Abstract
Background
Porcine reproductive and respiratory syndrome virus (PRRSV) is a RNA virus with high genetic variation. This virus causes significant economic losses in most pig-producing countries. The clinical presentation of PRRSV ranges from asymptomatic to devastating. In this study, we developed a sensitive and specific zip nucleic acid probe-based real-time PCR assay to evaluate the viremia of natural PRRSV-infected pigs in Taiwan. Serum samples were collected from 577 pigs aged 5–12 weeks. These include 444 clinically healthy pigs and 133 symptomatic pigs were confirmed to have porcine respiratory disease complex (PRDC).
Results
Viremia was quantified in 79 of the 444 (17.8%) clinically healthy pigs and in 112 of the 133 (84.2%) PRDC cases. Viremias were significantly more common in pigs with PRDC compared with the clinically healthy pigs (P <0.0001). These results suggest that a high viral load is a major feature of PRRSV-affected pigs.
Conclusions
ZNA probe-based real-time PCR can be a useful tool to diagnose symptomatic and asymptomatic PRRSV-infected pigs. The presence of this marker in a sample of animals with high PRRSV loads (>104.2 PRRSV genomes/μl of serum) seems to indicate that it correlates with the presence of PRDC in pigs.
Keywords: Porcine reproductive and respiratory syndrome virus, Viral load, Zip nucleic acid, Real-time PCR
Background
Porcine reproductive and respiratory syndrome (PRRS) causes significant economic losses in most pig-producing countries [1]. The causative agent, the PRRS virus (PRRSV), was identified in the early 1990s [1]. PRRSV is an enveloped, positive-strand RNA virus with a genome of approximately 15 kb, and it belongs to family Arteriviridae and order Nidovirales[1]. A remarkable amount of genetic variation has been observed among the PRRSVs isolates worldwide, particularly in Nsp2 [2-5], ORF5 [2-8] and the nucleocapsid (the ORF 7 product) [3,5,8,9]. The genetic characteristics of the PRRSV strains clearly indicate the existence of two major genotypes, the European type (EU genotype, type 1) and the North American type (NA genotype, type 2) [1].
During PRRSV infection, clinical disease is detectable in all of ages of pigs but is usually observed in nursery-grown pigs [1]. The clinical presentation of PRRSV can range from asymptomatic to devastating, with symptoms such as listlessness, emaciation, hyperpnea, dyspnea, chemosis, abortion, stillbirth and a reduction in semen quality [1]. However, infection with PRRSV predominantly exists at a subclinical level, participating as a cofactor in porcine respiratory disease complex (PRDC) and porcine circovirus associated disease (PCVAD) [10]. PRRSV can be detected using molecular methods from nasal fluid, salivary, serum and tonsil specimens from naturally infected pigs [11]. However, because PRRSV is common within the swine population, no quantitative real-time PCR assays have been described using the serum samples of both symptomatic and asymptomatic PRRSV-infected pigs.
The diagnosis of PRRSV infection has relied on probe-based real-time PCR [12-21], SYBR Green-based PCR [11,22-24], RT-PCR [1], virus isolation [1], immunohistochemistry [1] and serological methods [1]. Zip nucleic acids (ZNA) are oligonucleotide-oligocation conjugates with multiple cationic spermine moieties attached to the nucleic acid oligomer [25]. The melting temperature of a hybridized ZNA is easily predictable and increases linearly with the length of the oligocation [25]. ZNA were shown to enable specific and sensitive reactions when used as a primer for PCR and reverse transcription [26]. The present study describes a sensitive method for detecting type 2 PRRSV using real-time fluorescent quantitative PCR with ZNA probes.
Results
ZNA probe-based real-time PCR amplification and the limit of detection
Tenfold serial plasmid dilutions (101 to 106 copies/μl) were tested and used to construct the standard curve by plotting the logarithm of the plasmid copy number against the measured quantification cycles (Cq) values. The generated standard curve covered a linear range of six orders of magnitude of the standard plasmid DNA. The linear correlation (R2) between the Cq and the logarithm of the plasmid copy number was 0.996 (slope = −3.91) (Figure 1). To assess the limit of detection of the assay, 100 to 106 copies/μl of standard plasmid DNA per reaction were tested in 10 replicates. At more than 100 copies, 100% of the replicates were positive, and at 10 copies, 6 (60%) of the replicates were positive (Table 1).
Figure 1.
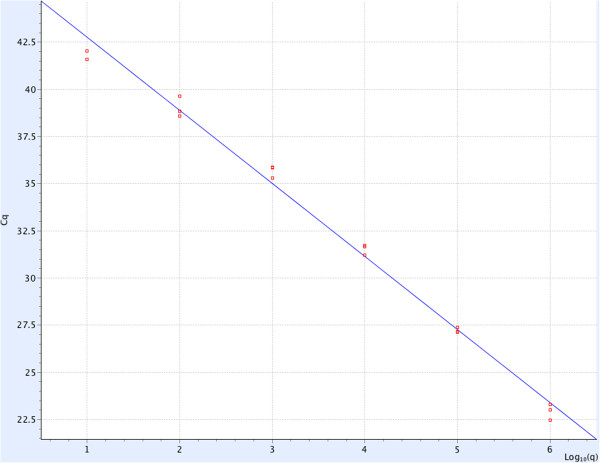
ZNA probe-based real-time PCR assay showing results obtained with serial dilutions of the PRRSV plasmid. Regression lines between the cycle threshold values plotted against the logarithm of the input copy number of the standard plasmid DNA. The dynamic range of the real-time PCR assay spanned 6 log units with a slope of −3.91 and an R2 value of 0.996.
Table 1.
Efficiency of the PRRSV ZNA probe-based real-time PCR assay
| Estimated PRRSV plasmid DNA copy no. | N | Mean Cq ± SD |
|---|---|---|
| 101 |
6/10a |
41.69 ± 0.42 |
| 102 |
10/10 |
39.19 ± 0.99 |
| 103 |
10/10 |
35.36 ± 0.55 |
| 104 |
10/10 |
31.28 ± 0.43 |
| 105 |
10/10 |
27.55 ± 0.55 |
| 106 | 10/10 | 22.63 ± 0.54 |
aNumber positive/number tested.
Reproducibility and specificity of the ZNA probe-based real-time PCR
The coefficient of variation of the mean Cq values in the within-run and between-run for standard plasmid DNA precision experiments ranged from 0.52 to 1.87% and from 0.85 to 1.98%, respectively (Table 2). The CV values in the within-run and between-run for clinical specimens that spanned the whole ranged from 0.43 to 4.70% and from 2.11 to 4.45%, respectively (Table 3). We analyzed other swine viruses to test the specificity of the ZNA probe-based real-time PCR. No specific amplifications were detected for any of these samples (data not shown).
Table 2.
Reproducibility of the PRRSV ZNA probe-based real-time PCR assay
| Concentration of the standard plasmid (copies/μl) |
Intra-assay variability |
Inter-assay variability |
||
|---|---|---|---|---|
| Mean Cq ± SD | CV% | Mean Cq ± SD | CV% | |
| 106 |
22.89 ± 0.43 |
1.87 |
23.95 ± 0.47 |
1.98 |
| 105 |
27.18 ± 0.14 |
0.52 |
27.75 ± 0.24 |
0.85 |
| 104 |
31.50 ± 0.28 |
0.88 |
32.06 ± 0.31 |
0.98 |
| 103 |
35.63 ± 0.31 |
0.87 |
36.34 ± 0.35 |
0.96 |
| 102 | 38.99 ± 0.56 | 1.43 | 40.50 ± 0.85 | 1.26 |
Table 3.
Results of the intra-assay and inter-assay testing for clinical specimens
| Pig no. |
Intra-assay variability |
Inter-assay variability |
||
|---|---|---|---|---|
| Mean Cq ± SDa | CV% | Mean Cq ± SD | CV% | |
| 1 |
2.09 ± 0.06 |
2.68 |
2.13 ± 0.05 |
2.11 |
| 2 |
2.98 ± 0.14 |
4.70 |
3.04 ± 0.14 |
4.45 |
| 3 |
4.85 ± 0.02 |
0.43 |
4.85 ± 0.16 |
3.39 |
| 4 |
6.01 ± 0.09 |
1.45 |
6.07 ± 0.17 |
2.72 |
| 5 | 2.75 ± 0.13 | 4.60 | 2.76 ± 0.10 | 3.51 |
aLog10 copy number per microliter of PRRSV.
Detection of the viral load in serum samples from in the PRRSV naturally infected pigs
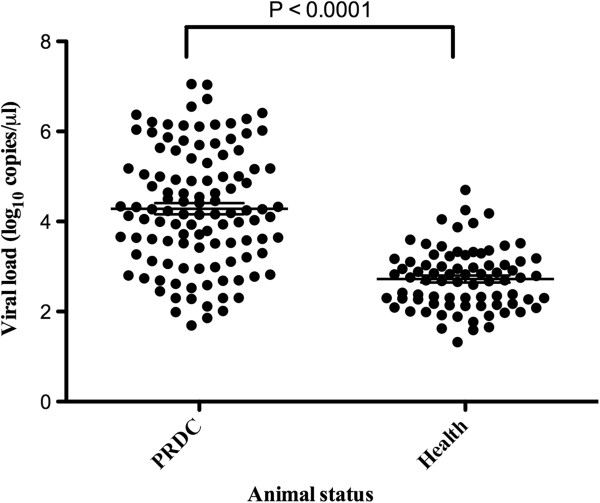
The assay was tested on serum from both healthy and PRDC pigs. As determined by ZNA probe-based real-time PCR, 112 (84.2%) of the 133 PRDC pigs and 79 (17.8%) of the 444 asymptomatic pigs were positive (Table 4). Using a chi-square test, the correlation between PRRSV and PRDC was calculated. Among the 577 pigs that were analyzed, a positive result for PRRSV appeared to be highly significantly correlated with the presence of PRDC (P <0.0001). In addition, the PRRSV load was significantly higher (P <0.0001) in the PRDC pigs (ranging from 1.69 to 7.05 log10 PRRSV genome/μl, median 4.21 log10) compared to that in the asymptomatic pigs (ranging from 1.32 to 4.70 log10 PRRSV genome/μl, median 2.75 log10) (Figure 2) (Table 4). These data indicated that the levels of viral load correlated with the severity of the clinical presentation of the PRRSV-infected pigs.
Table 4.
Number and descriptions of the PRRSV load results in serum collected from 2012 to 2013
|
Animal status |
|
||
|---|---|---|---|
| PRDC | Asymptomatic | P value | |
| Number of tested pigs |
133 |
444 |
|
| Number of positive pigs (%) |
112 (84.2) |
79 (17.8) |
<0.0001 |
| Mean ± SDa |
4.28 ± 1.31 |
2.72 ± 0.67 |
<0.0001 |
| Mediana |
4.21 |
2.75 |
|
| Rangea | 1.69 to 7.05 | 1.32 to 4.7 | |
aLog 10 Copies number per microliter in serum.
Figure 2.
Absolute copy number per microliter of PRRSV in serum from PRDC and clinically asymptomatic pigs. The serum of PRDC (n = 112) and asymptomatic pigs (n = 79) was analyzed using ZNA probe-based real-time PCR. The mean percentages (long horizontal lines) of the PRRSV load in the PRDC and asymptomatic pigs were compared. Error bars show SD. The PRRSV load was significantly higher (P < 0.0001) in the pigs with PRDC compared with the asymptomatic pigs, as assessed by unpaired, 2-tailed Student’s t-tests.
Discussion
The molecular diagnosis of PRRSV infection has relied on probe-based real-time PCR [12-21] and SYBR Green-based PCR [11,22-24]. This study first describes a new ZNA probe-based real-time PCR for PRRSV and evaluates this method as a diagnostic tool for PRRSV infection. This assay was sensitive, specific and reliable for the amplification of PRRSV cDNA, with a reproducible limit of detection of approximately 100 copies of target DNA per reaction. Both the intra- and inter-assay CVs for standard plasmid DNA and clinical specimens were satisfactorily low.
A novel type of modified oligonucleotides known as ZNA was recently developed as a method for the clinical diagnosis of pathogens, such as the hepatitis B virus [27]. ZNAs are able to discriminate between complementary sequence that are a perfect match and those that are mismatched by a single base pair [25]. ZNAs are particularly efficient at low magnesium concentrations, low primer concentrations and high annealing temperatures [25]. ZNA probes provide broad flexibility with respect to experimental design and represent an effective alternative to minor groove binder-DNA and locked nucleic acid probes [26].
The viral load is an indicator of active infection, virus-host interaction and disease progression [28]. The asymptomatic pigs are believed to serve as important reservoirs for the transmission of PRRSV to uninfected pigs [29]. Seventeen point eight percent (79/444) of the asymptomatic pigs were positive in this study. Similar findings (15.02%, 70/466) were reported in a study that employed traditional RT-PCR in China [29]. However, this is first study to report the viral load using serum samples in asymptomatic PRRSV-infected pigs using ZNA probe-based real-time PCR. Four asymptomatic PRRSV-infected pigs were detected at levels as high as 4 log10 viral copies/μl (Figure 2). This result may be explained by differences in the susceptibility to PRRSV infection.
The present study also reinforces a the previous suggestion that PRRSV is one of the major viral agent for PRDC [30,31]. PRRSV viraemia was detected in 84.2% (112/133) of the PRDC pigs, ranging from 1.69 to 7.05 log10 PRRSV genome/μl (mean 4.28 ± 1.31 log10, median 4.21 log10). In contrast to the symptomatic pigs, the viraemia of the asymptomatic pigs ranged from 1.32 to 4.7 log10 PRRSV genome/μl (mean 2.72 ± 0.67 log10, median 2.75 log10). Moreover, based on the present results, it can be concluded that when pigs are infected with PRRSV, the amount of PRRSV in serum samples is significantly higher in PRDC pigs. Similar reports had been made for another major viral agent of PRDC, PCV2 [32-34]. However, there were no previous studies performed for this disease. The results obtained in the present study indicated that the PRRSV load in serum might be used to assess the importance of PRRS infection in a symptomatic case of PRDC.
Conclusions
ZNA probe-based real-time PCR can be a useful tool to diagnose asymptomatic PRRSV-infected pigs. The presence of high PRRSV loads in a sample of animals (>104.2 PRRSV genomes/μl of serum) is correlated to the presence of PRDC in pigs.
Methods
Animal, specimen collection and sample preparation
Serum samples were collected from 577 pigs from middle and southern Taiwan from 2012 to 2013. These animals included 444 healthy pigs and 133 symptomatic pigs (aged from 5 to 12 weeks old) that showed a clinical history of PRDC, such as listlessness, emaciation, hyperpnea or dyspnea. RNA extraction and reverse transcription were performed according to the procedures outlined in Chomczynski [35] and Lin [36], respectively. This study protocol was approved by the Animal Care and Use Committee of the National Pingtung University of Science and Technology.
Primer and probe design
A conserved region of the M gene (the ORF 6 product) was identified in 125 nucleotide sequences from Asian (n = 74), European (n = 41), and American (n = 10) available from GenBank, which were aligned using the CLUSTAL W method and the MegAlign program (DNASTAR, Madison, WI). A 177-bp region was amplified from the ORF 6 gene of PRRSV using the primer pair PRRSV-M177F &-M177R (Table 5).
Table 5.
Primers and ZNA probe used in the multiplex real-time PCR assay for the M gene
| Primer or probe | Sequence (5′-3′) | Primer length (bp) | Amplicon length (bp) | Positiona |
|---|---|---|---|---|
| PRRSV-M177F |
CATTCTGGCCCCTGCCCA |
18 |
177 |
14698-14715 |
| PRRSV-M177R |
ACCACTCCYYGYTTDACAGCT |
21 |
14874-14854 |
|
| NA Probe | FAM-CTCGTGTTGGGTGGCAGA-ZNA4 BHQ1 | 18 | 14834-14851 |
aNucleotide numbering is based on the PRRSV isolate of strain VR-2332 (accession no. DQ176021).
Construction of the plasmid DNA standard curves
A DNA fragment of the ORF 6 gene was amplified from the PRRSV vaccine (Ingelvac® PRRS MLV, Boehringer Ingelheim) using conventional PCR. The PCR products were cloned using the T&A cloning kit (Yeastern Biotech Co., Ltd., Taipei, Taiwan) and sequenced. The PRRSV plasmids were purified using a plasmid miniprep purification kit (GMbiolab Co., Ltd., Taichung, Taiwan) and quantified by measuring the OD260 using a spectrophotometer (Hitachi U2900, Dallas, TX, USA). A standard curve was generated using 10-fold dilutions (100-106 copies/μl) of the standard plasmid DNA.
ZNA probe-based real-time PCR to detect PRRSV
The ZNA probe-based real-time PCR assays were performed using the LightCycler Nano (Roche Diagnostics, Mannheim, Germany). Each 10 μl reaction mixture contained 0.2 μM concentrations of the forward and reverse primers and 3 μl of the cDNA. The thermocycling conditions consisted of 10 min at 95°C and 45 cycles of 10 sec at 95°C, 10 sec at 55°C and 15 sec at 72°C. Each run included serial 10-fold dilutions of the standard plasmid DNA as a positive control and to construction the standard curve. A negative control that was missing the DNA template was included to detect any cross-contamination.
Reproducibility and specificity of ZNA probe-based real-time PCR
The intra- (within-run) and inter- (between runs) assay reproducibility were evaluated using 10-fold serial dilutions of the standard plasmid DNA (from 101-105 copies per reaction), tested in triplicate on three different days. The coefficients of variation of the absolute copy number obtained from each dilution were calculated. The reproducibility of the method was also evaluated by repeatedly testing the clinical samples, as previously described [37]. The specificity of the ZNA probe-based real-time PCR assay was assessed by testing nucleic acid extracts of porcine classical swine fever, porcine circovirus type 2, porcine parvovirus and porcine pseudorabies virus.
Statistical analysis
Student’s t-test was used to compare the viral loads between the various clinical symptom groups. The correlation between PRRSV and PRDC was evaluated using the chi-square test with Yate’s correction. P values <0.01 and <0.001 were considered significant and highly significant, respectively.
Abbreviations
PRRS: Porcine reproductive and respiratory syndrome; PRRSV: Porcine reproductive and respiratory syndrome virus; PRDC: Porcine respiratory disease complex; RT-PCR: Reverse transcription polymerase chain reaction; PCVAD: Porcine circovirus associated disease; ZNA: Zip nucleic acids; Cq: Quantification cycles.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CNL designed the ZNA probe, analyzed the experiments data and wrote the manuscript. WHL participated sample collection and performed the ZNA probe-based real-time PCR. LNH contributed to the RNA extraction and reverse transcription. SYW performed serum sample separation and arranged the data for statistical analysis. MTC managed the study, provided materials and reagents, contributed to the interpretation of the data and co-wrote the manuscript. All of the authors read and approved the final manuscript.
Contributor Information
Chao-Nan Lin, Email: cnlin6@mail.npust.edu.tw.
Wei-Hao Lin, Email: j7967633@gmail.com.
Li-Ning Hung, Email: cn7817@gmail.com.
Sheng-Yuan Wang, Email: april89000@gmail.com.
Ming-Tang Chiou, Email: mtchiou@mail.npust.edu.tw.
References
- Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. Porcine reproductive and respiratory syndrome virus (porcine arterivirus) 10. West Sussex: Wiley-Blackwell; 2012. [Google Scholar]
- Wu J, Li J, Tian F, Ren S, Yu M, Chen J, Lan Z, Zhang X, Yoo D, Wang J. Genetic variation and pathogenicity of highly virulent porcine reproductive and respiratory syndrome virus emerging in China. Arch Virol. 2009;154(10):1589–1597. doi: 10.1007/s00705-009-0478-6. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chen S, Zhang J, Zeng J, Guo X, Ge X, Zhang D, Yang H. Molecular variation analysis of porcine reproductive and respiratory syndrome virus in China. Virus Res. 2009;145(1):97–105. doi: 10.1016/j.virusres.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Li B, Fang L, Liu S, Zhao F, Jiang Y, He K, Chen H, Xiao S. The genomic diversity of Chinese porcine reproductive and respiratory syndrome virus isolates from 1996 to 2009. Vet Microbiol. 2010;146(3–4):226–237. doi: 10.1016/j.vetmic.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Shi Y, Hu Z, Xiong Z, Zhou Y, Jin X, Gu C, Hu X, Cheng G, Song N, Zhang W. Analysis of molecular variation of porcine reproductive and respiratory syndrome virus in Central China from 2006 to 2012. Arch Virol. 2013;158(3):717–721. doi: 10.1007/s00705-012-1542-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang X, Jiang P, Chen W, Wang K. Genetic variation analysis of porcine reproductive and respiratory syndrome virus isolated in China from 2002 to 2007 based on ORF5. Vet Microbiol. 2009;138(1–2):150–155. doi: 10.1016/j.vetmic.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Zhou YJ, Yu H, Tian ZJ, Li GX, Hao XF, Yan LP, Peng JM, An TQ, Xu AT, Wang YX. et al. Genetic diversity of the ORF5 gene of porcine reproductive and respiratory syndrome virus isolates in China from 2006 to 2008. Virus Res. 2009;144(1–2):136–144. doi: 10.1016/j.virusres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Shi M, Lam TT, Hon CC, Hui RK, Faaberg KS, Wennblom T, Murtaugh MP, Stadejek T, Leung FC. Molecular epidemiology of PRRSV: a phylogenetic perspective. Virus Res. 2010;154(1–2):7–17. doi: 10.1016/j.virusres.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Hao X, Lu Z, Kuang W, Sun P, Fu Y, Wu L, Zhao Q, Bao H, Cao Y, Li P. et al. Polymorphic genetic characterization of the ORF7 gene of porcine reproductive and respiratory syndrome virus (PRRSV) in China. Virol J. 2011;8:73. doi: 10.1186/1743-422X-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand RJ, Trible BR, Rowland RR. Pathogenesis of porcine reproductive and respiratory syndrome virus. Curr Opin Virol. 2012;2(3):256–263. doi: 10.1016/j.coviro.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Chung WB, Chan WH, Chaung HC, Lien Y, Wu CC, Huang YL. Real-time PCR for quantitation of porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 in naturally-infected and challenged pigs. J Virol Methods. 2005;124(1–2):11–19. doi: 10.1016/j.jviromet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Wasilk A, Callahan JD, Christopher-Hennings J, Gay TA, Fang Y, Dammen M, Reos ME, Torremorell M, Polson D, Mellencamp M. et al. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J Clin Microbiol. 2004;42(10):4453–4461. doi: 10.1128/JCM.42.10.4453-4461.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiboeker SB, Schommer SK, Lee SM, Watkins S, Chittick W, Polson D. Simultaneous detection of North American and European porcine reproductive and respiratory syndrome virus using real-time quantitative reverse transcriptase-PCR. J Vet Diagn Invest. 2005;17(2):165–170. doi: 10.1177/104063870501700211. [DOI] [PubMed] [Google Scholar]
- Revilla-Fernandez S, Wallner B, Truschner K, Benczak A, Brem G, Schmoll F, Mueller M, Steinborn R. The use of endogenous and exogenous reference RNAs for qualitative and quantitative detection of PRRSV in porcine semen. J Virol Methods. 2005;126(1–2):21–30. doi: 10.1016/j.jviromet.2005.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann JR, Hoff SJ, Yoon KJ, Burkhardt AC, Evans RB, Zimmerman JJ. Optimization of a sampling system for recovery and detection of airborne porcine reproductive and respiratory syndrome virus and swine influenza virus. Appl Environ Microbiol. 2006;72(7):4811–4818. doi: 10.1128/AEM.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Zhao JJ, Li N, Sun Y, Zhou YJ, Zhu Y, Tian ZJ, Tu C, Tong GZ, Qiu HJ. Simultaneous detection of Classical swine fever virus and North American genotype Porcine reproductive and respiratory syndrome virus using a duplex real-time RT-PCR. J Virol Methods. 2008;151(2):194–199. doi: 10.1016/j.jviromet.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Lurchachaiwong W, Payungporn S, Srisatidnarakul U, Mungkundar C, Theamboonlers A, Poovorawan Y. Rapid detection and strain identification of porcine reproductive and respiratory syndrome virus (PRRSV) by real-time RT-PCR. Lett Appl Microbiol. 2008;46(1):55–60. doi: 10.1111/j.1472-765X.2007.02259.x. [DOI] [PubMed] [Google Scholar]
- Xiao XL, Wu H, Yu YG, Cheng BZ, Yang XQ, Chen G, Liu DM, Li XF. Rapid detection of a highly virulent Chinese-type isolate of Porcine Reproductive and Respiratory Syndrome Virus by real-time reverse transcriptase PCR. J Virol Methods. 2008;149(1):49–55. doi: 10.1016/j.jviromet.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balka G, Hornyak A, Balint A, Benyeda Z, Rusvai M. Development of a one-step real-time quantitative PCR assay based on primer-probe energy transfer for the detection of porcine reproductive and respiratory syndrome virus. J Virol Methods. 2009;158(1–2):41–45. doi: 10.1016/j.jviromet.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NH, Chen XZ, Hu DM, Yu XL, Wang LL, Han W, Wu JJ, Cao Z, Wang CB, Zhang Q. et al. Rapid differential detection of classical and highly pathogenic North American Porcine Reproductive and Respiratory Syndrome virus in China by a duplex real-time RT-PCR. J Virol Methods. 2009;161(2):192–198. doi: 10.1016/j.jviromet.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Sinha A, Shen HG, Schalk S, Beach NM, Huang YW, Meng XJ, Halbur PG, Opriessnig T. Porcine reproductive and respiratory syndrome virus (PRRSV) influences infection dynamics of porcine circovirus type 2 (PCV2) subtypes PCV2a and PCV2b by prolonging PCV2 viremia and shedding. Vet Microbiol. 2011;152(3–4):235–246. doi: 10.1016/j.vetmic.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Inoue R, Tsukahara T, Sunaba C, Itoh M, Ushida K. Simple and rapid detection of the porcine reproductive and respiratory syndrome virus from pig whole blood using filter paper. J Virol Methods. 2007;141(1):102–106. doi: 10.1016/j.jviromet.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Martinez E, Riera P, Sitja M, Fang Y, Oliveira S, Maldonado J. Simultaneous detection and genotyping of porcine reproductive and respiratory syndrome virus (PRRSV) by real-time RT-PCR and amplicon melting curve analysis using SYBR Green. Res Vet Sci. 2008;85(1):184–193. doi: 10.1016/j.rvsc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Tian H, Wu J, Shang Y, Cheng Y, Liu X. The development of a rapid SYBR one step real-time RT-PCR for detection of Porcine Reproductive and Respiratory Syndrome Virus. Virol J. 2010;7:90. doi: 10.1186/1743-422X-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V, Voirin E, Paris C, Kotera M, Nothisen M, Remy JS, Behr JP, Erbacher P, Lenne-Samuel N. Zip Nucleic Acids: new high affinity oligonucleotides as potent primers for PCR and reverse transcription. Nucleic Acids Res. 2009;37(19):e130. doi: 10.1093/nar/gkp661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris C, Moreau V, Deglane G, Voirin E, Erbacher P, Lenne-Samuel N. Zip nucleic acids are potent hydrolysis probes for quantitative PCR. Nucleic Acids Res. 2010;38(7):e95. doi: 10.1093/nar/gkp1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar RM, Mollaie HR. Detection of HBV resistance to lamivudine in patients with chronic hepatitis B using Zip nucleic acid probes in Kerman, southeast of Iran. Asian Pac J Cancer Prev. 2012;13(8):3657–3661. doi: 10.7314/APJCP.2012.13.8.3657. [DOI] [PubMed] [Google Scholar]
- Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Res. 2002;30(6):1292–1305. doi: 10.1093/nar/30.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai JP, Wang YD, Tse H, Xiang H, Yuen KY, Che XY. Detection of asymptomatic antigenemia in pigs infected by porcine reproductive and respiratory syndrome virus (PRRSV) by a novel capture immunoassay with monoclonal antibodies against the nucleocapsid protein of PRRSV. Clin Vaccine Immunol. 2009;16(12):1822–1828. doi: 10.1128/CVI.00244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms PA, Halbur PG, Sorden SD. Three cases of porcine respiratory disease complex associated with porcine circovirus type 2 infection. Journal of Swine Health and Production. 2002;10(1):27–30. [Google Scholar]
- Yang CY, Chang TC, Lin CN, Tsai CP, Chiou MT. Study of Infectious Agents Involved in Porcine Respiratory Disease Complex in Taiwan. Taiwan Vet J. 2007;33(1):40–46. [Google Scholar]
- Brunborg IM, Moldal T, Jonassen CM. Quantitation of porcine circovirus type 2 isolated from serum/plasma and tissue samples of healthy pigs and pigs with postweaning multisystemic wasting syndrome using a TaqMan-based real-time PCR. J Virol Methods. 2004;122(2):171–178. doi: 10.1016/j.jviromet.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Olvera A, Sibila M, Calsamiglia M, Segales J, Domingo M. Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. J Virol Methods. 2004;117(1):75–80. doi: 10.1016/j.jviromet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Segales J, Calsamiglia M, Olvera A, Sibila M, Badiella L, Domingo M. Quantification of porcine circovirus type 2 (PCV2) DNA in serum and tonsillar, nasal, tracheo-bronchial, urinary and faecal swabs of pigs with and without postweaning multisystemic wasting syndrome (PMWS) Vet Microbiol. 2005;111(3–4):223–229. doi: 10.1016/j.vetmic.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Lin CN, Su BL, Wang CH, Hsieh MW, Chueh TJ, Chueh LL. Genetic diversity and correlation with feline infectious peritonitis of feline coronavirus type I and II: a 5-year study in Taiwan. Vet Microbiol. 2009;136(3–4):233–239. doi: 10.1016/j.vetmic.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N, Elia G, Martella V, Desario C, Campolo M, Trani LD, Tarsitano E, Tempesta M, Buonavoglia C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Vet Microbiol. 2005;105(1):19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]