Abstract
Toll-like and interleukin-1 (IL-1) family receptors recognize microbial or endogenous ligands and inflammatory mediators, respectively, and with the exception of Toll-like receptor 3 (TLR3), signal via the adaptor molecule myeloid differentiation factor 88 (MyD88). MyD88 is involved in oncogene-induced cell intrinsic inflammation and in cancer-associated extrinsic inflammation, and as such MyD88 contributes to skin, liver, pancreatic, and colon carcinogenesis, as well as sarcomagenesis. MyD88 is also protective, for example in oncogenic virus carcinogenesis or, acting downstream of IL-18R to strengthen mucosal repair, in azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colon carcinogenesis. Here, we discuss the mechanisms of the divergent effects of MyD88 and the balance of its protumor role in cancer-enhancing inflammation and immunity and its antitumor role in tissue homeostasis, repair, and immunity against the tumor or oncogenic pathogens.
Keywords: skin carcinogenesis, colon carcinogenesis, cancer and inflammation, oncogene-induced inflammation, Toll-like receptors, interleukin-1 family receptors
Inflammation and carcinogenesis
The idea that the contribution of inflammation and the immune system to carcinogenesis is a distinctive characteristic of cancer was originally proposed several years ago [1,2], and ‘avoiding immune destruction’ and ‘tumor promoting inflammation’ were recently highlighted as new hallmarks of cancer [3]. Inflammation is now known to contribute – both positively and negatively – to cancer initiation, progression, and dissemination, and has a pathogenic role in cancer-associated comorbidities such as anorexia, cachexia, and immunosuppression [4]. The effect of inflammation on cancer depends on activation of innate receptors by microbial or endogenous ligands. Many reports have shown that the adaptor protein MyD88 contributes to carcinogenesis in cancer models including cancer of the skin, liver, pancreas, and colon, as well as in sarcoma, by acting downstream of the TLRs or IL-1 family receptors. MyD88 signaling influences both the tumor promoting action of extrinsic inflammation mediated by infiltrating hematopoietic cells and the intrinsically induced inflammation mediated by oncogene-induced cell transformation (Figure 1). In other models, however, MyD88 has a protective effect. Here, we discuss these contrasting effects of MyD88 focusing on the recent elucidation of the underlying molecular mechanisms that are starting to shed light on the central role of innate receptors in the interaction of tissue cells with microbial commensals and endogenous inflammatory stimuli resulting in tissue homeostasis, wound healing, or carcinogenesis.
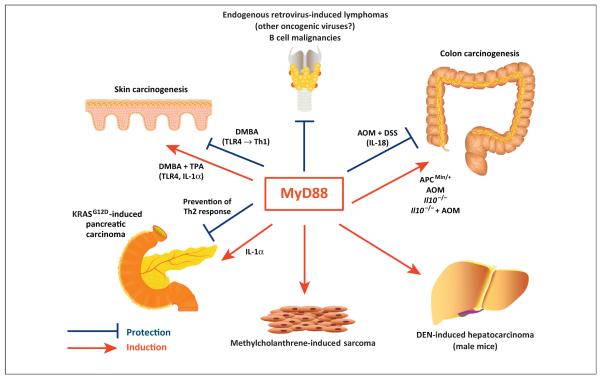
Figure 1.
Divergent roles of myeloid differentiation factor 88 (MyD88) in carcinogenesis. In various experimental carcinogenesis models, MyD88 signaling downstream of Toll-like receptors (TLRs) or interleukin-1 (IL-1)-family receptors has been associated with either suppression or enhancement of tumor formation. Some of these effects are also suggested by epidemiological or molecular studies in humans. The figure shows the major effects reported with indication of the proposed mechanisms when divergent effects for MyD88 were demonstrated in different experimental conditions for tumors originated from the same tissue. Please refer to the text of the review for details and references.
Infection-associated chronic inflammation is an etiopathogenic factor in up to a quarter of cancer cases worldwide, and infection with Helicobacter pylori, human papillomaviruses (HBVs), Epstein–Barr virus (EBV), and hepatitis B and C viruses is evident in the majority of these cases [5]. Other cancers originate in a setting of chronic inflammation not associated with infection, but secondary to tissue injury due to chemical or physical insults or hereditary disease. Changes in composition (dysbiosis) of the gut commensal microbiota or its interaction with immune cells can also modulate local and systemic inflammation, and this has been linked to cancer initiation [6,7]. Proinflammatory mediators are secreted not only by hematopoietic cells that infiltrate inflamed tissue (extrinsic inflammation) but also by epithelial, stromal, and endothelial cells (intrinsic inflammation). These mediators affect the proliferation and function of both hematopoietic and tissue cells. Chronic inflammation promotes cancer development through multiple mechanisms including genetic and epigenetic modifications arising from genomic instability and DNA damage, mediated in part by the local generation of reactive oxygen species (ROS) [8]. Tissue inflammation also promotes tumor growth by inducing tissue remodeling, supporting angiogenesis, and providing growth factors [2,8]. Cancer-induced inflammation is present in all established tumors, even those that did not originate in an inflamed tissue, as demonstrated by the presence of tumor-associated infiltrating hematopoietic cells [9]. Tumor-elicited inflammation provides a tumor promotion effect creating the conditions for tumor progression and dissemination as well as an immunosuppressive environment allowing the tumor to escape antitumor immunity [1]. Here, we examine the role of MyD88 at the interface between inflammation and cancer and discuss the balance of MyD88’s protumor role when involved in cancer-inducing inflammation or tumor-enhancing immune responses and its antitumor role when involved in the regulation of tissue homeostasis and repair or protective immune responses against the tumor itself or oncogenic pathogens.
MyD88-coupled TLRs and IL-1Rs as regulators of tissue homeostasis
The adaptor molecule MyD88 associates with all TLRs, with the exception of TLR3, as well as with all receptors for the IL-1 family of cytokines (Box 1). TLRs are considered to be the major family of innate or pattern recognition receptors (PRRs) that are activated by microbial products [microbe-associated molecular patterns (MAMPs)] and by endogenous ligands often released upon tissue damage and cell death [damage associated molecular patterns (DAMPs)]. TLRs play an important role in controlling the equilibrium of the host with commensal microbiota and in mediating the effects of the microbiota on local and systemic inflammation and immunity. TLRs also respond to endogenous ligands and are thus able to sense alterations in tissue homeostasis and to activate wound healing and tissue repair mechanisms [6]. Signaling through TLRs involves four different adaptor proteins. MyD88 is used by all TLRs except for TLR3, which uses a different adaptor, Toll-interleukin 1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF, also known as TIR-containing adapter molecule 1, TICAM1). TLR2 uses TIR-domain-containing adaptor protein (TIRAP also known as MyD88-adapter-like, MAL) in association with MyD88 whereas TLR4 utilizes two separate signaling pathways initiated by TIRAP in association with MyD88 for nuclear factor-κB (NF-κB)-mediated gene induction and TRIF-related adaptor molecule (TRAM, also known as TICAM2) associated with TRIF for type-I IFN production [10].
Box 1. Interleukin-1 cytokines (IL-1s) and IL-1 receptors (IL-1Rs).
Interleukin 1α/β were originally identified as endogenous pyrogens [25] and later extensively studied particularly for their proinflammatory functions as macrophage products. Subsequent studies identified other members with both proinflammatory and homeostatic functions of the IL-1 family of cytokines that is presently known to be composed of 11 proteins binding MyD88-coupled Ig-like receptors that are important in organism and tissue homeostasis, as well as in inflammation and immunity [100]. The IL-1 family includes some well-known proinflammatory cytokines such as IL-1 [IL-1α (IL-1F1) and IL-1β (IL-1F2)], IL-18 (IL-1F4), and IL-33 (IL-1F11) that also play an important role acting on T cells in controlling the differentiation of Th17, Th1, and Th2 cells, respectively [101]. All the members of the IL-1 receptor family signal through the adaptor MyD88 (Table I).
Table I.
IL-1s and IL-1Rs
| Name | Family name | Receptor | Co-receptor | Property |
|---|---|---|---|---|
| IL-1α | IL-1F1 | IL-1RI | IL-1RacP | Proinflammatory |
| IL-1β | IL-1F2 | IL-1RI | IL-1RacP | Proinflammatory, Th17 response |
| IL-1RA | IL-1F3 | IL-1RI | Antagonist for IL-1α, IL-1β | |
| IL-18 | IL-1F4 | IL-18Rα | IL-18Rβ | Proinflammatory, Th1 response |
| IL-36RA | IL-1F5 | IL-1Rrp2 | Antagonist for IL-36α, IL-36β, IL-36γ | |
| IL-36α | IL-1F6 | IL-1Rrp2 | IL-1RAcP | Proinflammatory in epithelial cells |
| IL-37 | IL-1F7 | (IL-18Rα?) | Binds IL-18 bp, anti-inflammatory | |
| IL-36β | IL-1F8 | IL-1Rrp2 | IL-1RAcP | Proinflammatory in epithelial cells |
| IL-36γ | IL1-F9 | IL-1Rrp2 | IL-1RAcP | Proinflammatory |
| IL-38 | IL-1F10 | IL-1Rrp2 | Similar to IL-36RA, inhibits Th17 | |
| IL-33 | IL-1F11 | ST2 | IL-1RAcP | Proinflammatory, Th2 response |
Signaling through MyD88 activates NF-κB, mitogen-activated protein kinases (MAPKs), the phosphatidylinositide 3-kinases (PI3K)-Akt pathway, and interferon regulatory factors (IRFs) 3, 5, and 7. MyD88 mutation at amino acid 265 is frequent in B cell malignancies and leads to constitutive activation of NF-κB signaling, confirming the importance of this axis in cancer pathogenesis [11,12]. MyD88 is also expressed at high amounts in several solid tumors and its expression is associated with poor prognosis in colorectal cancer [13,14]. TLR single nucleotide polymorphisms are associated with risk for stomach, prostate, and cervical cancers [15–18]. IL-1 polymorphisms are associated with lung and breast cancer [19,20], as well as gastric cancer in which IL-1 may play a role in the response to H. pylori infection [21]. IL-1β polymorphism is also associated with severity of anorexia/cachexia comorbidity and decreased survival in lung cancer patients [22].
Animals deficient for the gene encoding MyD88 have been used to study the role of inflammation in carcinogenesis. NF-κB is the primary signaling molecule downstream of MyD88 and data on how MyD88 deficiency affects carcinogenesis partly overlap with data on the role of NK-κB in cancer [23]. Also, the requirement for inflammasome activation for processing of pro-IL-1β and pro-IL-18 explains the partially overlapping results on intestinal dysbiosis, colitis, and colon carcinogenesis observed in mice deficient in genes encoding inflammasome components with those obtained in Myd88−/− mice unable to signal through the IL-1 receptor family [24].
A systemic role for IL-1 cytokines in regulation of homeostasis was recognized with the original description of IL-1 as an endogenous pyrogen [25]. The early work on the functions of PRRs, and TLRs in particular, was, however, focused on phagocytic and antigen-presenting cells. TLRs were recognized to activate these cell types and to act as initiators of inflammation and immunity via NF-κB- and IRF-mediated induction of proinflammatory cytokines, interferons, and chemokines. It was later recognized that TLRs are expressed by almost all cell types [26]. Stimulation of TLRs on both hematopoietic and non-hematopoietic cells directly affects cell survival and proliferation, in addition to inducing proinflammatory cytokines and chemokines, growth factors, and tissue remodeling enzymes. TLR stimulation of both normal and tumor cells can result in either cell proliferation or apoptosis [27–30]. TLR3 ligands often induce cell growth inhibition and apoptosis [28–30], whereas stimulation of fibroblasts with ligands of MyD88-coupled TLRs, e.g., the TLR5 ligand, flagellin, induces proliferation of serum-starved cells or prevents cell cycle exit upon serum withdrawal [27,28]. The survival/proliferative effect of TLR5 signaling is dependent on activation of PI3K-AKT signaling that targets the cell cycle inhibitor p27(kip1) for proteasome degradation [27,31]. At the same time, pro-survival BCL2 family members are induced [30]. TLR3 [poly(I:C)] and TLR4 [lipopolysaccharide (LPS)] ligands also induce proliferation, but only if endogenous type I IFN activity is blocked [27]. Thus, endogenous type I IFN can act as a switch between apoptotic and mitogenic TLR signaling pathways that are both upstream and downstream of AKT, increasing nuclear accumulation of p27, preventing cell proliferation, and activating apoptotic caspases [27].
Both TLR and IL-1R signaling contribute to tissue homeostasis, including tissue repair and regeneration, and this is partly explained by the pro-survival effects of MyD88 signaling. Myd88−/− mice show altered or delayed skin wound healing [32], colon tissue repair [33], and liver regeneration [34]. MyD88 signaling downstream of TLRs expressed in epithelial, parenchymal, or hematopoietic cells may play a role in these repair mechanisms but the inability of Myd88−/− mice to respond to the IL-1 cytokine family is probably also involved. For example, in the damaged skin, keratinocytes produce IL-1, which promotes proliferation in both an autocrine and paracrine way by inducing mitogenic factors; upon wound healing, however, IL-1 limits its action by inducing IL-1 receptor antagonist (IL-1RA) in dermal fibroblasts via activation of the peroxisome proliferator-activated receptor β/δ (PPAR β/δ) [35]. Il1a was also reported as a target gene of p63, a member of the p53 family that is a major regulator of keratinocyte proliferation and epidermal maintenance [36]. Also, in the colon epithelium, as discussed below, the protective effect of MyD88 is, at least in part, mediated by IL-18, another member of the IL-1 family [37]. The activation of TLRs and the induction of IL-1 family cytokines in the regulation of tissue homeostasis may be mediated by endogenous ligands (DAMPs) or, in the case of skin and intestine, by commensal MAMPs. It is of interest that whereas gut commensals regulate inflammation and tissue homeostasis both locally and systemically [6], the control of skin inflammation and immunity is compartmentalized and depends on skin commensal bacteria but not on gut bacteria [38].
MyD88 in skin carcinogenesis
Skin carcinogenesis can be studied using a two-stage model that involves treatment with the mutagen 7,12-dimethylbenz[a]anthracene (DMBA) followed by repeated skin paintings with the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA) [39–42]. This leads to formation of benign papillomas with activating mutations of v-Ha-ras Harvey rat sarcoma viral oncogene homolog (HRAS) 10–15 weeks after treatment [43]. A proportion of these papillomas become locally invasive squamous cell carcinomas [43]. In this model, Myd88−/− mice are more resistant than wild type mice to the formation of papillomas. A deficiency in TLR4, but not TLR2 or TLR9, signaling on immune and radioresistant cells is partly responsible for the phenotype in Myd88−/− mice [40]. TLR4 does not respond to microbial LPS but instead is activated by the endogenous ligand, high mobility group box-1 (HMGB1), which is released by necrotic keratinocytes, most likely during the tumor promotion phase, and induces an inflammatory response needed for tumor development [40]. Although TLR4 is required for carcinogenesis in the DMBA/TPA model, it is protective during initiation of cutaneous chemical carcinogenesis induced by DMBA alone. This is achieved through induction of an antitumor T helper 1 (Th1) response, which diverts to a protumor Th17 response in the absence of TLR4 [44]. However, other studies have shown that the tumor-promoting inflammation and epidermal hyperplasia induced by TPA and also by repeated treatment with DMBA are mediated by IL-1α [45,46]. Topical treatment with TPA also induces MyD88-dependent production of the immunosuppressive enzyme, indoleamine 2,3 dioxygenase, which induces T cell suppression and enhances tumor formation [47]. Overall, these studies showed that MyD88, coupled to either TLR4 or IL-1R, plays an instrumental role in skin tumor promotion.
Coste et al. [41] have proposed an alternative cell intrinsic role for MyD88 in skin carcinogenesis that involves MyD88 binding to the extracellular regulated MAP kinase (ERK), thus preventing its degradation by MAP kinase phosphatase 3 (MKP3) and amplifying activation of the canonical RAS pathway. These data are consistent with previous reports that RAS associates with TNF receptor-associated factor 6 (TRAF6) and other signaling molecules downstream of MyD88 in TLR and IL-1R signaling pathways, contributing to the activation of NF-κB, MAPK, and PI3K [48–51]. MyD88 inactivation in many human tumor cell lines suppresses proliferation [44], which is consistent with its role in cell survival and proliferation [27,30,31]. In many human RAS-driven lung cancers, the TRAF6 gene is amplified and overexpressed and its product is required for constitutive NF-κB activation, anchorage-independent cell growth, and tumor formation [52]. Thus, MyD88, in an autonomous way or downstream of constitutive activation of TLRs or IL-1Rs, may cooperate with TRAF6 signaling to enable unrestricted proliferation of tumor cells.
Mice in which the Myd88 gene was specifically deleted in keratinocytes show resistance to skin carcinogenesis, indicating that MyD88 signaling in the epithelial cells also contributes to tumor formation [42]. Activation of the epidermal growth factor receptor (EGFR)–RAS–MAPK pathway in keratinocytes and the associated inflammatory process involving secretion of chemokines and cytokines are central to the skin carcinogenesis process [53,54]. Thus, the role of MyD88 was studied in RAS-transformed mouse keratinocyte cultures [42]. Both Myd88−/− and Il1r−/− keratinocytes that express oncogenic RAS are hyperproliferative and secrete EGFR ligands. However, unlike RAS-expressing wild type keratinocytes, the gene-targeted keratinocytes failed to upregulate proinflammatory genes or downregulate differentiation markers [42]. Overall, the differentiation and proinflammatory effects of oncogenic RAS in keratinocytes is dependent on an autocrine loop of IL-1α release, IL-1R activation, and MyD88 signaling, which leads to IkBα phosphorylation and NF-κB activation (Figure 2). Both the induction of proinflammatory and tissue remodeling mediators, as well inhibition of differentiation, were dependent on NF-κB activation. In contrast to RAS-transformed mouse embryonic fibroblasts and human melanoma cells that contain a v-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutation, in which MyD88 cooperates with RAS to induce ERK activation [41], RAS-transformed keratinocytes from Myd88−/−or Il1r−/− mice fail to activate NF-κB but not ERK. RAS itself can activate NF-κB via noncanonical kinases such as IKKε or TANK-binding kinase 1 (TBK1) or via the complexes of mucosa associated lymphoid tissue lymphoma translocation gene 1 (MALT1) and B cell CLL/lymphoma 10 (BCL10) with a member of the CARD recruited membrane associated protein (CARMA) family [55,56]. However, in RAS-transformed keratinocytes, direct activation of NF-κB by RAS appears to play a secondary role if any, and the maintenance of high nuclear levels of activated NF-κB is completely dependent on the IL-1α–MyD88 autocrine loop [42]. Production of IL-1α is downstream of EGFR signaling through MAPK activation [57,58], and stimulation of keratinocytes with EGFR ligands induces secretion of proinflammatory mediators through the IL-1α autocrine loop [42] as also described in other cell types [59]. Although IL-1α is constitutively expressed in keratinocytes and released upon cell necrosis [60], accumulation of Il1a transcripts is highly induced by RAS transformation and IL-1α production by the transformed keratinocytes may be through secretion or release by a fraction of necrotic cells in the cell culture [42]. Although high levels of Il1b transcripts are also expressed in RAS-transformed keratinocytes, IL-1β is not secreted, most likely because the inflammasome is not activated in these cells to process pro-IL-1β [42].
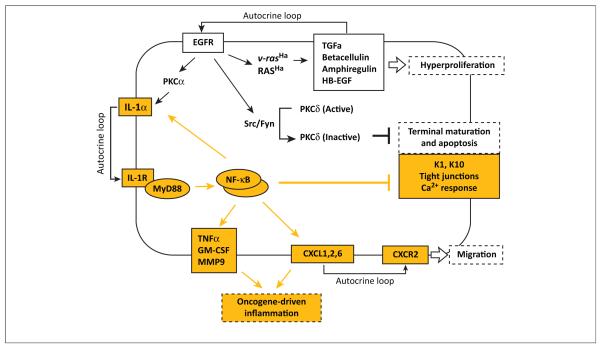
Figure 2.
Rat sarcoma viral oncogene homolog (RAS) activation converts normal keratinocytes to an initiated phenotype through a series of potentially reversible autocrine signals. Cultured mouse keratinocytes transduced with an oncogenic RAS vector have revealed several autocrine loops essential for tumor initiation [42]. Upregulation of epidermal growth factor receptor (EGFR) ligands and EGFR activity stimulates proliferation and activates the Src/Fyn pathway to tyrosine phosphorylate and inactivate PKCδ, reducing its role as an essential component of the keratinocyte death pathway, thus inhibiting the terminal stage of differentiation [102]. Tumor initiation by oncogenic RAS, through cross-activation by EGFR and its signaling through protein kinase C (PKC)α, causes release of interleukin-1α (IL-1α), activation of interleukin-1 receptor (IL-1R) signaling through myeloid differentiation factor 88 (MyD88), phosphorylation of IκBα and nuclear translocation of p65, p50, and RelB, activating nuclear factor-κB (NF-κB) transcriptional activity. Among the downstream effectors are chemokine (C-X-C motif) receptor 2 (CXCR2) ligands, other chemokines, proinflammatory cytokines, and tissue-remodeling enzymes that characterize the oncogene-associated intrinsic inflammation, as well as the suppression of keratinocyte differentiation markers that characterize the initiated phenotype [42]. Pathways dependent on the IL-1α–MyD88 autocrine loop are highlighted in yellow.
Overall, the recent studies on the mechanisms by which MyD88 affects skin carcinogenesis demonstrated a contribution at the level of both extrinsic inflammation that sustains tumor promotion and keratinocyte intrinsic inflammation that involves an IL-1α-mediated feedback loop. Also, MyD88 signaling contributed to the molecular pathways of RAS-induced keratinocyte transformation reminiscent of the role of RAS and other oncogenes in cancer-associated inflammation discussed below.
IL-1α and MyD88 in RAS transformation of keratinocytes
RAS activation in keratinocytes induces the secretion of soluble mediators with proinflammatory, mitogenic, angiogenic, and tissue remodeling activity. Thus, RAS shares with other pro-oncogenes such as rearranged during transfection (RET), BRAF, and v-myc myelocytomatosis viral oncogene homolog (MYC) not only the ability, when activated by mutation or overexpression, to deregulate cell cycling leading to cell transformation and uncontrolled tumor growth but also through the release of soluble mediators to affect the stromal cells and the interaction between the tumor and microenvironment. Epithelial cells produce inflammatory cytokines, chemokines, metalloproteases, and angiogenic and growth factors that attract and activate hematopoietic cells and act in an autocrine or paracrine manner to maintain the proinflammatory secretory response. When activated or overexpressed in epithelial cells, oncogenes such as RAS can induce this pathway of inflammation, establishing what has been appropriately defined as the oncogene-driven connection between cancer and inflammation [61]. Several chemokines that bind chemokine (C-X-C motif) receptor 2 (CXCR2) are among the proinflammatory factors dependent upon MyD88 in RAS-transformed keratinocytes. A number of studies establish a cell intrinsic protumorigenic function for CXCR2 [54,62]. In addition, CXCR2-mediated recruitment of Gr1+CD11b+ cells can foster tumor growth and metastasis [63–65]. This proinflammatory secretory pattern is often associated, although not in RAS-transformed keratinocytes, with cellular senescence, an essentially irreversible growth arrest induced by cellular aging or oncogenic stimuli [66]. A potent inducer of senescence is the DNA damage response, particularly to double-strand DNA breaks that recruit the DNA repair factor, ataxia telangiectasia mutated (ATM), leading to formation of morphologically detectable nuclear foci (DNA segments with alteration reinforcing senescence, DNA-SCARS), activation of p53 and Rb in the DNA-SCARS, and cell cycle arrest or apoptosis [67]. The senescence-associated secretory phenotype (SASP) is associated with senescence and involves secretion of various inflammatory cytokines, including IL-1α, IL-6, CXCR2 ligands, and metalloproteases, and a gene expression pattern reminiscent of that induced by oncogenes [68]. Similar to what is observed in RAS-transformed keratinocytes [42], the SASP is amplified by a positive feedback loop mediated by IL-1α and thus it is dependent on MyD88 signaling [69]. SASP requires DNA damage but is independent of p53 [68]. Cellular senescence is interpreted as a brake for the early events of oncogene-induced carcinogenesis [70,71]. However, when these growth arrest mechanisms fail, oncogene-induced DNA damage and the associated intrinsic inflammatory pathways activated by a SASP-like response may promote carcinogenesis.
For tumor progression in skin, a tissue in which the inherent epithelial maturation program leads to cell death, terminal differentiation must be abrogated. RAS transformation of keratinocytes through autocrine IL-1α–MyD88–NF-κB signaling not only induces a proinflammatory phenotype but also inhibits differentiation as highlighted by lack of expression of the genes encoding the suprabasal keratins K1 and K10 as well as of other genes characteristic of keratinocyte differentiation [42]. The IL-1α autocrine loop is also responsible for the downregulation of several genes involved in cell-to-cell adhesion and tight junction formation, in particular several cadherins and components of the desmosomes [42]. Thus, the IL-1α autocrine loop in RAS-transformed cells appears responsible for the ability of RAS to induce ras-related C3 botulinum toxin substrate (RAC)-and MAPK-mediated cell dissociation [72], which is a major component of the epithelium–mesenchyme transition (EMT), although alone insufficient to induce the full EMT phenotype. These data suggest that NF-κB activation may contribute to the dedifferentiation of keratinocytes during the progression from benign papilloma to malignancy [73].
In most neoplastic cell types, NF-κB has a survival effect. However, the data in RAS-transformed mouse keratinocytes indicate that NF-κB activation induced by the IL-1α–MyD88 autocrine loop does not interfere with RAS-mediated induced proliferation and survival [42]. Additionally, in human epidermal cells NF-κB has been paradoxically shown to mediate cell cycle arrest by activated RAS [74], preventing RAS-mediated carcinogenesis. Thus, intrinsic signaling through MyD88 downstream of IL-1R in RAS-transformed keratinocytes is largely responsible for the activation of NF-κB, which plays complex roles in different phases of epidermal tumor formation. In particular, these pathways contribute to the secretion of soluble mediators affecting tumor–stroma interaction and also to the deregulation of cellular differentiation. It is likely that similar molecular pathways may be involved in RAS-induced tumors in other tissues and in the mechanisms of cell transformation and intrinsic inflammation mediated by other oncogenes.
Tumor-enhancing effects of MyD88 signaling in other models of carcinogenesis
In addition to skin carcinogenesis, MyD88 contributes to other models of chemical carcinogenesis including 3′-methyl-cholanthrene (MCA)-induced sarcoma [39] and diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC) in male mice [75]. Inflammation was not considered to contribute to the MCA-induced sarcoma model and increased susceptibility to sarcoma formation was observed [75] in mice deficient for the proinflammatory tumor necrosis factor (TNF) that are resistant to skin carcinogenesis [76], indicating that TNF functions to protect the host against MCA-induced sarcoma. Furthermore, the MCA model has been widely used for the demonstration of immunosurveillance and immune editing, which are driven by MyD88-regulated cytokines such as TNF and IFN [77]. Thus, the finding that Myd88−/− mice are resistant to MCA-induced carcinogenesis indicates that inflammation-dependent tumor formation and immunoediting may co-exist in the same model and that MyD88 may have opposite effects in the different aspects of the interaction of inflammation and immunity with tumor formation[39]. The intrinsic or extrinsic mechanisms by which MyD88 affects sarcoma formation remain to be defined [39].
In mice given the chemical carcinogen DEN, increase of serum IL-6 and induction of HCC are predominant in males, reflecting the similar gender disparity observed in humans. Estrogen inhibits secretion of IL-6 from Kupffer cells exposed after DEN treatment. IL-6 production is likely to be triggered by the release from necrotic cells of ligands for both MyD88-coupled receptors and the DNAX-activation protein 12 (DAP12)-coupled triggering receptor expressed on myeloid cells (TREM-1) [75,78]. Thus, both Myd88−/− and Trem1−/− male mice are resistant to DEN-induced hepatocarcinogenesis [75,78].
The role of MyD88 in KRAS-induced pancreatic ductal carcinoma is more complex. In both mice and human patients, carcinoma development is dependent on KRASG12D-induced NF-κB activation by an IL-1α–MyD88 autocrine loop, similar to that observed in skin carcinogenesis [79]. However, MyD88 inhibition in vivo exacerbates pancreatic inflammation and malignant progression by enhancing the capacity of dendritic cells to promote carcinogenesis by inducing a protumorigenic Th2-type response [80] providing another example of the opposite effects of MyD88 on tumor initiation and progression by targeting inflammation-induced carcinogenesis or modulating antitumor immunity.
Divergent roles of MyD88 signaling in colon carcinogenesis
MyD88 can play a role in carcinogenesis by acting downstream of TLR or IL-1R signaling both in transformed cells, or in stromal or inflammatory cells, in the tumor microenvironment. The complex role of MyD88 in cancer is best exemplified by studies of colon carcinogenesis (Figure 3). In ApcMin/+ mice that carry a heterozygous truncation mutation at codon 850 of the tumor suppressor adenomatous polyposis coli (Apc) gene and generate spontaneous intestinal tumors from loss of heterozygosity, MyD88 contributes to adenoma growth and progression [81]. Furthermore, Myd88−/− mice subjected to multiple injections of the carcinogen AOM have reduced colon tumor growth compared to wild type controls [81]. MyD88 signaling is also required for AOM-enhanced colon carcinogenesis in Il10−/− mice [82]. Although Myd88−/− mice are viable and apparently normal in the absence of infection, early studies [83] highlighted an important role for MyD88 in maintaining intestinal homeostasis, in that γ-irradiation or administration of DSS resulted in severe ulceration and inflammation, accompanied by bleeding and lethality. Recognition of luminal commensal bacteria by TLRs is crucial for colonic epithelial cell regeneration upon DSS injury [33,84,85]. The ability of MyD88 to signal through NF-κB suggests that its role in controlling mucosal homeostasis may be dependent on the activity of this transcription factor on enterocyte survival. In addition to its anti-apoptotic and proliferative functions, NF-κB has been causally implicated with the inflammation associated with cancer [86]. Mice with enterocyte-specific depletion of IKKβ, a key regulator of NF-κB, developed a lower number of colonic polyps than wild type mice when treated with AOM followed by tumor promotion with DSS [86]. Also, when IKKβ was specifically deleted in myeloid cells, the size of the polyps was decreased, suggesting that NF-κB-dependent myeloid-derived factors such as cytokines promote tumor growth [86].
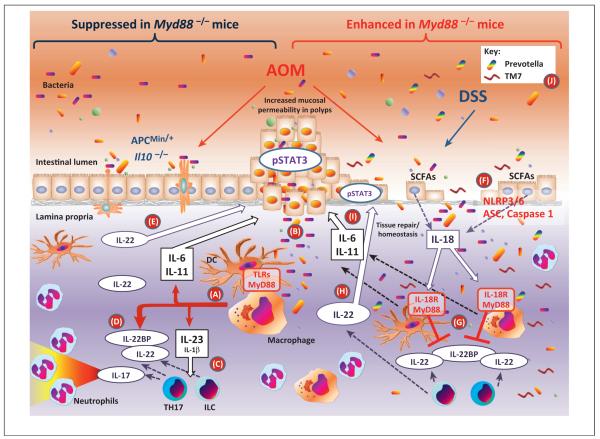
Figure 3.
Divergent roles of myeloid differentiation factor 88 (MyD88) in colon carcinogenesis. MyD88 deficiency in the models of colon carcinogenesis shown on the left side of the figure in which mucosa damage is limited [e.g., repeated treatments with the azoxymethane (AOM) carcinogen, ApcMin/+ or Il10−/− mice] and protects against tumor growth, probably by limiting in hematopoietic cells the inflammatory response downstream of Toll-like receptors (TLRs) triggered by commensal bacteria or their products (pathway A) [24,33,81,82]. Once polyps are formed, they are characterized by increased permeability allowing bacterial trans-mucosal translocation (B) and tumor promotion due to an increased inflammatory response, dominated by the production of interleukin-23 (IL-23) that induces T helper 17 (Th17) cells and innate lymphoid cells (ILCs) to produce IL-22 and IL-17 (C) [93]. IL-22 is important in mucosal homeostasis and repair [92] and is regulated at the level of production as well as by the amount of IL-22 binding protein (IL-22BP) produced (D) [91]. However, persistence of IL-22 after mucosal repair prolongs epithelial cell proliferation favoring polyp formation (E) [91]. Thus, IL-22-deficient mice have decreased and IL-22BP-deficient mice have increased carcinogenesis in these models [91]. In the AOM/dextran sulfate sodium (DSS) model of colon carcinogenesis shown on the right side of the figure, the extensive mucosal damage and erosion induced by DSS allow massive trans-mucosal microbial translocation with an excessive inflammatory response [24]. Microbes, in part through the release of short chain fatty acids (SCFAs) [103], induce epithelial cells to produce IL-18 (F) that has a mucosa protective effect by blocking the production of IL-22BP (G), thus increasing the bioavailability of IL-22 (H) for mucosa tissue repairs [91]. MyD88-deficient mice lack the IL-18 protective effect because of the role of MyD88 in IL-18R signaling while they are able to produce colon damaging and tumor-inducing inflammatory cytokines such as IL-11 and IL-6 (I) even in the absence of TLR and IL-1R signaling [37]. Thus, MyD88-deficient mice have a dramatically increased susceptibility to AOM/DSS-induced colitis and carcinogenesis [37,83–85]. This phenotype is in part reproduced by mice unable to produce IL-18 (Il18−/−), to process pro-IL-18 (Nlrp3−/−, Nlrp6−/−, ASC−/−, or Caspase1−/−) or to signal in response to IL-18 (Il18r−/− ) [89,90]. Also, deficiency of IL-18 functions results in dysbiosis (J) with expansion of colitis-inducing bacteria such as TM7 and Prevotella spp. [90]. In the AOM/DSS model, IL-22-deficient mice show increased and IL-22BP-deficient mice decreased susceptibility to colitis-associated cancer [91].
Unlike what is described in most other tumor models, intact MyD88 protects against development of colonic polyps in the AOM/DSS model of colitis-associated cancer (CAC) [37]. Myd88−/− mice are more susceptible to AOM/DSS-induced colitis and form more polyps that are larger and have histological characteristics of more aggressive adenomas and carcinomas with frequent mutation in the β-catenin gene [37]. Although mice deficient for single TLRs in some cases show increased susceptibility to colitis, they never showed an increased incidence of AOM/DSS-induced polyps as observed in the Myd88−/− mice with the possible exception of TLR2 deficiency [37,87,88]. By contrast, incidence of CACs after AOM/DSS treatment of mice lacking IL-18 and IL-18R1 is comparable to that observed in Myd88−/− mice, suggesting that the susceptibility to colitis and CAC in Myd88−/− mice is at least in part due to an inability to signal through the IL-18R [37]. Susceptibility to AOM/DSS-induced colon carcinogenesis is also increased in mice lacking Caspase-1, the inflammasome adaptor protein PYCARD (ASC), or the nucleotide-binding domain, leucinerich repeat and pyrin domain containing protein 3 (NLRP3) inflammasome, all of which are unable to process pro-IL-18 [89]. In mice deficient for the NLRP6 inflammasome, which are susceptible to spontaneous and DSS-induced colitis, IL-18 levels were decreased and the bacterial phyla Bacteroidetes (Prevotellaceae) and TM7 were expanded in the fecal microbiota [90]. Susceptibility to colitis could be transferred from Nlrp6−/− mice to wild type mice by transfer of the microbiota during cross-fostering or co-housing; however, the role of the two expanded bacterial species in colitis remains correlative. Pro-IL-18 is produced by intestinal epithelial cells and processed by the NLRP3 or NLRP6 inflammasomes in response to mucosal damage. IL-18 exerts a protective effect on mucosal homeostasis by down-regulating the production of IL-22 binding protein (IL-22BP) by colonic dendritic cells [91]. IL-22 is produced by innate lymphoid cells or adaptive Th17 and Th22 cells and induces proliferation of intestinal epithelial cells during damage repair [92]. IL-22 bioavailability in the tissue is determined not only by the amount of IL-22 produced but also negatively by production of IL-22BP, which is constitutive but reduced by damage-induced IL-18 [91]. Because IL-22 has a proliferative effect, failure to downregulate IL-22 after damage repair may be detrimental and favor carcinogenesis [91]. Thus, Il22 bp−/−/ApcMin/+ mice develop an increased number of intestinal tumors whereas Il22−/− mice develop fewer colon tumors [91], mimicking the phenotype of Myd88−/− mice [81] that are unable to signal through IL-18R and to downregulate the production of IL-22BP. Regulation of IL-22 availability by IL-18 probably explains the decreased tumor formation in Myd88−/−/ApcMin/+ mice and in AOM-treated Myd88−/− mice. In these two models of carcinogenesis, mucosal damage-induced inflammation has a limited role on tumor initiation but the proliferative effect of IL-22 facilitates the initiation of tumor dependent on loss of heterozygosity in the Apc locus or on AOM-induced mutation of β-catenin or other loci. Once tumor lesions are initiated, they are characterized by defective expression of barrier proteins, local invasion by bacteria and bacterial products, and activation of inflammatory response through MyD88-coupled TLRs, which is required for tumor progression, characterized by IL-23, IL-17, and IL-6 production [93]. The mechanisms underlying colon carcinogenesis are, however, distinct in the CAC model of AOM/DSS-induced carcinogenesis. In this model, a single treatment with the genotoxic AOM is followed by several cycles of DSS treatments that facilitate tumor promotion by inducing extensive mucosal damage, widespread microbial invasion, and inflammation. Myd88−/−, Il18−/−, and Il18r−/− mice are unable to repair the DSS-induced mucosal damage [37,81], in part due to the inability of IL-18 signaling to increase the bioavailability of IL-22 by down-regulating IL-22BP [91]. This leads to increased and persistent microbial translocation and dysbiosis, and even in the absence of TLR and IL-1R signaling, increased production of the protumorigenic IL-6 and IL-11 cytokines. As a result of altered colonic homeostasis, Myd88−/−, Il18−/−, and Il18r−/− mice, as well as Il22−/− mice, develop a higher number of, and more aggressive, polyps than wild type mice, whereas Il22 bp−/− mice develop less polyps, probably because of the protective effect of IL-22 on mucosa repair following DSS treatment [24,37,90,91].
MyD88 functions during oncogenic virus infection
As already discussed, MyD88 participates in carcinogenesis through its involvement in mediating the homeostatic functions of TLRs and IL-1Rs. Because TLRs are instrumental in the response and resistance to infectious pathogens, it is expected that MyD88 affects the response to tumor-inducing pathogens. This is supported by the ability of DNA-oncogenic viruses, such as HPV16, hepatitis B virus, and EBV, to efficiently and persistently abolish expression of the endoplasmic DNA sensor TLR9 in epithelial cells, plasmacytoid dendritic cells, and B lymphocytes, respectively [94–96]. Suppression of TLR9 might prevent an efficient innate response against oncogenic viruses and facilitate the establishment of a chronic infection, which is considered a necessary condition for cervical and other virus-induced cancers. The RNA sensor TLR7 plays an important role in the response to oncogenic retroviruses. C57BL/6 mice are resistant to tumor formation induced by endogenous retroviruses but several immunodeficient C57BL/6 strains, including Myd88−/− and Tlr3−/−Tlr7−/−Tlr9−/− mice, spontaneously develop lymphomas caused by integration of infectious ecotropic murine leukemia viruses. These viruses are generated by rearrangement of the defective endogenous retroviruses Emv2 on C57BL/6 mouse chromosome 8 with several endogenous retroviral sequences on other chromosomes [97,98]. Signaling through the MyD88-coupled TLRs by bacterial MAMPs enhances the transcription of endogenous retroviral sequences, thus favoring rearrangement and generation of fully competent infectious viruses that are vertically transmitted through blood and milk and that by integrating next to oncogene sequences induce lymphoma formation [97,98]. MyD88 is also required for optimal B cell response and formation of antibodies able to either neutralize the virus or modify the gut commensal microbiota, thus indirectly affecting virus rearrangement, persistence, and vertical transmission [97–99].
Concluding remarks
MyD88 acts at the interface of tissue homeostasis and cancer. Myd88−/− mice have allowed investigators to identify novel pathways in carcinogenesis. Although dissecting the precise mechanisms underlying the in vivo phenotype of Myd88−/− mice is challenging, models of skin and colon carcinogenesis have provided insight into MyD88 function in carcinogenesis. As expected, MyD88 signaling downstream of innate receptors is important for activation of extrinsic inflammation mediated by infiltrating hematopoietic cells. In this context of cancer-inducing inflammation, MyD88 can promote tumor formation. It is also emerging that MyD88 signaling, most often in association with the IL-1R, can regulate a proinflammatory feedback mechanism that is involved in tissue repair responses, as well as in the intrinsic inflammation associated with oncogene activation, cell transformation, and senescence. This feedback appears to be required to maintain NF-κB activation and possibly other transcription factors in the inflamed tissues. The molecular mechanisms of cell and DNA damage response that activates this feedback mechanism and the relative roles of IL-1α, IL-1β, and possibly other members of the IL-1 family are incompletely understood. A noteworthy finding is the role of the IL-1 family member IL-18 in protecting the colonic mucosa and favoring mucosal damage repair through two apparently unrelated mechanisms: regulation of intestinal microbiota composition and of IL-22 bioavailability by preventing IL-22BP production. Recent insights into IL-1 function in the skin and other tissues, and of IL-18 in the colon, may be the tip of the iceberg, and IL-1 family members may play a general role in tissue homeostasis, and in the response to cellular stress and genotoxicity. The regulation of antitumor adaptive immunity by innate receptor activation and MyD88 signaling has a complex effect on cancer depending on the immune activating or suppressive effects of the innate responses and on the antitumor or protumor class of immune response elicited. Innate resistance and adaptive immunity may limit infectivity of oncogenic microorganisms but could also be responsible for the chronic inflammatory pathology that is associated with certain infections, e.g., those caused by hepatitis viruses or H. pylori, and predisposes to cancer. Innate receptor activation also regulates the host response to commensal microbiota modifying its compositions and modulating inflammatory and immune responses both locally and systemically. Only recently the relevance of this regulation for cancer promotion and response to therapy has been recognized and investigated. A better knowledge of the role of innate receptors activated by pathogenic and commensal microorganisms or by endogenous activators of inflammation in regulation of tissue and immune homeostasis will help in the understanding of cancer biology and in the identification of targets for cancer prevention and therapy.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH).
References
- 1.Zitvogel L, et al. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 2.Colotta F, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 6.Goldszmid RS, Trinchieri G. The price of immunity. Nat. Immunol. 2012;13:932–938. doi: 10.1038/ni.2422. [DOI] [PubMed] [Google Scholar]
- 7.Jobin C. Colorectal cancer: CRC – all about microbial products and barrier function? Nat. Rev. Gastroenterol. Hepatol. 2012;9:694–696. doi: 10.1038/nrgastro.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schetter AJ, et al. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grivennikov SI, et al. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeda K, et al. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 11.Ngo VN, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treon SP, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N. Engl. J. Med. 2012;367:826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 13.Wang EL, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br. J. Cancer. 2010;102:908–915. doi: 10.1038/sj.bjc.6605558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Je EM, et al. Mutational and expressional analyses of MYD88 gene in common solid cancers. Tumori. 2012;98:663–669. doi: 10.1177/030089161209800518. [DOI] [PubMed] [Google Scholar]
- 15.El-Omar EM, et al. Polymorphisms in Toll-like receptor genes and risk of cancer. Oncogene. 2008;27:244–252. doi: 10.1038/sj.onc.1210912. [DOI] [PubMed] [Google Scholar]
- 16.Hold GL, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905–912. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Roszak A, et al. Involvement of Toll-like Receptor 9 polymorphism in cervical cancer development. Mol. Biol. Rep. 2012;39:8425–8430. doi: 10.1007/s11033-012-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens VL, et al. Genetic variation in the toll-like receptor gene cluster (TLR10-TLR1-TLR6) and prostate cancer risk. Int. J. Cancer. 2008;123:2644–2650. doi: 10.1002/ijc.23826. [DOI] [PubMed] [Google Scholar]
- 19.Engels EA, et al. Systematic evaluation of genetic variants in the inflammation pathway and risk of lung cancer. Cancer Res. 2007;67:6520–6527. doi: 10.1158/0008-5472.CAN-07-0370. [DOI] [PubMed] [Google Scholar]
- 20.Han W, et al. Multiplex genotyping of 1107 SNPs from 232 candidate genes identified an association between IL1A polymorphism and breast cancer risk. Oncol. Rep. 2010;23:763–769. [PubMed] [Google Scholar]
- 21.Sugimoto M, et al. Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori. J. Gastroenterol. Hepatol. 2009;24:1725–1732. doi: 10.1111/j.1440-1746.2009.06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jatoi A, et al. The cancer anorexia/weight loss syndrome: exploring associations with single nucleotide polymorphisms (SNPs) of inflammatory cytokines in patients with non-small cell lung cancer. Support. Care Cancer. 2010;18:1299–1304. doi: 10.1007/s00520-009-0748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiDonato JA, et al. NF-kappaB and the link between inflammation and cancer. Immunol. Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 24.Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat. Rev. Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J. Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat. Rev. Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 27.Hasan UA, et al. Cell proliferation and survival induced by Toll-like receptors is antagonized by type I IFNs. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8047–8052. doi: 10.1073/pnas.0700664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan UA, et al. Toll-like receptor signaling stimulates cell cycle entry and progression in fibroblasts. J. Biol. Chem. 2005;280:20620–20627. doi: 10.1074/jbc.M500877200. [DOI] [PubMed] [Google Scholar]
- 29.Salaun B, et al. TLR3 can directly trigger apoptosis in human cancer cells. J. Immunol. 2006;176:4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 30.Salaun B, et al. Toll-like receptors’ two-edged sword: when immunity meets apoptosis. Eur. J. Immunol. 2007;37:3311–3318. doi: 10.1002/eji.200737744. [DOI] [PubMed] [Google Scholar]
- 31.Monick MM, et al. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J. Immunol. 2001;166:4713–4720. doi: 10.4049/jimmunol.166.7.4713. [DOI] [PubMed] [Google Scholar]
- 32.Macedo L, et al. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am. J. Pathol. 2007;171:1774–1788. doi: 10.2353/ajpath.2007.061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakoff-Nahoum S, et al. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Iimuro Y, et al. Role of innate immune response in liver regeneration. J. Gastroenterol. Hepatol. 2007;22(Suppl. 1):S57–S58. doi: 10.1111/j.1440-1746.2006.04651.x. [DOI] [PubMed] [Google Scholar]
- 35.Chong HC, et al. Regulation of epithelial-mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J. Cell Biol. 2009;184:817–831. doi: 10.1083/jcb.200809028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barton CE, et al. Novel p63 target genes involved in paracrine signaling and keratinocyte differentiation. Cell Death Dis. 2010;1:e74. doi: 10.1038/cddis.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salcedo R, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J. Exp. Med. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naik S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swann JB, et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 2008;105:652–656. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittal D, et al. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 2010;29:2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coste I, et al. Dual function of MyD88 in RAS signaling and inflammation, leading to mouse and human cell transformation. J. Clin. Invest. 2010;120:3663–3667. doi: 10.1172/JCI42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cataisson C, et al. IL-1R-MyD88 signaling in keratinocyte transformation and carcinogenesis. J. Exp. Med. 2012;209:1689–1702. doi: 10.1084/jem.20101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darwiche N, et al. Expression profile of skin papillomas with high cancer risk displays a unique genetic signature that clusters with squamous cell carcinomas and predicts risk for malignant conversion. Oncogene. 2007;26:6885–6895. doi: 10.1038/sj.onc.1210491. [DOI] [PubMed] [Google Scholar]
- 44.Yusuf N, et al. Protective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer Res. 2008;68:615–622. doi: 10.1158/0008-5472.CAN-07-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee WY, et al. Interleukin-1 alpha mediates phorbol ester-induced inflammation and epidermal hyperplasia. FASEB J. 1994;8:1081–1087. [PubMed] [Google Scholar]
- 46.Li X, et al. Interleukin-1alpha up-regulation in vivo by a potent carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) and control of DMBA-induced inflammatory responses. Cancer Res. 2002;62:417–423. [PubMed] [Google Scholar]
- 47.Muller AJ, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc. Natl. Acad. Sci. U.S.A. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caunt CJ, et al. Ras controls tumor necrosis factor receptor-associated factor (TRAF)6-dependent induction of nuclear factor-kappa b. Selective regulation through receptor signaling components. J. Biol. Chem. 2001;276:6280–6288. doi: 10.1074/jbc.M006772200. [DOI] [PubMed] [Google Scholar]
- 49.McDermott EP, O’Neill LA. Ras participates in the activation of p38 MAPK by interleukin-1 by associating with IRAK, IRAK2, TRAF6, and TAK-1. J. Biol. Chem. 2002;277:7808–7815. doi: 10.1074/jbc.M108133200. [DOI] [PubMed] [Google Scholar]
- 50.Wang KZ, et al. TRAF6 activation of PI 3-kinase-dependent cytoskeletal changes is cooperative with Ras and is mediated by an interaction with cytoplasmic Src. J. Cell Sci. 2006;119:1579–1591. doi: 10.1242/jcs.02889. [DOI] [PubMed] [Google Scholar]
- 51.Xu H, et al. Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J. Biol. Chem. 2003;278:36334–36340. doi: 10.1074/jbc.M305698200. [DOI] [PubMed] [Google Scholar]
- 52.Starczynowski DT, et al. TRAF6 is an amplified oncogene bridging the RAS and NF-kappaB pathways in human lung cancer. J. Clin. Invest. 2011;121:4095–4105. doi: 10.1172/JCI58818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mueller MM. Inflammation in epithelial skin tumours: old stories and new ideas. Eur. J. Cancer. 2006;42:735–744. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Cataisson C, et al. Inducible cutaneous inflammation reveals a protumorigenic role for keratinocyte CXCR2 in skin carcinogenesis. Cancer Res. 2009;69:319–328. doi: 10.1158/0008-5472.CAN-08-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahanivong C, et al. Protein kinase C alpha-CARMA3 signaling axis links Ras to NF-kappa B for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. Oncogene. 2008;27:1273–1280. doi: 10.1038/sj.onc.1210746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harb. Perspect. Biol. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hobbs RM, Watt FM. Regulation of interleukin-1alpha expression by integrins and epidermal growth factor receptor in keratinocytes from a mouse model of inflammatory skin disease. J. Biol. Chem. 2003;278:19798–19807. doi: 10.1074/jbc.M300513200. [DOI] [PubMed] [Google Scholar]
- 58.Mascia F, et al. EGFR regulates the expression of keratinocyte-derived granulocyte/macrophage colony-stimulating factor in vitro and in vivo. J. Invest. Dermatol. 2010;130:682–693. doi: 10.1038/jid.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Streicher KL, et al. Activation of a nuclear factor kappaB/interleukin-1 positive feedback loop by amphiregulin in human breast cancer cells. Mol. Cancer Res. 2007;5:847–861. doi: 10.1158/1541-7786.MCR-06-0427. [DOI] [PubMed] [Google Scholar]
- 60.Cohen I, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borrello MG, et al. Inflammation and cancer: the oncogene-driven connection. Cancer Lett. 2008;267:262–270. doi: 10.1016/j.canlet.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 62.Wang B, et al. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- 63.Shang K, et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS ONE. 2012;7:e51848. doi: 10.1371/journal.pone.0051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acharyya S, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jamieson T, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Invest. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Campisi J. Cellular senescence: putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 2011;21:107–112. doi: 10.1016/j.gde.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodier F, et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 2011;124:68–81. doi: 10.1242/jcs.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orjalo AV, et al. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharpless NE, DePinho RA. Cancer: crime and punishment. Nature. 2005;436:636–637. doi: 10.1038/436636a. [DOI] [PubMed] [Google Scholar]
- 71.Halazonetis TD, et al. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 72.Edme N, et al. Ras induces NBT-II epithelial cell scattering through the coordinate activities of Rac and MAPK pathways. J. Cell Sci. 2002;115:2591–2601. doi: 10.1242/jcs.115.12.2591. [DOI] [PubMed] [Google Scholar]
- 73.Loercher A, et al. Nuclear factor-kappaB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res. 2004;64:6511–6523. doi: 10.1158/0008-5472.CAN-04-0852. [DOI] [PubMed] [Google Scholar]
- 74.Dajee M, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 75.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 76.Moore RJ, et al. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat. Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 77.Schreiber RD, et al. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 78.Wu J, et al. The proinflammatory myeloid cell receptor TREM-1 controls Kupffer cell activation and development of hepatocellular carcinoma. Cancer Res. 2012;72:3977–3986. doi: 10.1158/0008-5472.CAN-12-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ling J, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ochi A, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J. Exp. Med. 2012;209:1671–1687. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 82.Uronis JM, et al. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Araki A, et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterol. 2005;40:16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 84.Brown SL, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J. Clin. Invest. 2007;117:258–269. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pull SL, et al. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. U.S.A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greten FR, et al. IKKb links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Lowe EL, et al. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS ONE. 2010;5:e13027. doi: 10.1371/journal.pone.0013027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukata M, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elinav E, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber S, et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–263. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zenewicz LA, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grivennikov SI, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fathallah I, et al. EBV latent membrane protein 1 is a negative regulator of TLR9. J. Immunol. 2010;185:6439–6447. doi: 10.4049/jimmunol.0903459. [DOI] [PubMed] [Google Scholar]
- 95.Vincent IE, et al. Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS ONE. 2011;6:e26315. doi: 10.1371/journal.pone.0026315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hasan UA, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 2007;178:3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 97.Young GR, et al. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature. 2012;491:774–778. doi: 10.1038/nature11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu P, et al. Nucleic acid-sensing Toll-like receptors are essential for the control of endogenous retrovirus viremia and ERV-induced tumors. Immunity. 2012;37:867–879. doi: 10.1016/j.immuni.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 99.Kane M, et al. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boraschi D, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur. Cytokine Netw. 2011;22:127–147. doi: 10.1684/ecn.2011.0288. [DOI] [PubMed] [Google Scholar]
- 101.Guo L, et al. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 2012;33:598–606. doi: 10.1016/j.it.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Denning MF, et al. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C delta. J. Biol. Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 103.Kalina U, et al. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur. J. Immunol. 2002;32:2635–2643. doi: 10.1002/1521-4141(200209)32:9<2635::AID-IMMU2635>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]