insulin sensitivity, eosinophils have been shown to be important in sustaining AAM in the VAT of mice on HFD, by the localized release of IL-4 and IL-13 (3). More recently attention has focused on the role of innate lymphoid cell types (ILC) that are present in the VAT, in particular type 2 ILC. Interestingly one such ILC2 population was identified in fat-associated lymphoid clusters in both mice and humans (4). A recent study in mice has eloquently identified a role for ILC2, induced in response to helminth infection or exogenous treatment with the ILC2-eliciting cytokine IL-33, in the localization of eosinophils within VAT and the local expansion of AAM (5). Herein we show IL-25-responsive innate cells, including IL-13 producing ILC2 and NKT cells regulate weight and glucose homeostasis in mice on HFD via the induction and maintenance of eosinophils and AAM in the VAT.
MATERIALS AND METHODS
Animals
C57BL6/J and Rag1 deficient C57BL6/J mice (Rag1−/−) were purchased from Jackson Laboratories (Bar Harbor, MD, USA). Jα18 deficient (Jα18−/−) mice were on a C57BL6/J background. CD1d deficient (Cd1d−/−), IL-13eGFP reporter mice (6), IL-13 deficient mice (Il13−/−) (7) and IL-17BR deficient mice (Il17br−/−) (6) were backcrossed to C57BL6/J background in house. IL-13eGFP (6) mice were crossed with Il4KN2 mice (8) in-house. In all studies, male mice of 7-9 weeks of age were used. Animals were housed in a specific pathogen-free facility in individually ventilated and filtered cages under positive pressure. All animal experiments were performed in compliance with the Irish Medicines Board regulations and approved by the Trinity College Dublin’s BioResources ethical review board.
High fat diet, in vivo glucose testing and interventions
Age-matched mice were fed a high fat diet (60% kcal fat; D12492; Research Diets, Inc.) or control diet (10% kcal fat) for 8-16 weeks as indicated. Glucose tolerance was assessed in mice fasted overnight and challenged with 2 g/kg glucose i.p. and blood analyzed by a glucose meter (Abbott Laboratories, IL, USA). After 8 weeks on HFD mice were injected on days 0, 2 and 4 with 0.4 μg recombinant mouse IL-25 (R&D Systems, Abingdon, UK), 20 μg α-Galactosylceraminde (α-Gal-Cer; Avanti Polar Lipids Inc., AL, USA) or 20 μg sulfatides (Matreya LLC, PA, USA) and weight monitored for 6 days. For neutralizing experiments 250 μg/mouse anti-CD90.2 mAb or rat IgG2b isotype control (BioXCell) were administered i.p. every 3 days at commencement of feeding HFD.
Flow cytometry
VAT from lean and obese mice was mechanically shredded and incubated with 1 mg/ml collagenase D from Clostridium histolyticum (Roche Applied Science, West Sussex, UK) and a single cell suspension prepared. Surface marker expression was assessed by flow cytometry with data collection on a CyAn (Beckman Coulter) and data analyzed using FlowJo software (Tree Star). IL-4 expression in the cells was identified in IL-4 reporter mice (Il4KN2) using Biolegend mAb; huCD2-biotin (TS1/8). Th2 cells were identified as IL-4 expressing CD4+, stained with ebioscience mAb; CD4-efluor V450 (Ly-2). IL-13 expression was determined using IL-13eGFP reporter mice (6). IL-5 expression was determined by intracellular staining. Briefly, cells were fixed and permeablised using the BD Cytofix/Cytoperm kit (BD Biosciences) and stained with BDBiosciences mAb; IL-5-PE (TRFK5). To identify ILC2 cells were stained with BD Biosciences mAbs; CD8-APC (Ly-2), B220-APC (RA3-6B2), F4/80-APC (BM8), ICOS-PE (7E.17G9), Siglec-F-APC (E50-2440); eBiosciences mAbs; CD4-APC (RM4-5), CD11b-APC (M1/70), Gr-1-APC (RB6-8CS), FcεR1-APC (MAR-1) and T1/ST2-FITC mAb (DJ8: MD biosciences). To identify eosinophils and AAM cells were stained with BD Biosciences mAbs; Siglec-F-PE (E50-2440), F4/80-APC (BM8) and eBiosciences mAb; CD11b-PerCP (M1/70). NKT cells were stained with eBiosciences mAbs; TCRβ-APC (H57-597) and NK1.1-FITC (pk136) and PBS-57-ligand complexed CD1d tetramers (NIH Tetramer Core Facility). Expression of IL-17BR and CD90.2 were determined using IL-17BR-APC (752101; R&D Systems) and CD90.2-PECy7 (53-2.1;eBiosciences). Prior to surface staining all cells were incubated with LIVE/DEAD Fixable Aqua stain (Molecular probes, Invitrogen) to isolate dead cells. Using appropriate isotype-controls, quadrants were drawn and data were plotted on logarithmic scale density-plots.
Cell sorting and adoptive transfer
C57BL/6J mice were treated with 2μg recombinant mouse IL-25 i.p. for 3 days and the peritonteal exudate cells collected and stained for ILC2 isolation. ILC2 were gated as above and sorted on a Mo-Flo cell sorter. The purity of the sorted ILC2 was >97%.
C57BL/6J mice were treated with α-Gal-Cer or sulfatides i.p. and spleen cells recovered and stained for type I and type II NKT cells, as above, and sorted on a Mo-Flo cell sorter. The purity of the sorted populations revealed a purity of >97%.
For ILC2, type I and type II NKT cells and in vitro generated Th2 cells (9), 200,000 cells were transferred i.p. on day 0 and day 2. Organs were collected on day 6.
Statistics
Statistical analysis was performed using GraphPad InStat®. Results are presented as mean +/− SEM. Differences, indicated as two-tailed P values, were considered significant when P>0.05 as assessed by unpaired Student’s t test with Welch correction applied as necessary.
RESULTS AND DISCUSSION
The absence of IL-13 is associated with weight gain due to significantly reduced eosinophils and AAM in VAT
The CAM versus AAM polarization state of macrophages in the VAT governs local insulin sensitivity and prevents the development of metabolic syndrome and type 2-diabetes (10). Recently, Wu et al. demonstrated that IL-4 release from eosinophils, induced by IL-5, could induce weight loss in obese mice, through sustaining AAM polarization in VAT (3). In addition, another of the type 2 cytokines, IL-13, has been identified as particularly important in glucose homeostasis, a dysregulation of which is implicated in the generation of type 2-diabetes and weight gain (5, 11). Indeed, mice deficient in IL-13 (Il13−/−) fed a HFD have markedly exacerbated weight gain when compared to comparable treated WT animals (Fig. 1A; (11)), with Il13−/− mice demonstrating dysregulated glucose metabolism and glucose intolerance when compared to WT counterparts (Fig. 1B; (11)). We analyzed the cellular composition of the VAT in Il13−/− mice on HFD and observed a significant (P<0.05) decrease in both eosinophils and AAM compared to WT mice (Supplemental Fig. 1A), which would contribute to the glucose intolerance and exacerbated weight gain in Il13−/− animals. In addition to the observed localized effects on glucose metabolism, a key role for IL-13 in hepatic glucose production suggests that IL-13 exhibits an effect beyond that observed in modulating inflammation (11).
Figure 1. IL-13 produced by type 2 cells, regulates weight gain and glucose tolerance in mice.
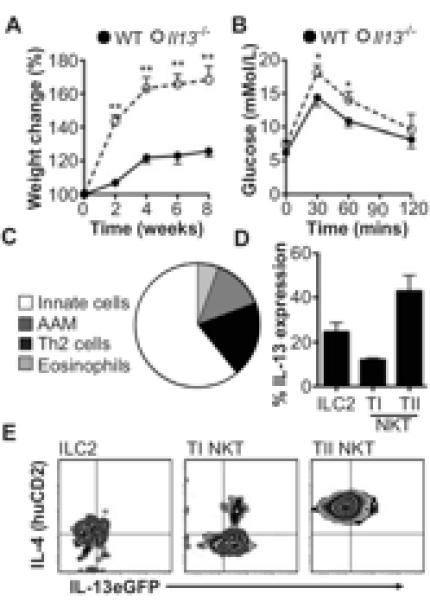
(A) Weight gain, expressed as a percentage from starting weight, in Il13−/− and WT mice on HFD for 8 weeks. (B) Blood glucose tolerance test. (C) Percentage of IL-13+ eosinophils, AAM, Th2 cells or innate cells and (D) IL-13 expressing innate cells in VAT. (E) IL-4 and/or IL-13 expressing ILC2, type I or type II NKT cells in Il4KN2/Il13eGFP mice. Data are representative of n=2-6 (+/− SEM) from 2 independent experimental replicates (*P<0.05, ** P<0.01).
IL-13 is produced by a number of immune cells that can be found in VAT, such as Th2 cells, however, work has recently focused on ILC2 (4, 6) as innate cellular sources of IL-13. Indeed a role for ILC2 in regulating weight gain through the release of IL-5 and IL-13 thereby sustaining eosinophils and AAM in VAT was recently described (5). Due to the clear importance of IL-13 in regulating weight gain we used dual IL-4/IL-13 reporter mice (Il4KN2/Il13eGFP (6, 8)), to determine the predominant cellular sources of IL-13 and also IL-4 in VAT in obese mice. In adipose tissue, lineage marker (CD3, CD4, CD8, CD11b, CD11c, CD19, F4/80, Gr-1, FcεR1) negative innate cells are the major producers of IL-13 (Fig. 1C). In VAT ILC2 (Lineage −ICOS+T1/ST2+IL-7Rα+) and NKT (TCRβ+NK1.1+) subpopulations are the prominent IL-13 producing cells (Fig. 1D). NKT cells are present in VAT of both mice and humans (12), and are defined as classical type I invariant NKT cells, which specifically express the receptor Vα14Jα18 in mice, or non-classical type II NKT cells with a variable TCR repertoire (13). We show that while type II NKT cells are the predominant source of IL-13, type I NKT cells predominately expressed IL-4 in the VAT, whilst eosinophils are the major source of IL-5 (Fig. 1D,E; data not shown). Some recent studies have demonstrated that type I NKT cells are depleted in VAT of obese individuals (12, 14). Furthermore, mice deficient in both populations of NKT cells (Cd1d−/−) and specifically type I NKT cells (Jα18−/−) have shown increased weight gain and elevated fasting glucose, whilst transferring type I NKT cells back into Jα18-deficient mice improves glucose homeostasis (12, 14). However, other studies have shown no role for NKT cells in either weight loss or glucose homeostasis (15). The role for type II NKT cells is less defined, although a recent study suggests that type II NKT cells initiate inflammation in the adipose tissue, which may result in insulin resistance (16). A variety of factors are postulated to explain discrepancies between studies on NKT cells and obesity in mice (17). In the context of this study, male C57BL6/J congenic Cd1d−/− or Jα18−/− mice have no significant alteration in either diet-induced obesity or glucose homeostasis when compared to WT mice maintained in our animal facility (data not shown).
IL-25 induces weight loss and IL-13-producing cells in obese mice
As outlined above using the Il4KN2/Il13eGFP mice, ILC2 and type II NKT cells are prominent cellular sources of IL-13 in the VAT from both obese and lean mice (Fig. 1D-E; data not shown). As the type 2 cytokine IL-25 drives expansion of ILC2 (6, 18), we treated obese mice with IL-25 and monitored metabolic and inflammatory changes. In addition, mice were also treated with the glycolipid ligand α-Gal-Cer to induce type I NKT cells (19) or sulfatides (3′-sulfated b-galactosylceramide) to induce type II NKT cells (20). Exogenous treatment of obese mice with each reagent preferentially expanded the frequency of respective cell types in VAT (Fig. 2A-C).
Figure 2. Innate type 2 cells are induced in response to IL-25, α-Gal-Cer and sulfatides.
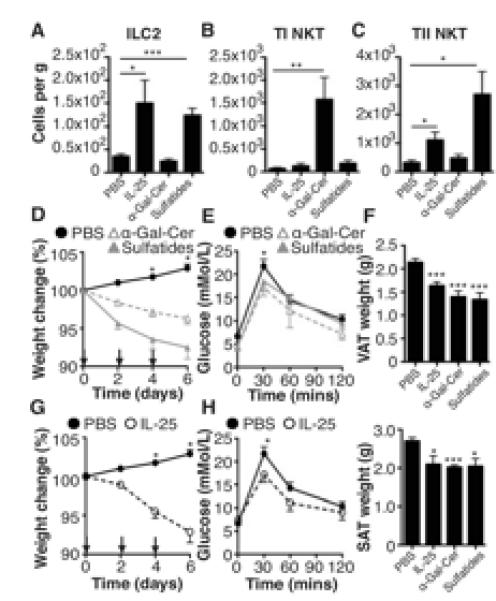
Obese WT mice were treated with PBS, IL-25, α-Gal-Cer or sulfatides on days 0, 2 and 4. The cellular composition of VAT was assessed by flow cytometry, including expression of (A) ILC2 (Lineage −ICOS+T1/ST2+), (B) type I NKT cells (TCRβ+NK1.1+ CD1d tetramers+), (C) type II NKT cells (TCRβ+NK1.1+ CD1d tetramers−). Weight (D,G) and (E,H) glucose tolerance was monitored in the groups treated with α-Gal-Cer, sulfatides or IL-25. (F) Visceral (VAT) and subcutaneous (SAT) adipose tissues were excised and weighed. Data are representative of n=2-6 (+/− SEM) from 2 independent experimental replicates (*P<0.05, **P<0.01, ***P<0.001).
Administration of α-Gal-Cer specifically induces type I NKT cells, while administration of sulfatides induced type II NKT cells and also ILC2 (Fig. 2A-C). Whilst all treatments significantly (P<0.001) induced eosinophilia in the fat, only treatment with IL-25 significantly (P<0.05) induced AAM (Supplemental Fig. 1B). The ability of IL-25 and sulfatides to induce both ILC2 and type II NKT cells suggests an intricate interplay between these cell types. Indeed, a role for IL-25 in inducing both IL-13 producing ILC2 and IL-17RB+ NKT cells has been reported in mouse models of airways hyperreactivity and colitis (21, 22). Interestingly, whilst we show that both type I and type II NKT cells express IL-17BR, only type II NKT cells appear to be induced by IL-25 treatment (Fig. 2B,C; Supplemental Fig. 2).
In addition to the clear effects of governing cellularity of the VAT, we show that inducing either type I or type II NKT cells in obese mice induces significant (P<0.05) weight loss (Fig. 2C), with a significant (P<0.01) reduction in fasting glucose, almost returning levels to those seen in mice fed normal chow (data not shown), and improved glucose tolerance (Fig. 2D), suggesting improved glucose homeostasis in response to expansion of type I or type II NKT cells. This weight loss in obese mice only, is associated with significant (P<0.05-0.001) reduction in the size of both visceral and subcutaneous adipose tissue (Fig. 2F; Supplemental Fig. 1C) and in the case of sulfatides, is not associated with a corresponding reduction in food or water intake, although food and water intake do appear slightly reduced in mice treated with α-Gal-Cer (Supplemental Fig. 1D).
Due to the ability of IL-25 to drive the expansion of ILC2 and NKT cells (Fig. 2A), both of which are able to drive weight loss (5, 12), we assessed the ability of IL-25 to induce weight loss. Treating obese mice with recombinant IL-25 induced significant (P<0.05) weight loss and glucose intolerance (Fig. 2E,F). Treatment with IL-25, whilst resulting in significantly decreased VAT and SAT (Fig. 2F; Supplemental Fig. 1C), does not alter food or water intake (Supplemental Fig. 1D) suggesting that the weight loss observed is due to the altered cellularity of the adipose tissue. It is also noteworthy that treatment with IL-25 does not alter weight gain in mice fed a control diet (Supplemental Fig. 1E). Furthermore, in support for a role for IL-25 in metabolic homeostasis mice deficient in the IL-25 receptor, IL-17BR (Il17br−/−), have exacerbated weight gain when on a HFD and are glucose intolerant (Supplemental Fig. 1F,G).
Depletion of ILC exacerbates weight gain in mice
We have identified a role for IL-25-induced IL-13-producing ILC in regulating the cell composition of VAT and inducing weight loss. To confirm a role for ILC2 specifically in metabolic homeostasis we used T and B cell deficient Rag1−/− mice and treated the mice with anti-CD90.2 mAb to block ILC2 (23). It is noteworthy that Rag1−/− mice are capable of gaining weight in response to HFD (Fig. 3A), indicating that weight gain is independent of adaptive immunity. In Rag1−/− mice treated with anti-CD90.2 mAb there was significantly (P<0.05) greater weight following HFD treatment relative to control mAb-isotype treated Rag1−/− mice with reduced glucose tolerance (Fig. 3A,B). This was apparent not only in total body weight, but in addition, anti-CD90.2 treated mice had significantly (P<0.05) increased subcutaneous and visceral adipose tissue deposits (Fig. 3C-E). Interestingly, anti-CD90.2 treatment also significantly (P<0.05) enhanced weight gain in mice fed a control diet (Supplemental Fig. 2A). However, treatment of either CD or HFD fed mice did not alter food or water intake (Supplemental Fig. 2B,C). Anti-CD90.2 mAb-treated mice had significant reductions in ILC2 (P<0.01) (Fig. 3C), with attendant reductions in eosinophils and AAM in VAT of Rag1−/− mice treated with anti-CD90.2 (Supplemental Fig. 2D), suggesting the depletion of ILC2 alone can affect eosinophil and subsequent AAM polarization independently of NKT cells, which are absent in Rag1−/− mice. This provides further evidence that in this mouse model of obesity, ILC2 play an important role in regulating weight gain by promoting localized eosinophilia and AAM polarization. However, these studies in Rag1−/− mice do not allow for the interaction of T or B cells with other cells, which may be of significant importance in normal animals.
Figure 3. Depletion of innate lymphoid cells exacerbates weight gain in Rag-1−/− mice.
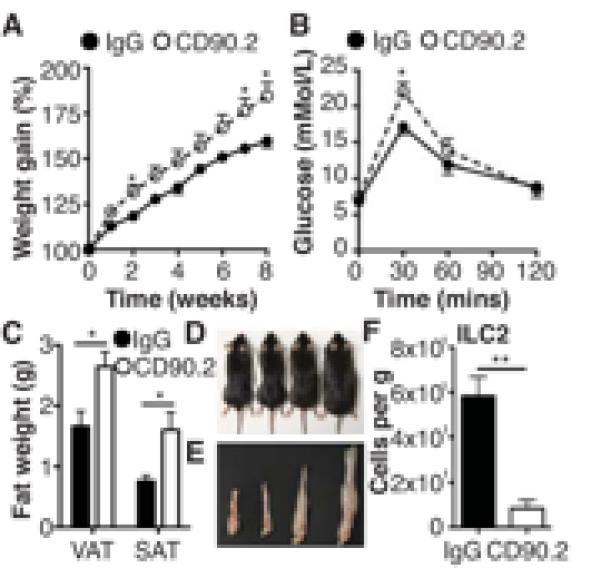
(A) Rag1−/− mice were treated with control IgG or anti-CD90.2 mAb every 3 days and weight monitored over 8 weeks while on a HFD and (B) glucose tolerance tested. VAT and SAT were excised and weighed (C) and mice (D) and VAT (E) photographed (L-R; CD + IgG, CD + CD90.2, HFD + IgG, HFD + CD90.2). (F) Expression in VAT of ILC2 (Lineage −T1/ST2+ICOS+) expressed per gram of adipose tissue. Data are representative of n=6 (+/− SEM) from two separate experiments (*P<0.05, **P<0.01).
To elucidate the relative roles for ILC2 and NKT cell types in diet-induced obesity and glucose homeostasis, we transferred ILC2, type I or type II NKT cells into obese mice. In accordance with previously published data (12), transfer of type I NKT cells induces a transient weight loss and improves glucose tolerance (Fig. 4). Transfer of ILC2 caused a transient weight loss and improvement in glucose tolerance comparable to that observed in mice receiving type I NKT cells (Fig. 4A, B, Supplemental Fig. 2E). Of particular interest is the effect of transferring type II NKT cells, which induces a greater and more prolonged weight loss and marked glucose tolerance (Fig. 4A, B, Supplemental Fig. 2E). Transfer of ILC2, type I or type II NKT cells induced eosinophilia and AAM polarization in the VAT relative to control mice, with transfer of ILC2 predominantly induce AAM while type I and type II NKT cells induce high levels of both eosinophils and AAM (Supplemental Fig. 2F). It is noteworthy that the transfer of in vitro expanded Th2 cells does no induce significant weight loss or alter glucose tolerance. These data show that while all three innate cell types play an important role in regulating weight gain and glucose homeostasis, there appears to be a hierarchy of action, with type II NKT cells potentially, on a cell transfer basis, an more important role.
Figure 4. Adoptive transfer of ILC2 and NKT cells induces weight loss.
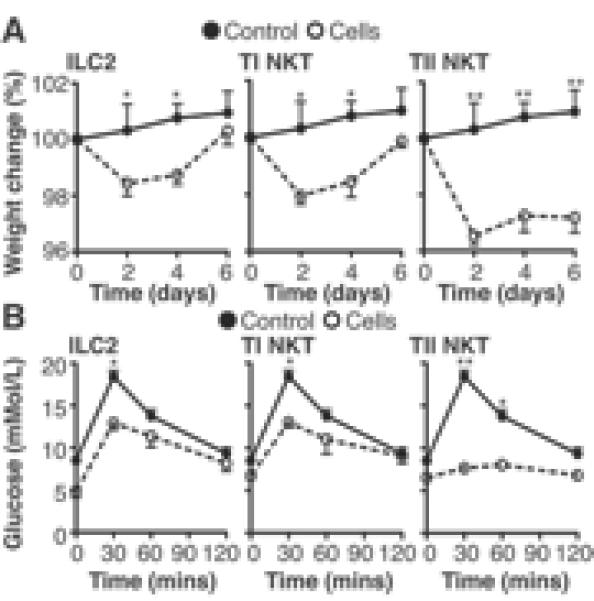
(A) Weight change in obese C57BL6/J mice after transfer of 200,000 ILC2, type I NKT or type II NKT cells i.p. on day 0 and day 2, displayed against mice given a transfer of 200,000 Th2 cells and (B) blood glucose tolerance analyzed on day 6. Data are representative of n=6 (+/− SEM) from 2 individual experimental replicates (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
The interaction between the innate immune system and obesity is a prominent area of research due to the current health crisis associated with the obesity epidemic. In addition to providing mechanistic insights, this study suggests a future potential role for modulating cytokine activity in the adipose tissue of obese patients, either to upregulate IL-25 or IL-13 directly, thereby artificially promoting ILC2 and NKT cell expansion and localized eosinophila and AAM polarization. It would also be prudent to assess the effects of upregulating these cytokines on other metabolic organs. This study raises the potential for IL-25 and IL-25 elicited cells as therapeutics for stabilizing glucose homeostasis and weight.
Supplementary Material
Acknowledgements
The authors would like to thank Alan Nolan for his technical assistance. Prof Masaru Taniguchi (Yokohama, Japan) kindly provided Jα18−/− mice and we are grateful to the NIH Tetramer Core Facility for providing CD1d tetramers.
REFERENCES
- 1.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annual review of immunology. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 2.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 3.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nature immunology. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 5.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–432. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 8.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangan NE, Dasvarma A, McKenzie AN, Fallon PG. T1/ST2 expression on Th2 cells negatively regulates allergic pulmonary inflammation. European journal of immunology. 2007;37:1302–1312. doi: 10.1002/eji.200636520. [DOI] [PubMed] [Google Scholar]
- 10.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, Inouye K, Barlow JL, Ji Y, Mizgerd JP, Qi L, Shi H, McKenzie AN, Lee CH. Direct control of hepatic glucose production by interleukin-13 in mice. The Journal of clinical investigation. 2013;123:261–271. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. The Journal of experimental medicine. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, Sun S, Xu A, Bhargava P, Yang L, Lam KS, Gao B, Lee CH, Kersten S, Qi L. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. The Journal of biological chemistry. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotas ME, Lee H, Gillum MP, Annicelli C, Guigni BA, Shulman GI, Medzhitov R. Impact of CD1d deficiency on metabolism. PLoS One. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nature reviews. Immunology. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukens JR, Kanneganti T. Fat chance: not much against NKT cells. Immunity. 2012;37:447–449. doi: 10.1016/j.immuni.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. European journal of immunology. 2009;39:1893–1901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 19.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 20.Donath MY. When metabolism met immunology. Nature immunology. 2013;14:421–422. doi: 10.1038/ni.2591. [DOI] [PubMed] [Google Scholar]
- 21.Satoh M, Andoh Y, Clingan CS, Ogura H, Fujii S, Eshima K, Nakayama T, Taniguchi M, Hirata N, Ishimori N, Tsutsui H, Onoe K, Iwabuchi K. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PloS one. 2012;7:e30568. doi: 10.1371/journal.pone.0030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camelo A, Barlow JL, Drynan LF, Neill DR, Ballantyne SJ, Wong SH, Pannell R, Gao W, Wrigley K, Sprenkle J, McKenzie AN. Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. Journal of gastroenterology. 2012;47:1198–1211. doi: 10.1007/s00535-012-0591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature immunology. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


