Abstract
The retinoblastoma protein is essential for accurate DNA replication, and its loss is commonly associated with cancer. It emerges that this protein also regulates another stage of the cell cycle.
Just as a team of explorers needs a detailed map to guide them on their journey, a cell uses information held within its DNA, and packaged into chromosomes, for guidance throughout its life. It is therefore crucial that genetic information is faithfully maintained for the lifetime of the cell and of its progeny. The tumour-suppressor protein retinoblastoma is known for its role in regulating DNA replication and so maintaining genome stability1,2. Three papers3–5 published in Genes & Development now also implicate this protein in mediating proper distribution of genetic information between the two daughter cells during mitotic cell division.
Once a cell divides, its genetic information instructs it what shape to assume, what functions to perform, how quickly to proliferate again or whether to stop dividing altogether. Indeed, some of the genes that regulate the cell-division cycle serve as brakes to halt the cell’s journey, allowing it to differentiate and settle into its final tissue destination. Incorrect copying of the genetic instructions or damage to the DNA harbouring these instructions could lead the cell to the wrong destination or, worse, to an endless journey — a crisis that warrants cell death.
Tumour-suppressor proteins stop cell division under such circumstances. For instance, the retinoblastoma protein (Rb) — the loss of which can cause human eye tumours, as well as most other types of cancer1 — can stop cell proliferation by limiting the expression of genes that promote DNA replication during the S phase of the cell cycle1,2 (Fig. 1). It thus puts a strong brake on cell division that is removed only when the cell has little chance to pass on erroneous or incomplete genetic instructions.
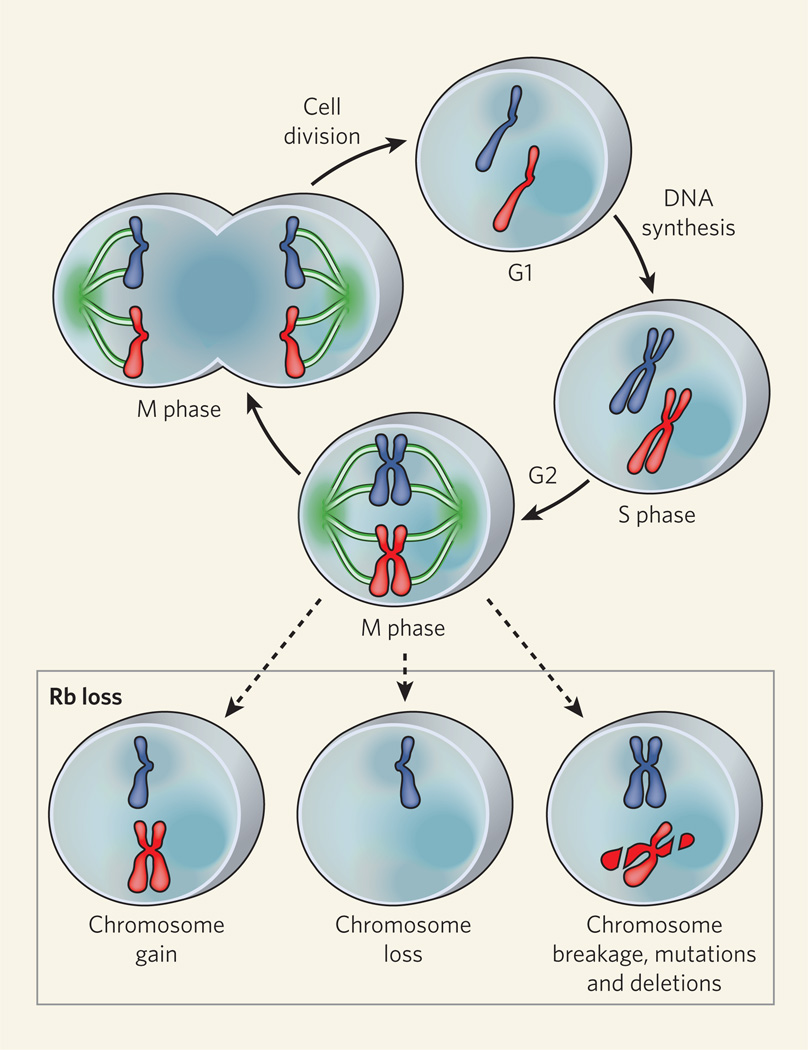
Figure 1. Retinoblastoma and the cell cycle.
Dividing cells undergo cycles of division, in which the S phase (when DNA replicates) is separated from the M phase (when mitotic cell division occurs) by two gap phases, G1 and G2. The role of the retinoblastoma (Rb) protein in regulating the transition from G1 to the S phase is well established. Three studies3–5 now implicate this protein in mediating chromosome dynamics during the M phase. Outcomes of Rb deficiency during mitosis include chromosome gain, chromosome loss and chromosome breakage.
Just as essential as faithful DNA replication is the accurate distribution of the replicated chromosomes between the two daughter cells during cell division. (By analogy, if members of the explorers’ team decide to go their separate ways, accurate copies of the map would be of little use if they were not equally distributed among the explorers.)
Chromosomes are equally segregated into the daughter cells during mitosis, or the M phase of the cell cycle (Fig 1). There have been hints2 that Rb also plays a direct part in segregating chromosomes during mitosis, although it remained unclear whether mitotic defects associated with loss of this protein were an indirect consequence of errors committed during the preceding S phase. Could it be that Rb mediates both accurate and timely DNA replication and proper distribution of chromosomes in mitosis? The answer is yes, according to the three new studies3–5.
For chromosomes to physically move through the viscous environment of the cell, they must condense into relatively small and stiff shapes. Just like folding a map into a compact form allows its easy storage and protects it against damage during travel, presumably condensation minimizes drag and potential breakage while the chromosomes are pushed and pulled during segregation to the opposite sides of the dividing cell. Coschi et al.3 demonstrate in mice that Rb facilitates chromosome condensation, independently of its role in DNA replication. When combined with an additional mutation in another tumour-suppressor protein, mitotic defects caused by the loss of Rb resulted in highly aggressive cancers. The involvement of Rb in chromosome condensation therefore seems to be crucial for its tumour-suppressive activity.
Manning and colleagues4 report that Rb is also required for the functioning of specialized chromosomal regions called centromeres. Chromosomes attach to the mitotic spindle through centromeres, which move along the spindle to the opposite ends of the dividing cell. Rb mediates the proper condensation and orientation of centromeres on duplicated chromosomes and thus their attachment to the spindle. Strikingly, Manning et al. find that, when the Rb function is compromised, the levels of chromosome mis-segregation rise to those measured in tumour cells.
The stability of chromosomes must also be maintained during mitosis, when chromosomes can break. Van Harn et al.5 provide evidence that mouse fibroblast cells lacking functional Rb and its related proteins p107 and p130 are more prone to chromosome breakage. On cell division, broken chromosomes are haphazardly distributed between the two daughter cells, which can lead to cells that are missing genetic information on some chromosomes or that carry duplicate copies of some chromosome regions (Fig. 1).
Apart from implicating Rb in the control of mitotic chromosomes, the three studies have another theme in common: loss of Rb function does not doom cells to death. Consequently, when the function of this protein is compromised, low-level defects in any of these mitotic processes can ensue, setting the stage for inheritance of altered genetic instructions at each cycle of cell division, and incrementally increasing the likelihood of generating a renegade cell with uncontrollable proliferative potential.
How exactly does Rb facilitate chromosome condensation, ensure centromere function and protect chromosomes from breakage? Of particular interest is the molecular machinery that works on chromosomes to condense them. What precise role does condensation have on centromere function? Although general chromosome condensation is abnormal in Rb-deficient cells, chromosomes nevertheless achieve sufficient compaction and progress through many cell divisions. To treat cancer, might it be possible to target the chromosome-condensation machinery to completely stall mitosis and so stop tumour-cell division? The new findings3–5 give us reason to explore this possibility.
References
- 1.Weinberg RA. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 2.Sage J, Straight AF. Genes Dev. 2010;24:1329–1333. doi: 10.1101/gad.1948010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coschi CH, et al. Genes Dev. 2010;24:1351–1363. doi: 10.1101/gad.1917610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manning AL, Longworth MS, Dyson NJ. Genes Dev. 2010;24:1364–1376. doi: 10.1101/gad.1917310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Harn T, et al. Genes Dev. 2010;24:1377–1388. doi: 10.1101/gad.580710. [DOI] [PMC free article] [PubMed] [Google Scholar]