Abstract
The Second International Workshop on Advances in Electrocorticography (ECoG) was convened in San Diego, CA, USA, on November 11–12, 2010. Between this meeting and the inaugural 2009 event, a much clearer picture has been emerging of cortical ECoG physiology and its relationship to local field potentials and single-cell recordings. Innovations in material engineering are advancing the goal of a stable long-term recording interface. Continued evolution of ECoG-driven brain–computer interface technology is determining innovation in neuroprosthetics. Improvements in instrumentation and statistical methodologies continue to elucidate ECoG correlates of normal human function as well as the ictal state. This proceedings document summarizes the current status of this rapidly evolving field.
Keywords: Electrocorticography, Brain–computer interface, High-frequency oscillations, Brain mapping, Seizure detection, Gamma-frequency electroencephalography, Neuroprosthetics, Subdural grid
1. Introduction
1.1. Anthony Ritaccio
The Second International Workshop on Advances in Electrocorticography (ECoG) was convened in San Diego, CA, USA, on November 11–12, 2010, as a satellite event of the annual meeting of the Society for Neuroscience. Building on the success of the First International Workshop [1], the program was expanded to a 2-day format to adequately represent the explosive growth in knowledge in both clinical and experimental realms. In the year between these gatherings, a much clearer picture has emerged of cortical ECoG physiology and its relationship to local field potentials and single-cell recordings. Similarly, there has been rapid evolution in material engineering of active and passive sensor technology. ECoG continues to evolve as a preeminent direct neural interface in both animal and human brain–computer interfaces (BCIs). Improvements in instrumentation available to the clinical epileptologist, continued elucidation of pathological high-frequency oscillations, and the demonstration of “microseizures” in submillimeter domains redefine the epileptogenic zone and may soon prove transformative in epilepsy surgery planning. Prescient research into the aforementioned developments was at the core of our second gathering. We give our greatest thanks to the authoritative multi-international faculty of ECoG “scale chauvinists,” who gave of their time and expertise to present their work as well as contribute to these representative proceedings.
2. Emerging understanding of electrocorticography physiology: What is inside the electrocorticography signal?
2.1. Kai J. Miller, Josef Parvizi
For many years, observations made with the electroencephalogram (EEG) have made us believe that the brain electrophysiology is about synchronized rhythmic activity of neuronal populations. However, because of the remote distance of the scalp EEG electrode from the cortical surface (~20 mm), the captured electrophysiological signals are necessarily averaged across a large area of the cortical mantle, and therefore any asynchronized pattern of activity within a population of neurons is largely lost to spatial averaging in the scalp EEG measurement.
In contrast to scalp EEG, ECoG offers a closer look into the dynamics of electrophysiological signals within a focal cortical tissue it records from. Given the relative size of ECoG electrodes (~2 mm in diameter), the recording area underneath each electrode resembles the size of a typical voxel in the current neuroimaging methods (i.e., 10 mm3), which contains ~10–20 functional columns [2] and ~105 neurons and 109 synapses [3]. Thus, ECoG measures neuronal populations on a much more local scale than recordings from the scalp.
On the basis of earlier work, it is understood that current dipoles between cortical lamina produce macroscale field potentials [4]. Properties of the physiology underlying the current source density (CSD) in different cortical lamina were established experimentally in the late 1970s and early 1980s [5]. We now know that the propagating action potentials in axons and axonal terminals do not contribute strongly to the CSD at spatial scales of ~50–300 μm or greater, for example, the scales where local field potentials (LFP) are pooled from or over which ECoG potentials are averaged. Instead, it is the dendritic synaptic current exchange (i.e., influx and efflux) that modulates the CSD and, by extension, the LFP and the ECoG signal. This has recently been substantiated by simultaneous in vivo recordings of the intracellular potential and the LFP, showing that they are tightly coupled temporally, independent of the spiking pattern of the neuron [6]. As such, one might directly infer properties about the underlying neuronal statistics from the shape of the changes in the electric potential [7,8].
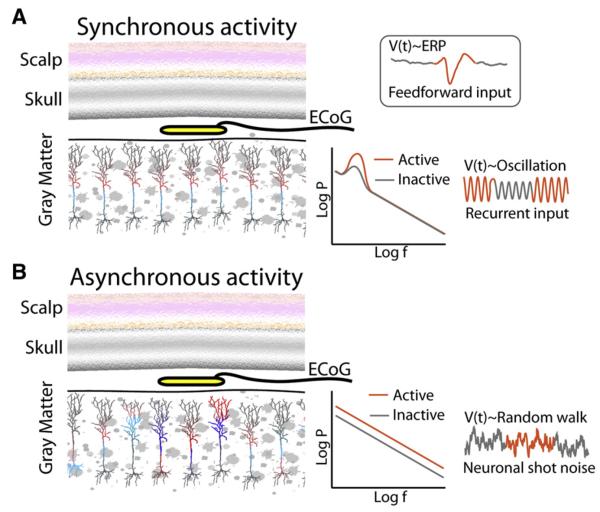
A way to think about the properties of ECoG signals is the degree to which synaptic inputs are in concert and synchronized across a population of neurons, compared with the asynchronous pattern of complex local inputs within a population of neurons (Fig. 1). Although the EEG is best in capturing fluctuating rhythms, the ECoG trace of asynchronous inputs, when averaged, appears as a type of random walk in time and contains a mixture of various processes such as event-related potentials, rhythmic oscillations, and asynchronous local activity.
Fig. 1.
How synaptic input to a neuronal population might be reflected in the ECoG potential time series V(t). (A) Synchronized activity, feedforward input revealed by transient, multiphasic, event-related potential changes, or recurrent feedback oscillatory inputs revealed by changes in peaked aspects of the power versus frequency spectrum. (B) Asynchronous, local activity revealed by broadband changes in the power versus frequency spectrum. P, power; f, frequency.
In the ECoG, changes in the way local neurons interact with one another will appear as a “speeding up” of the random walk, difficult to see when looking at the raw potential but apparent as broadband, power-law changes when looking in the frequency domain (Fig. 1) [7]. Synchronized changes, by contrast, can be visually apparent in the raw tracing—even if the synchronization is relatively weak, averaging augments the synchronized portion, whereas the other aspects are diminished. If the synchronization is tied to an oscillatory process of some kind (such as the one in the loop between the cortex and sub-cortical structures), then a “rhythm” will emerge, and the synchronization and desynchronization of the rhythmic process will be revealed as changing amplitude of a sinusoid in the time series and as a peak in the frequency domain. If the synchronization is tied to a feedforward process, such as thalamic input to V1 following a visual stimulus, the time series may reflect a multiphasic, time-locked response, the “event-related potential,” where the different portions of the event-locked time series will likely reflect initial input to cortical pyramidal neurons from the thalamus, and then synchronized lateral inhibition. The measured ECoG voltage time series will be then a mixture of random walk-like changes reflecting asynchronous local cortical processing, sinusoidal changes reflecting synchronized oscillatory feedback, and event-locked multiphasic characteristic deflections reflecting feedforward synchronized volleys tied to a specific input event. Moreover, when a slower rhythm predominates in a cortical region, its phase of activity can easily modulate the amplitude of the higher-frequency signals, that is, the so-called phase–amplitude coupling, which has been shown to be significant during cognitive processes [9,10].
Emerging understanding of macroscale neurophysiology using ECoG will have to focus on isolating its different subcomponents (e.g., event-related potentials, rhythmic oscillations, and asynchronous local activity) from the voltage time series. For instance, application of sophisticated pattern recognition to the event-related phenomenon can be used to capture the specific identity of visual stimuli on a single-trial basis [11]; examining the frequency domain, rhythmic interarea oscillations can be revealed as peaked phenomena at specific frequencies that are time-locked to our engagement or disengagement in a task [12,13]; or very local cortical processing, such as the changes in the random walk-type process of the voltage time series, can be isolated from broadband changes across the entire frequency range [14], which is most easily revealed at high frequencies, where the peaked effect of oscillatory changes are not present [15,16].
3. Contemporary clinical electrocorticography
3.1. Chronic electrocorticography in epilepsy
3.1.1. Robert B. Duckrow
Although a majority of people with epilepsy experience relief from recurrent seizures with medical therapy, there remains a substantial population whose seizures are intractable. Of that portion with symptomatic and localization-related epilepsies, surgical therapy based on the principle of focal seizure onset is reasonably effective. If a discrete region of seizure onset can be localized and safely removed, the hope of seizure freedom, reduced medication burden, and improved quality of life can be offered. Invasive intracranial EEG recording was developed when it became clear that scalp EEG recordings were inadequate for this purpose [17].
Intracranial recording of the electrocorticogram is performed for clinical purposes [18] using 2.3-mm-diameter disk electrodes made of platinum or stainless steel embedded in silicone elastomer. Commonly used electrode arrays are linear, curvilinear, or rectilinear with 5- or 10-mm center-to-center spacing. These are threaded between the dura and arachnoid or placed on the exposed surface of the brain through burr holes or craniotomy with the connecting wires exiting through scalp incisions. Signals are obtained from deep structures using penetrating linear probes with multiple cylindrical contacts of 1.1-mm diameter and variable length and spacing. Digital acquisition commonly resolves signals from 0.1 to 100 Hz.
The cerebral activity measured with intracranial electrodes is interpreted by defining recurring electrographic patterns at seizure onset and correlating them with the eventual outcome of resective surgery. When seizure onset is associated with pathological lesions defined by brain imaging, resection of involved areas can lead to seizure control in 60–80% of patients [19].
The challenge of contemporary ECoG is the definition of the seizure onset zone when brain images show no discrete lesion or when subtle abnormalities are multifocal, as is common with malformations of cortical development. Intracranial recording continues to provide the best spatial and temporal resolution of epileptiform activity but suffers from sparse sampling and a resulting inability to distinguish seizure onset patterns from those propagated from a distant source. This interpretive approach to extratemporal nonlesional epilepsy results in seizure control only 30–50% of the time [19]. Although this can be explained by sampling error, it may also suggest the need for an alternative hypothesis of seizure generation.
Functional networks are being defined in the brain in the resting state and during task performance [20]. If regional networks are involved in the epileptic process, seizures may be generated by the interaction of these regions [21]. By acting together, regions forming an epileptic network can define dependent multifocal seizure onset. This suggests that interruption of a pathway (edge) or region (node) could disable the network. However, given the resiliency of complex networks, it is possible that all major nodes of an epileptic network must be disrupted to achieve seizure freedom, as implied by the necessity to resect both foci in many patients with dual pathology [22].
Although the likelihood of obtaining useful information from the intracranial EEG is high, our ability to localize the epileptogenic zone will require an adequate definition of the structure and function of brain networks that initiate and maintain epileptic seizures. Future effective application of intracranial ECoG will have to meet this challenge.
3.2. Intraoperative electrocorticography: Applications and limitations
3.2.1. Andrew J. Cole
Electrocorticography, defined as recording the EEG directly from the surface of the brain, can be performed in the extraoperative setting, using grids, strips, or pedestal-based systems, or in the intraoperative setting, typically using either frame-based or array-based recording electrodes. Here, we discuss the utility of intraoperative ECoG.
The indications for intraoperative ECoG include the identification of epileptogenic cortex based on the recording of spontaneous activity; the assessment of completeness of resection, judged by the presence of persistent or de novo spikes; the recognition of epileptic discharge during stimulus-based functional brain mapping in the setting of epilepsy, tumor, or vascular neurosurgical procedures; and, on occasion, to reduce the need for high-morbidity, chronic intracranial investigation.
Important technical issues directly impact the performance of intraoperative ECoG, including the presence of a noisy electrical environment, the limited time window in which to examine the cortical physiology, anesthetic effects on cortical activity, challenges related to co-registering electrodes with anatomic markers in underlying lesions, and limited ability to sample because of the amount of time available and the size and location of the craniotomy. In addition, real-time interpretation is required to provide useful and actionable feedback to the neurosurgeon.
The interpretation of results of activation techniques during corticography should be undertaken with caution. Typical activation techniques include anticonvulsant withdrawal, physiological activation such as hyperventilation and photic stimulation, and pharmacological activation, including the use of pentylenetetrazole, methohexital, alfentanyl, or etomidate. Application of these agents may increase spike frequency, increase the size of the cortical area producing spikes, or increase the number of cortical sites producing spikes independently. These activation effects are listed in decreasing order of reliability with respect to defining the epileptogenic zone.
With respect to localization of the epileptic zone, spontaneous spikes may arise from an area exceeding the critical zone. Stimulation-induced afterdischarge has not been demonstrated to be reliable in identifying epileptic cortex. Stimulation-induced habitual seizures are likely to provide reliable lobar localization but have limited utility in elucidating sublobar localization. Stefan et al. [23] have noted that reproduction of the patient's typical warning seems to be a reliable tool for identifying the seizure onset zone in the mesial temporal lobe.
The use of ECoG to define the limits of resection elicits strong feelings based on few data. Most studies suggest that residual spikes predict poor outcome in lesional epilepsy surgery. In an important study, McKhann et al. [24] showed that residual hippocampal spikes predicted poorer outcome in mesial temporal lobe epilepsy surgery. Palmini et al. [25] have shown that focal cortical dysplasias are often characterized by active spikes, repetitive bursting, and electrographic seizures and that uniformly poor outcome can be expected in those with residual postresection epileptic discharge, presumably indicating incomplete resection of the developmental abnormality. De novo postresection spikes are likely to be benign, perhaps related to acute surgical injury [26].
Electrocorticography is frequently used to perform intraoperative functional mapping of eloquent cortex. Examination of somatosensory evoked potentials may be useful to define the central sulcus, whereas interruption of function is the hallmark of stimulation of language cortex. Motor evoked potentials may be assessed while the patient is anesthetized, providing a useful means to identify primary motor cortex. In each of these cases, ongoing ECoG is critical to assess generation of afterdischarges and seizures that might confound interpretation of the mapping data and place the patient at considerable risk. Careful choice of anesthetic agent and avoidance of inhalational agents is critical to successful ECoG mapping.
Intraoperative ECoG will continue to play an important role in advanced neurosurgical treatment at tertiary care centers. New real-time analytical approaches are desperately needed to improve our ability to fully identify the epileptic zone and assess the completeness of resection. Identification of better surrogates for epileptogenic cortex such as high-frequency discharges could potentially reduce the need for high-morbidity chronic intracranial investigations of patients with focal epilepsy in the future.
4. Emerging electrocorticography methodologies
4.1. Instrumentation for emerging electrocorticography applications
4.1.1. Peter Brunner
Traditional clinical applications that use ECoG signals all depend on visual inspection. These traditional applications include diagnosis of epilepsy and other disorders of the central nervous system [27], localization of epileptogenic cortex [28], and mapping of eloquent cortex prior to resective brain surgery [29]. In all these applications, highly trained clinical investigators base their diagnoses on visual inspection of recorded behavioral patterns and neurophysiological signals. Recent studies have described promising techniques that could replace or enhance these traditional procedures. These emerging techniques (e.g., brain–computer interfaces, or BCIs [30], passive mapping of eloquent cortex [16], and automated seizure detection [31]) subject ECoG features (that are often not readily accessible through visual inspection) to produce automated real-time computer analyses, visualization, and feedback.
The use of these emerging techniques has been impeded by the technical characteristics (i.e., resolution, sampling rate, filtering) of current clinical bedside monitoring systems, which have been optimized to provide a visual impression comparable to that of their mechanical predecessors, rather than to provide the most detailed representation of the ECoG signals.
In response to this issue, several manufacturers have begun to design dedicated systems for research purposes. These research systems record signals acquired using EEG, ECoG, or microelectrode recordings in real time from up to 512 channels, sampled at up to 50 kHz with very high sensitivity (e.g., 24-bit resolution, 250-mV sensitivity). Such systems not only acquire data but also can communicate them in real time to external software. Thus, these systems focus on the requirements of emerging clinical applications.
These dedicated research systems are usually not intended to fully replace current clinical bedside monitoring systems. Thus, integration of these research systems in a clinical environment requires additional hardware (e.g., splitter boxes) to interface with existing clinical bedside monitoring systems. The bulk of this additional hardware in addition to the existing head stages sets practical limits to emerging ECoG applications, particularly with respect to increasing the number of possible recording channels. In response, some manufacturers have begun to design splitter boxes that integrate head stages and splitter boxes.
Using these research-grade systems, groups around the world are now beginning to demonstrate the efficacy of emerging clinical applications [32–35]. Translation of these clinical demonstrations into clinical practice will eventually require optimized integration, miniaturization, and wireless communication so that the resulting systems can be implanted chronically.
In summary, the role of ECoG-based instrumentation is evolving from its original role, which was focused solely on clinical bedside monitoring, to support of new and more complex applications including BCIs, real-time functional mapping, and automated seizure detection.
4.2. Sensors (including current limitations and solutions)
4.2.1. Emerging passive sensor technology for electrocorticography
4.2.1.1. Justin Williams
There has been emerging interest in recording higher-density potentials from the surface of the brain, for both scientific [36–38] and translational [39–41], as well as clinical [42], applications. The use of these devices thus far has been centered on modified commercially available electrode grids that have transitioned from more closely spaced macroelectrodes [38] to high-density microelectrodes [41,42]. The push in the field is to continue this progression toward custom-fabricated devices that rely on microfabrication capabilities to produce new sensor arrays with a host of capabilities. These devices, collectively termed microECoG arrays, take advantage of microelectromechanical (MEMS) processing techniques to produce arrays that are ultrahigh-density and are built on flexible insulating substrates [36,37,43]. The use of flexible insulating substrates (as opposed to traditional stiff silicon) allows for the devices to conform to the convoluted surface of the brain, thereby providing intimate contact of each electrode with the cortical surface [37,43]. Additionally, many thin-film polymers have unique material properties that allow them to “stick” to the brain tissue or have surfaces that are easily modified with chemical treatments [43]. There are a number of different materials that can be used, with polyimide, parylene, liquid crystal polymer, and polydimethylsiloxane (PDMS) being the most popular because of their combination of flexibility, dielectric properties, biocompatibility, and amenability to MEMS manufacturing methods [37,43–45]. One of the main advantages of using flexible thin-film electronics materials is that they are amenable to rapid prototyping manufacturing processes [43]. This allows for a wide range of electrode geometries to be produced that can accommodate different species (from mice to rats to primates) and different brain areas [36,37,43]. Fig. 2 illustrates the variety of electrode arrays that can be produced. The use of rapid prototyping processes also makes possible the idea that “personalized” electrode arrays could be produced in future clinical applications [43].
Fig. 2.
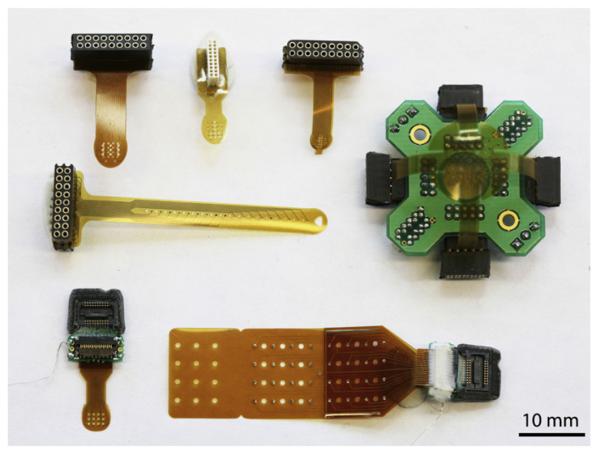
Flexible thin-film microECoG devices can be made into a variety of sizes and configurations to accommodate various size species, brain regions, and insertion methods.
Thin-film microECoG devices also have the potential to open up a number of new research paradigms. The thin-film electrode substrate can be fabricated with holes throughout the substrate wherever it is not necessary for insulating electrode traces [43,46]. This significantly reduces the footprint of the overall device and also allows for the integration of other types of sensors. For example, penetrating intracortical microelectrode arrays can be inserted through the center of the microECoG array to provide a three-dimensional sampling of neural activity from the underlying brain [46]. This type of preparation could provide insights into the relationship between single-unit and field activity in different cortical layers with the microECoG signals recorded at the overlying brain surface. Lastly, polymers such as parylene and PDMS are optically transparent. Coupling these transparent substrate microECoG arrays with contemporary window brain imaging techniques opens up a number of possibilities for linking microECoG electrophysiology with optical measures of underlying vascular and glial function. Additionally, these approaches lend themselves to the use of optogenetic manipulation of the neural tissue [47]. Overall, the use of thin-film electronic approaches for making ultraflexible, high-density microECoG arrays provides unique opportunities in both basic science and future clinical applications.
4.2.2. High-density micro-electrocorticography using flexible silicon electronics
4.2.2.1. Jonathan Viventi
Current implantable brain devices for clinical and research applications require that each electrode is individually wired to a separate electronic system. Establishing a high-resolution interface over broad regions of the brain is infeasible under this constraint, as an electrode array with thousands of passive contacts would require thousands of wires to be individually connected. To overcome this limitation, we have developed new implantable electrode array technology that incorporates active, flexible electronics. This technology has enabled extremely flexible arrays of 720 and soon, thousands of multiplexed and amplified sensors spaced as closely as 250 μm apart, connected using just a few wires. These devices yield an unprecedented level of spatial and temporal microECoG resolution for recording and stimulating distributed neural networks.
We have demonstrated a 360-channel active electrode array capable of sampling a 10×9-mm area of cortex with high spatial resolution (500-μm spacing) and high temporal resolution (>10 kS/s) while requiring only 39 wires. This technology can be readily scaled to much larger sizes, such as 80×80 mm, with 25,600 electrodes, while maintaining a sampling rate >1.2 kS/s.
MicroECoG is one of the many possible applications of this technology, which also includes cardiac, peripheral nerve, and retinal prosthetic devices. Using this technology, we have produced examples of retinotopic and tonotopic maps from in vivo recordings. We have also observed finely detailed spatial and temporal patterns from a feline model of acute neocortical epileptiform spikes and seizures induced with local administration of the GABA antagonist picrotoxin. These spatial and temporal patterns may give rise to seizures and suggest new stimulation paradigms to treat epilepsy.
5. Emerging electrocorticography applications
5.1. Functional mapping
5.1.1. General principles of functional brain mapping with electrocorticography
5.1.1.1. Nathan E. Crone, Anna Korzeniewska, Mackenzie C. Cervenka, Dana Boatman-Reich
When neurosurgical resections for medically refractory partial seizures and other focal brain diseases involve tissue near functionally critical cortical areas, it is important to map cortical function at a spatial resolution that is comparable to that of the planned resection and to that of functional brain networks. Although functional magnetic resonance imaging (fMRI) offers a potential replacement for the Wada test when determining hemispheric lateralization, it remains controversial whether it should be used to guide surgical resections. For this purpose, electrocortical stimulation mapping (ESM) remains the gold standard for both intraoperative and extraoperative functional mapping, in large part because it allows clinicians to test the functional impact of discrete, temporary, and reproducible cortical “lesions.” ESM is thus thought to identify tissue that is critical to function, and not merely participatory [48]. This distinction is widely considered an important advantage over all methods that rely on cortical activation, although it is based on assumptions that ESM does not perturb the function of cortical networks outside the stimulation site and that other sites cannot support the same function, that is, no functional reserve or plasticity. Because sequential testing of individual cortical sites with ESM is often time consuming and can elicit afterdischarges and even seizures that interfere with functional mapping, clinicians have for many years been interested in using passive ECoG recordings from the same implanted electrodes to map cortical function. This approach has a number of important potential advantages, including the ability to simultaneously and rapidly assess cortical function at all electrode sites without artificially perturbing the brain. In contrast to fMRI, this approach can also capture the fine temporal dynamics by which different cortical sites are activated, potentially yielding more insight into the functional role of each region.
One of the challenges to overcome with ECoG functional mapping has been to decompose extraordinarily complex EEG signals into components that can be used to reliably measure functional brain activation. A variety of phase-locked and non-phase-locked signal components have been used for this purpose. Recent studies have emphasized the utility of increases in signal energy in a broad range of high gamma frequencies (~60–200 Hz) as an index of cortical activation during a variety of tasks [49–51]. Activity in this frequency range is highly correlated with blood oxygen level-dependent (BOLD) responses [52,53] and with average firing rates in cortical neuronal populations [54,55]. Although the generating mechanisms for this activity and the nature of its relationship to neural computation are hotly debated [7,56,57], it appears to have great practical value as a ubiquitous index of overall activity in local neuronal populations. Moreover, in contrast to the all-or-none information often provided by ESM, ECoG high-gamma activity may also provide a graded measure of local population activity that can be used to estimate the degree to which individual recording sites contribute to overall activity in cortical networks responsible for normal brain function. This could allow clinicians to estimate the capacity of the overall network to continue functioning despite the resection of individual components.
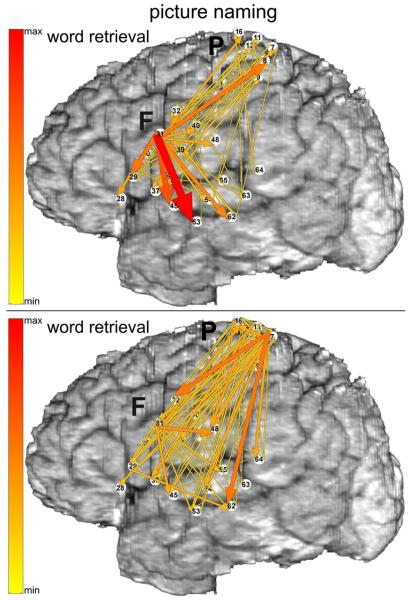
Through use of advanced multichannel analyses of ECoG recordings, it may also be possible to capture the temporal dynamics of task-related functional interactions between the components of cortical networks (Fig. 3) [58]. This could help identify the nodes of these networks that are most important for function and, thus, crucial to preserve during surgical resections. Although these and other developments in ECoG functional mapping offer exciting opportunities to gain new insights into brain function and to use these insights to guide clinical practice, it will be important to continue testing the validity of this new mapping technique with respect to the effects of both “virtual” lesions (ESM) and surgical resections (postoperative neurological outcomes).
Fig. 3.
Event-related functional interactions revealed with ECoG during picture naming. The task was performed with spoken (top) versus signed (bottom) responses. Functional interactions were analyzed with multivariate autoregressive modeling of signal interactions based on Granger causality. Event-related increases in functional interactions at high gamma frequencies (70–115 Hz) are illustrated for an interval corresponding to object recognition and word retrieval (i.e., from visual stimulus onset to median onset latency of spoken or signed responses). Arrows indicate the directions and intensities of increased interactions that are statistically significant. The width and color of each arrow both represent linearly the magnitude of the interaction. Color scale (left) is of the same range for all interactions (top and bottom), scaled from maximal to minimal. Ten percent of the smallest interactions is not shown. Reprinted from: Korzeniewska A, Franaszczuk PJ, Crainiceanu CM, Kuś R, Crone NE. Dynamics of large-scale cortical interactions at high gamma frequencies during word production: event related causality (ERC) analysis of human electrocorticography (ECoG). NeuroImage 2011;56:2218-37. Copyright 2011, with permission from Elsevier.
5.1.2. Using electrocorticography signals for real-time brain mapping
5.1.2.1. Anthony Ritaccio
The observations that ECoG amplitudes in certain frequency bands carry substantial information about motor, language, and visual tasks [12,16,59–62] and are fMRI congruent [63,64] have generated interest in a novel passive brain mapping technology. The applicability of functional mapping for clinical purposes by recording macroscopic local field potentials representing task-driven neuronal populations remains limited by the need for highly trained personnel and sophisticated offline analysis techniques as well as the absence of real-time capability.
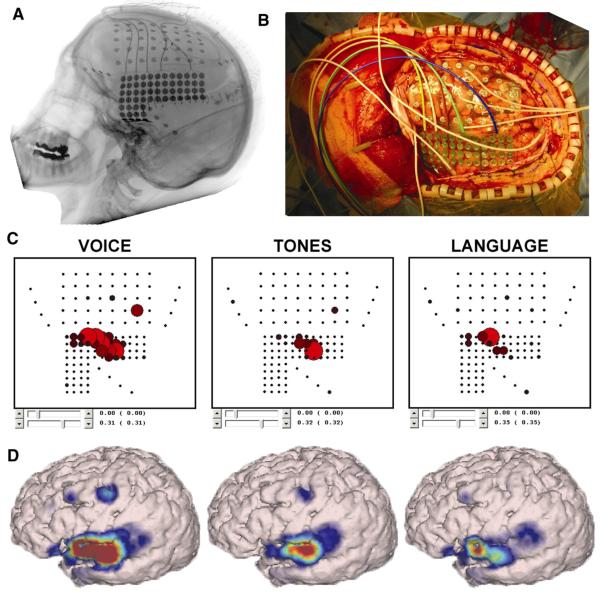
We have recently demonstrated a comprehensive evaluation of a robust and practical procedure for real-time functional mapping from subdural electrodes [33]. The procedure is based on our BCI2000 software platform (http://www.bci2000.org) [65] and SIGFRIED (SIGnal modeling For Real-time Identification and Event Detection) signal processing technology [66]. SIGFRIED can detect and visualize task-related changes in real time without any a priori parameterization (e.g., of frequency bands). To provide a basis for visual feedback of task-related signal changes, the SIGFRIED procedure establishes a statistical model of baseline “resting” data over minutes. In response to visual cues, patients then perform simple motor tasks (hand or tongue movements), receptive language tasks (passive listening to narrated passage), or expressive language tasks (verb generation). For each grid contact and 500-ms period, the time series ECoG signal is converted into the frequency domain. Frequencies between 70 and 100 Hz are then submitted to SIGFRIED. During online processing, SIGFRIED uses the established baseline model to repetitively calculate the likelihood that the signal at each grid contact during activity is statistically different from the modeled baseline signals, generating (in real time and seconds) a probability color map coregistered to the grid localization on the patient's magnetic resonance image (Fig. 4).
Fig. 4.
Real-time assessment of auditory and receptive language nodes: patient with epilepsy with ECoG electrodes implanted over left frontal, parietal, and temporal cortex. A lateral X-ray (A) and an operative photograph (B) depict the configuration of two grids (one 40-contact frontal grid, one 68-contact temporal grid) and three 4-contact strips. A passive mapping procedure (SIGFRIED) identified eloquent language cortex by contrasting task-related changes during listening to voices and tones (C, D). The results are presented in two intuitive interfaces: a two-dimensional interface that mimics the electrode grid and a three-dimensional anatomically correct interface.
In our recent multicenter study [33], we found that the SIGFRIED procedure identifies motor sites in at least the same contacts or their immediate neighbors compared with electrocortical stimulation (ECS) mapping. Similar congruency has been made with ECS in the language domain [67] and in the operating room environment [68].
It is likely that passive ECoG mapping will play an important adjunctive role at the bedside and in the operating room in the near future. Based on its procedural simplicity, rapidity (minutes), safety (passive recording), and relatively low expense, this methodology has the potential to complement and potentially replace currently used clinical methods used for functional localization prior to invasive brain surgery. The system is currently in evaluation by a number of surgical epilepsy centers in the United States and Europe.
5.2. Pathological (ictal) high-frequency recordings in epilepsy
5.2.1. Brian Litt
For more than 50 years, the neurophysiology of epilepsy has been defined by the concept of the “epileptic focus,” a 3- to 10-cm3 contiguous volume of tissue “required” to generate clinical seizures. Experimental findings over recent years have replaced this view with one of a distributed cellular network whose basic components and scale are not well understood. High-frequency recordings obtained at up to 30 kHz (usually down-sampled) from arrays of 40-μm microwires interspersed between standard centimeter-scale clinical contacts are changing our view of the physiological substrate of seizures. From work in our laboratory and others, oscillations in the ripple (100–200 Hz) and fast ripple (>200 Hz) ranges appear to be bio-markers for epileptic brain: fast ripples in the hippocampus and ripples in the neocortex. We also report “microseizures,” seen only in submillimeter domains recorded by microwires, rhythmic, “chirping” events that evolve temporally and spatially like seizures but are confined to single microwires in broad arrays and often do not spread to adjacent contacts 1 mm away. These observations suggest that epileptic seizures may arise from “clouds” of cortical column-scale regions whose independent oscillations may be synchronized in some fashion to generate clinical events in much larger volumes of tissue. Current work in our group, not yet published, suggests that these microseizure events may actually be spiral waves confined to the neocortex, when measured by very high-resolution multidimensional arrays in acute seizures precipitated by GABA antagonists, like picrotoxin.
These findings suggest the need for more versatile, higher-resolution devices for recording over large regions of the neocortex and deeper structures to work out the actual topology and dynamics of epileptic networks. These findings also indicate that current clinical methods for localizing and treating seizures with surgery and implantable devices might be less effective, in part, because of their limited temporal and spatial resolution. It is important to note that more complex, higher-resolution recording and stimulation devices currently under development in our laboratory generate their own challenges, such as how to collect, process, and interpret massive streams of data from thousands of channels sampled at greater than 10 kHz/channel. These challenges have given birth to another active area of research in our laboratory: the use of automated, unsupervised methods to detect, map, and track high-frequency oscillations, ripples, fast ripples, and microseizures, as well as other high-bandwidth phenomena, during seizure generation and propagation. Such signals include multiunit activity, field potentials, and widespread intracranial EEG. These challenges are similar in many ways to those faced by researchers in the field of brain–computer interfacing.
Looking toward the future, there is mounting evidence that more flexible, multiscale devices for recording and modulating brain activity over larger regions will be necessary to understand seizure generation over the heterogeneity of conditions known as “epilepsy,” as well as better algorithms for interpreting their output. It is also clear that the devices, techniques, and algorithms created through this research are certain to benefit those in related fields of research such as brain–computer interfacing.
5.3. Brain–computer interfaces
5.3.1. Electrocorticography-based brain–computer interfacing in humans
5.3.1.1. Gerwin Schalk
All conventional methods to communicate with or control our environment require motor function. Whether we speak, type on a keyboard, or communicate through body language, we rely on muscles. Unfortunately, many neuromuscular or neurological conditions [such as amyotrophic lateral sclerosis (ALS)] affect muscular control. Thus, people affected by these conditions are impeded or unable to communicate. Those most seriously affected lose all muscle function, including eye movements and respiration, and become effectively “locked in” to their bodies. Efforts in the field of BCI research aim to restore some of these lost functions by detecting the intent of a person directly from brain signals and by using the result to control external devices such as word processors and cursor movement. The sensor modalities that have most commonly been used in BCI studies to date are EEG recordings from the scalp and single-neuron recordings from within the cortex. Over the past decade, an increasing number of studies have also explored the use of ECoG activity.
Thus far, the majority of ECoG-based BCI studies have been conducted with patients with intractable epilepsy who are invasively monitored to localize their seizure focus and to identify eloquent cortex. Also, a minority of experiments have used patients undergoing an awake craniotomy or have used nonhuman primates. ECoG has attracted substantial and increasing interest because it has been shown to reflect specific details of actual and imagined actions and because its technical characteristics should readily support robust and chronic implementations of BCI systems in humans.
The protocol of an ECoG-based BCI study usually has two parts. In the first part, the ECoG feature(s) (e.g., amplitudes at particular ECoG frequencies measured at particular locations) to be used for BCI control is chosen. In the second part, the feature(s) is used for online BCI control of cursor movement or another output. Several human ECoG-based BCI studies using such protocols have been described to date [11,69–81]. These studies present encouraging results. Specifically, they demonstrate that ECoG allows people to rapidly acquire accurate brain-based control of a computer cursor in one or two dimensions, or to spell words at a rate of more than 20 characters per minute. In terms of speed and/or acquisition of control, these results substantially exceed those expected by similar systems using scalp-recorded EEG.
In summary, ECoG is generating substantial excitement for its potential to support basic neuroscience research and powerful BCI systems. Ultimately, clinically practical ECoG-based BCI systems must be wholly implantable and function reliably for many years. Although these systems have not yet been developed, the extensive work needed to develop and validate them has already begun. Its successful completion could lead to ECoG-based BCI systems of great value to people with severely disabling disorders.
5.3.2. Brain–computer interfacing in animal models
5.3.2.1. Dan Moran
All of the previous ECoG BCI studies in monitored human patients with epilepsy used subdural electrodes. As the goal of the monitoring is to identify the seizure focus and then surgically remove it, opening the dura and exposing the central nervous system is an appropriate risk. However, in animal models designed specifically for ECoG BCI studies, the arrays can be placed epidurally. The dura's conductivity is comparable to that of cerebrospinal fluid; thus, the only effect it will have on recordings is the small increased distance between the recording sites and the brain (1–2 mm). With the dura kept intact, the safety of the implant is significantly enhanced (reduced incidence of meningitis or encephalitis). Furthermore, because the outer dura is attached to the skull, there is no relative motion between the electrode and the surrounding tissue unlike penetrating single-unit electrodes or subdural ECoG electrodes. Given these advantages, epidural ECoG appears to be an optimal recording modality for BCI applications.
In our most recent studies, we have had nonhuman primates implanted for 15 months with chronic epidural ECoG electrodes. With high-gamma-band activity (75–105 Hz), these subjects performed accurate two- and three-dimensional BCI control of a computer cursor. The electrode impedance as well as the ECoG signal-to-noise ratio remained stable over the duration of the implant. When explanted after 15 months, the underlying dura looked like it did on the day of the initial implant. Likewise, the underlying brain was in pristine condition with no sign of deterioration or attachment to the overlying dura. Through biofeedback and neural plasticity, the subjects were able to learn to independently modulate the brain activity under electrodes placed as close as 3 mm apart. Overall, epidural ECoG is a very pragmatic modality for BCI applications having the proper balance of safety, invasiveness, stability, speed, and accuracy.
6. Conclusion
6.1. Gerwin Schalk
Electrocorticography is the technique of recording from or stimulating the brain using electrodes that are placed subdurally or epidurally. ECoG has been used for decades for select clinical purposes—most commonly to identify functional and epileptic brain areas in people with epilepsy—and on occasion for research. The important role of ECoG for basic research and its potential to create a new range of clinical applications have long been greatly underappreciated, not only because access to ECoG recordings is limited in humans, but more importantly because it has long been assumed that only recordings from individual neurons carry important details about cortical function. Research over the past several years, including the work summarized in this proceedings article, has changed this view by highlighting the important capacities of ECoG for research and clinical applications. Enthusiasm for the ECoG platform continues to grow rapidly and is documented by an explosion in the number of high-quality research articles. We expect that increased sophistication in signal acquisition, signal analysis, and interpretation of ECoG signals will continue to be strong drivers in the further development of the field of ECoG-based research and that the resulting increase in understanding will serve to establish ECoG as an important technique for characterizing normal as well as abnormal brain function.
Acknowledgments
This research was partially supported by the NIH (R01-NS40596 (N.C.), K24-DC010028 (D.B.), R01-EB000856 (G.S.), R01-EB006356 (G.S.)), the U.S. Army Research Office (W911NF-07-1-0415 (G.S.) and W911NF-08-1-0216 (G.S.)), the NASA graduate student research program (K.J.M.), and the Stanford University NeuroVentures Program (J.P.).
References
- [1].Ritaccio A, Brunner P, Cervenka MC, et al. Proceedings of the first international workshop on advances in electrocorticography. Epilepsy Behav. 2010;19:204–15. doi: 10.1016/j.yebeh.2010.08.028. [DOI] [PubMed] [Google Scholar]
- [2].Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–22. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- [3].Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384:312–20. [PubMed] [Google Scholar]
- [4].Nunez PL, Cutillo BA. Neocortical dynamics and human EEG rhythms. Oxford Univ. Press; New York: 1995. [Google Scholar]
- [5].Mitzdorf U. Current source–density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- [6].Okun M, Naim A, Lampl I. Intracellular recordings in awake rodent unveil the relation between local field potential and neuronal firing. Society for Neuroscience meeting; Chicago, IL, USA. 2009; Abstracts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miller KJ, Sorensen LB, Ojemann JG, den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput Biol. 2009;5:e1000609. doi: 10.1371/journal.pcbi.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miller KJ. Broadband spectral change: evidence for a macroscale correlate of population firing rate? J Neurosci. 2010;30:6477–9. doi: 10.1523/JNEUROSCI.6401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Canolty RT, Edwards E, Dalal SS, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–8. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Voytek B, Canolty RT, Shestyuk A, Crone NE, Parvizi J, Knight RT. Shifts in gamma phase-amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Hum Neurosci. 2010;4:191. doi: 10.3389/fnhum.2010.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brunner P, Ritaccio AL, Emrich JF, Bischof H, Schalk G. Rapid communication with a “P300” matrix speller using electrocorticographic signals (ECoG) Front Neurosci. 2011;5:5. doi: 10.3389/fnins.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Crone NE, Miglioretti DL, Gordon B, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis: I. Alpha and beta event-related desynchronization. Brain. 1998;121:2271–99. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- [13].Pfurtscheller G. Event-related desynchronization (ERD) and event related synchronization (ERS) Williams & Wilkins; Baltimore: 1999. [Google Scholar]
- [14].Miller KJ, Zanos S, Fetz EE, den Nijs M, Ojemann JG. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J Neurosci. 2009;29:3132. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Miller KJ, Shenoy P, den Nijs M, Sorensen LB, Rao RN, Ojemann JG. Beyond the gamma band: the role of high-frequency features in movement classification. IEEE Trans Bio-med Eng. 2008;55:1634. doi: 10.1109/TBME.2008.918569. [DOI] [PubMed] [Google Scholar]
- [16].Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis: II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt. 12):2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- [17].Crandall PH, Walter RD, Rand RW. Clinical applications of studies on sereotactically implanted electrodes in temporal-lobe epilepsy. J Neurosurg. 1963;20:827–40. doi: 10.3171/jns.1963.20.10.0827. [DOI] [PubMed] [Google Scholar]
- [18].Spencer S, Nguyen D, Duckrow R. Invasive EEG in presurgical evaluation of epilepsy. In: Shorvon S, Perucca E, Engle J, editors. The treatment of epilepsy. 3rd ed Blackwell; Oxford: 2009. pp. 767–98. [Google Scholar]
- [19].Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–37. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- [20].Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- [21].Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–27. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- [22].Kim DW, Lee SK, Nam H, et al. Epilepsy with dual pathology: surgical treatment of cortical dysplasia accompanied by hippocampal sclerosis. Epilepsia. 2010;51:1429–35. doi: 10.1111/j.1528-1167.2009.02403.x. [DOI] [PubMed] [Google Scholar]
- [23].Stefan H, Pauli E, Eberhard F, Ugrinovich R, Buchfelder M. “Tailoring” resections in drug refractory temporal lobe epilepsy. Nervenarzt. 1996;67:306–10. [PubMed] [Google Scholar]
- [24].McKhann GM, II, Schoenfeld-McNeill J, Born DE, Haglund MM, Ojemann GA. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J Neurosurg. 2000;93:44–52. doi: 10.3171/jns.2000.93.1.0044. [DOI] [PubMed] [Google Scholar]
- [25].Palmini A, Gambardella A, Andermann F, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37:476–87. doi: 10.1002/ana.410370410. [DOI] [PubMed] [Google Scholar]
- [26].MacDonald DB, Pillay N. Intraoperative electrocorticography in temporal lobe epilepsy surgery. Can J Neurol Sci. 2000;27(Suppl. 1):S85–96. doi: 10.1017/s031716710000072x. [DOI] [PubMed] [Google Scholar]
- [27].Gibbs F, Lennox W, Gibbs E. The electro-encephalogram in diagnosis and in localization of epileptic seizures. Arch Neurol Psychiatry. 1936;36:1225–35. [Google Scholar]
- [28].Penfield W, Erickson T, Thomas C. Epilepsy and cerebral localization: a study of the mechanism, treatment and prevention of epileptic seizures. Arch Intern Med. 1942;70:916–7. [Google Scholar]
- [29].Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- [30].Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. Electroencephalogr Clin Neurophysiol. 1991;78:252–9. doi: 10.1016/0013-4694(91)90040-b. [DOI] [PubMed] [Google Scholar]
- [31].Gotman J. Automatic seizure detection: improvements and evaluation. Electroencephalogr Clin Neurophysiol. 1990;76:317–24. doi: 10.1016/0013-4694(90)90032-f. [DOI] [PubMed] [Google Scholar]
- [32].Sellers EW, Vaughan TM, Wolpaw JR. A brain–computer interface for long-term independent home use. Amyotroph Lateral Scler. 2010;11:449–55. doi: 10.3109/17482961003777470. [DOI] [PubMed] [Google Scholar]
- [33].Brunner P, Ritaccio AL, Lynch TM, et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15:278–86. doi: 10.1016/j.yebeh.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Morrell MJ, the RNS System Pivotal Investigators Results of a multi-center double blinded randomized controlled pivotal investigation of the RNS™ system for treatment of intractable partial epilepsy in adults. Presented at the 63rd Annual Meeting of the American Epilepsy Society; Boston, MA. December 6–8; 2009. [Google Scholar]
- [35].Guger C, Daban S, Sellers E, et al. How many people are able to control a P300-based brain-computer interface (BCI)? Neurosci Lett. 2009;462:94–8. doi: 10.1016/j.neulet.2009.06.045. [DOI] [PubMed] [Google Scholar]
- [36].Hollenberg BA, Richards CD, Richards R, Bahr DF, Rector DM. A MEMS fabricated flexible electrode array for recording surface field potentials. J Neurosci Methods. 2006;153:147–53. doi: 10.1016/j.jneumeth.2005.10.016. [DOI] [PubMed] [Google Scholar]
- [37].Rubehn B, Bosman C, Oostenveld R, Fries P, Stieglitz T. A MEMS-based flexible multichannel ECoG-electrode array. J Neural Eng. 2009;6:036003. doi: 10.1088/1741-2560/6/3/036003. [DOI] [PubMed] [Google Scholar]
- [38].Neelon MF, Williams J, Garell PC. The effects of auditory attention measured from human electrocorticograms. Clin Neurophysiol. 2006;117:504–21. doi: 10.1016/j.clinph.2005.11.009. [DOI] [PubMed] [Google Scholar]
- [39].Leuthardt EC, Freudenberg Z, Bundy D, Roland J. Microscale recording from human motor cortex: implications for minimally invasive electrocorticographic brain–computer interfaces. Neurosurg Focus. 2009;27:E10. doi: 10.3171/2009.4.FOCUS0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rouse AG, Moran DW. Neural adaptation of epidural electrocorticographic (EECoG) signals during closed-loop brain computer interface (BCI) tasks. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:5514–7. doi: 10.1109/IEMBS.2009.5333180. [DOI] [PubMed] [Google Scholar]
- [41].Kellis SS, House PA, Thomson KE, Brown R, Greger B. Human neocortical electrical activity recorded on nonpenetrating microwire arrays: applicability for neuro-prostheses. Neurosurg Focus. 2009;27:E9. doi: 10.3171/2009.4.FOCUS0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Stead M, Bower M, Brinkmann BH, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–97. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Thongpang S, Richner TJ, Brodnick SK, et al. A microelectrocorticography platform and deployment strategies for chronic BCI applications. Clin EEG Neurosci. 2011;42:259–65. doi: 10.1177/155005941104200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hassler C, von Metzen RP, Ruther P, Stieglitz T. Characterization of parylene C as an encapsulation material for implanted neural prostheses. J Biomed Mater Res B. 2010;93:266–74. doi: 10.1002/jbm.b.31584. [DOI] [PubMed] [Google Scholar]
- [45].Rubehn B, Stieglitz T. In vitro evaluation of the long-term stability of polyimide as a material for neural implants. Biomaterials. 2010;31:3449–58. doi: 10.1016/j.biomaterials.2010.01.053. [DOI] [PubMed] [Google Scholar]
- [46].Toda H, Suzuki T, Sawahata H, Majima K, Kamitani Y, Hasegawa I. Simultaneous recording of ECoG and intracortical neuronal activity using a flexible multichannel electrode-mesh in visual cortex. Neuroimage. 2011;54:203–12. doi: 10.1016/j.neuroimage.2010.08.003. [DOI] [PubMed] [Google Scholar]
- [47].Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–9. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. 2007;17:477–89. doi: 10.1007/s11065-007-9046-6. [DOI] [PubMed] [Google Scholar]
- [49].Crone NE, Korzeniewska A, Franaszczuk PJ. Cortical gamma responses: searching high and low. Int J Psychophysiol. 2011;79:9–15. doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cervenka MC, Boatman-Reich DF, Ward J, Franaszczuk PJ, Crone NE. Language mapping in multilingual patients: electrocorticography and cortical stimulation during naming. Front Hum Neurosci. 2011;5:13. doi: 10.3389/fnhum.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jerbi K, Ossandon T, Hamame CM, et al. Task-related gamma-band dynamics from an intracerebral perspective: review and implications for surface EEG and MEG. Hum Brain Mapp. 2009;30:1758–71. doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–7. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- [53].Khursheed F, Tandon N, Tertel K, Pieters TA, Disano MA, Ellmore TM. Frequency-specific electrocorticographic correlates of working memory delay period fMRI activity. Neuroimage. 2011;56:1773–82. doi: 10.1016/j.neuroimage.2011.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–36. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J Neurosci. 2009;29:13613–20. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gaona CM, Sharma M, Freudenburg ZV, et al. Nonuniform high-gamma (60–500 Hz) power changes dissociate cognitive task and anatomy in human cortex. J Neurosci. 2011;31:2091–100. doi: 10.1523/JNEUROSCI.4722-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Korzeniewska A, Franaszczuk PJ, Crainiceanu CM, Kus R, Crone NE. Dynamics of large-scale cortical interactions at high gamma frequencies during word production: event related causality (ERC) analysis of human electrocorticography (ECoG) Neuroimage. 2011;56:2218–37. doi: 10.1016/j.neuroimage.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aoki F, Fetz EE, Shupe L, Lettich E, Ojemann GA. Changes in power and coherence of brain activity in human sensorimotor cortex during performance of visuomotor tasks. Biosystems. 2001;63:89–99. doi: 10.1016/s0303-2647(01)00149-6. [DOI] [PubMed] [Google Scholar]
- [60].Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clin Neurophysiol. 2001;112:565–82. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- [61].Lachaux JP, Rudrauf D, Kahane P. Intracranial EEG and human brain mapping. J Physiol Paris. 2003;97:613–28. doi: 10.1016/j.jphysparis.2004.01.018. [DOI] [PubMed] [Google Scholar]
- [62].Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–80. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- [63].Lachaux JP, Fonlupt P, Kahane P, et al. Relationship between task-related gamma oscillations and BOLD signal: new insights from combined fMRI and intracranial EEG. Hum Brain Mapp. 2007;28:1368–75. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hermes D, Miller KJ, Vansteensel MJ, Aarnoutse EJ, Leijten FS, Ramsey NF. Neurophysiologic correlates of fMRI in human motor cortex. Hum Brain Mapp. 2011 Jun 20; doi: 10.1002/hbm.21314. EPub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain–computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- [66].Schalk G, Brunner P, Gerhardt LA, Bischof H, Wolpaw JR. Brain–computer interfaces (BCIs): detection instead of classification. J Neurosci Methods. 2008;167:51–62. doi: 10.1016/j.jneumeth.2007.08.010. [DOI] [PubMed] [Google Scholar]
- [67].Wu M, Wisneski K, Schalk G, et al. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010;66:E407–9. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- [68].Roland J, Brunner P, Johnston J, Schalk G, Leuthardt EC. Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy Behav. 2010;18:123–8. doi: 10.1016/j.yebeh.2010.02.017. [DOI] [PubMed] [Google Scholar]
- [69].Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW. A brain–computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1:63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- [70].Leuthardt EC, Miller KJ, Schalk G, Rao RP, Ojemann JG. Electrocorticography-based brain computer interface: the Seattle experience. IEEE Trans Neural Syst Rehabil Eng. 2006;14:194–8. doi: 10.1109/TNSRE.2006.875536. [DOI] [PubMed] [Google Scholar]
- [71].Wilson JA, Felton EA, Garell PC, Schalk G, Williams JC. ECoG factors underlying multimodal control of a brain-computer interface. IEEE Trans Neural Syst Rehabil Eng. 2006;14:246–50. doi: 10.1109/TNSRE.2006.875570. [DOI] [PubMed] [Google Scholar]
- [72].Felton EA, Wilson JA, Williams JC, Garell PC. Electrocorticographically controlled brain–computer interfaces using motor and sensory imagery in patients with temporary subdural electrode implants: report of four cases. J Neurosurg. 2007;106:495–500. doi: 10.3171/jns.2007.106.3.495. [DOI] [PubMed] [Google Scholar]
- [73].Schalk G, Miller KJ, Anderson NR, et al. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008;5:75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hinterberger T, Widman G, Lal TN, et al. Voluntary brain regulation and communication with electrocorticogram signals. Epilepsy Behav. 2008;13:300–6. doi: 10.1016/j.yebeh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- [75].Blakely T, Miller KJ, Zanos SP, Rao RP, Ojemann JG. Robust, long-term control of an electrocorticographic brain–computer interface with fixed parameters. Neurosurg Focus. 2009;27:E13. doi: 10.3171/2009.4.FOCUS0977. [DOI] [PubMed] [Google Scholar]
- [76].Vansteensel MJ, Hermes D, Aarnoutse EJ, et al. Brain–computer interfacing based on cognitive control. Ann Neurol. 2010;67:809–16. doi: 10.1002/ana.21985. [DOI] [PubMed] [Google Scholar]
- [77].Miller KJ, Schalk G, Fetz EE, den Nijs M, Ojemann JG, Rao RP. Cortical activity during motor execution, motor imagery, and imagery-based online feedback. Proc Natl Acad Sci U S A. 2010;107:4430–5. doi: 10.1073/pnas.0913697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yanagisawa T, Hirata M, Saitoh Y, et al. Real-time control of a prosthetic hand using human electrocorticography signals. J Neurosurg. 2011;114:1715–22. doi: 10.3171/2011.1.JNS101421. [DOI] [PubMed] [Google Scholar]
- [79].Krusienski DJ, Shih JJ. Control of a brain–computer interface using stereotactic depth electrodes in and adjacent to the hippocampus. J Neural Eng. 2011;8:025006. doi: 10.1088/1741-2560/8/2/025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Krusienski DJ, Shih JJ. Control of a visual keyboard using an electrocorticographic brain–computer interface. Neurorehabil Neural Repair. 2011;25:323–31. doi: 10.1177/1545968310382425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Leuthardt EC, Gaona C, Sharma M, et al. Using the electrocorticographic speech network to control a brain–computer interface in humans. J Neural Eng. 2011;8:036004. doi: 10.1088/1741-2560/8/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]