Abstract
Modified vaccinia virus Ankara (MVA), developed >30 years ago as a highly attenuated candidate smallpox vaccine, was recloned from a 1974 passage and evaluated for safety and immunogenicity. Replication of MVA is impaired in most mammalian cells, and we found that mice with severe combined immunodeficiency disease remained healthy when inoculated with MVA at 1,000 times the lethal dose of vaccinia virus derived from the licensed Dryvax vaccine seed. In BALB/c mice inoculated intramuscularly with MVA, virus-specific CD8+ T cells and antibodies to purified virions and membrane protein components of the intracellular and extracellular infectious forms of vaccinia virus were induced in a dose-dependent manner. After one or two inoculations of MVA, the T cell numbers and antibody titers equaled or exceeded those induced by percutaneous injection of Dryvax. Antibodies induced by MVA and Dryvax were neutralizing and inhibited virus spread in cultured cells. Furthermore, vaccinated mice were protected against lethal intranasal challenge with a pathogenic vaccinia virus. B cell-deficient mice unable to generate antibodies and β2-microglobulin-deficient mice unable to express MHC class I molecules for a CD8+ T cell response were also protectively vaccinated by MVA. In contrast, mice with decreased CD4 or MHC class II expression and double-knockout mice deficient in MHC class I- and II-restricted activities were poorly protected or unprotected. This study confirmed the safety of MVA and demonstrated that the overlapping immune responses protected normal and partially immune-deficient animals, an encouraging result for this candidate attenuated smallpox vaccine.
The last endemic case of smallpox occurred in 1977, after which vaccination largely ceased. Concerns that variola virus might be used as a biological weapon, however, have reawakened interest in protective vaccines and therapeutics (1). The licensed smallpox vaccines, consisting of live vaccinia virus, confer long-lasting immunity against closely related orthopoxviruses, including variola virus, but routinely produce pustular skin lesions and infrequent but severe side reactions (2, 3). Consequently, the vaccine is contraindicated for many millions of people and their close contacts with histories of eczema, atopic dermatitis, immunodeficiency, or heart disease. Licensure of a smallpox vaccine that may be safely used to immunize those with risk factors has a high priority.
Attenuated strains of vaccinia virus were developed in the 1960s in response to the need for a safer smallpox vaccine (2). One such candidate vaccine, Modified Vaccinia Virus Ankara (MVA), was administered to ≈100,000 people in Germany (4, 5), although it was never evaluated in a smallpox endemic area because of the progress being made in eradicating the disease with existing vaccines. Interest in MVA resurfaced when it was shown to efficiently express recombinant genes and protectively immunize experimental animals against a variety of viral diseases (6-9). MVA was developed by >570 serial passages in chicken embryo fibroblasts (CEFs), during which it incurred multiple DNA deletions (10-12). MVA replicates poorly or undetectably in human and most other mammalian cells (13-15). The block in replication occurs at a late step in virus assembly (6, 13) and is caused by multiple gene defects (16). Because of its extreme attenuation, no adverse effects were reported even when high doses of MVA were given to immune-deficient non-human primates (17).
Major advances in virology and immunology, which have occurred since MVA was originally developed, can help in its evaluation as a candidate smallpox vaccine. Studies of vaccinia virus have led to the characterization of two distinct infectious forms (18). The initial infectious form, known as the intracellular mature virion (IMV), has an outer membrane acquired early during morphogenesis and can be released by cell lysis. An additional membrane surrounds a subset of IMV, which are then transported to the outside of the intact cell and called cell-associated enveloped virions (CEVs). CEVs that detach from the cell are called extracellular enveloped virions (EEVs). The CEVs and EEVs have the same fragile outer membrane and are thought to be responsible for spread to adjacent and more distant cells, respectively. The IMV form is very stable in the environment and hence may enable virus spread from animal to animal. Smallpox is thought to spread mainly by the upper respiratory track route. The outer membranes of IMV and CEV/EEV contain different viral proteins and, therefore, immune responses to both forms of virus provide optimal protection in animal models of orthopoxvirus infections (19, 20). Evidence shows that cell-mediated immune responses are important in the recovery of naïve animals from sublethal infections (21, 22), whereas antibodies may have a prophylactic role against lethal infections (23, 24).
Because smallpox has been eradicated, candidate vaccines can no longer be tested for efficacy in humans. Additional studies are needed to compare MVA with currently licensed smallpox vaccine in non-human primate and rodent models and to develop correlates of immunity. Mice have numerous advantages, including the availability of immunological reagents and strains with genetically defined immune deficiencies. Recent studies indicated that MVA induces cellular and humoral immune responses to vaccinia virus in mice (25, 26). The present study was initiated with the following objectives: first, to reclone MVA from a stock that had been last propagated in 1974, a date that preceded known cases of bovine spongiform encephalitis; second, to determine the safety of the MVA clone in mice with severe combined immunodeficiency disease (SCID); third, to compare the MVA clone and the currently licensed New York City Board of Health (Dryvax, Wyeth) strain of vaccinia virus for induction of specific antibody and cell-mediated immune responses and ability to protect immune-competent mice against a lethal intranasal (i.n.) challenge with a pathogenic strain of vaccinia virus; and fourth, to evaluate the ability of MVA to protectively vaccinate mice with specific immune deficiencies.
Materials and Methods
Viruses. MVA, from the 572nd passage in primary CEF harvested on February 22, 1974, was received from A. Mayr (Ludwig-Maximilians-Universitat, Munich). A vial of smallpox vaccine (Dryvax, Wyeth Ayerst Laboratories, Marietta, PA) from the Centers for Disease Control and Prevention was reconstituted, and the titer was determined as 5 × 107 plaque-forming units (pfu) per ml. A frozen vaccine seed stock (New York City Board of Health) was obtained from Wyeth Ayerst Laboratories. Vaccinia virus strain Western Reserve (WR) is available from American Type Culture Collection as VR-1354. Wyeth and WR strains of vaccinia virus were grown in HeLa cells and titered in BS-C-1 cells as described (27); MVA was grown and titered in CEF (27).
Cells. Specific pathogen-free premium eggs from Charles River Laboratories (North Franklin, CT) or Hy-Vac (Abel, IA) were from B&E Eggs (Ephrata, PA). MRC-5 and HeLa cells were from American Type Culture Collection. Certified reagents were used for the cultivation and maintenance of CEF and passages of MVA and included γ-irradiated trypsin (27250-018, Invitrogen) and Eagle's MEM (12-662F, Cambrex, Walkersville, MD) supplemented with γ-irradiated FBS (100-106, Gemini Biological Products, Woodland, CA), glutamine (17-605E, Cambrex), streptomycin sulfate (S0890, Sigma), and neomycin (N1142, Sigma).
Mice. Six- to nine-week-old female mice from Taconic Farms included T cell- and B cell-deficient C.B-17 SCID mice (CB17SC-M); BALB/c (BALB) and B cell-deficient Jh (001147-M) mice on a BALB/c background; C57BL/6 (B6) and β2m (B2MN5-M) β2-microglobulin-deficient, Cd4 (001055-M) CD4-deficient, Abb(H2-Ab1) (ABBN5-M) MHC class II-deficient, and Abb/B2m (004080-MM) MHC class I- and II-deficient mice.
Measurement of Serum Antibody. Two-fold serial dilutions of serum were incubated for 2 h at room temperature with 106 pfu of sucrose-gradient-purified vaccinia virus WR that had been fixed with 2% paraformaldehyde in individual wells of a 96-well plate. Antibody was detected with anti-mouse peroxidase (Roche Molecular Biochemicals) and BM Blue substrate (Roche Applied Science). A370 and A492 were determined with a Spectra Max Plus Spectrophotometer (Molecular Devices), and endpoints were defined as the maximum serum dilution at which the absorbance was >0.10 after subtraction of background wells assayed in the absence of vaccinia virus. For an enhanced-sensitivity ELISA, the times of incubation of serum dilutions and peroxidase were increased to overnight and 5 h, respectively. ELISA titers to baculovirus-produced recombinant vaccinia virus proteins L1 and A33, obtained from J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen (University of Pennsylvania, Philadelphia) were determined as discussed above, except that plates were coated overnight at 4°C with L1 (0.04 μg/ml) or A33 (0.09 μg/ml) proteins in PBS.
Neutralization and Anti-Comet Test. Neutralization titers were determined by incubating sera with a vaccinia virus that expresses enhanced GFP, infecting cells, and then measuring fluorescence by flow cytometry (28). IC50 values were calculated with prism software (GraphPad, San Diego). For an enhanced sensitivity assay, the virus multiplicity was decreased from 0.25 to 0.125 pfu/cell.
For the anti-comet test, BS-C-1 cells were infected with the IHD strain of vaccinia virus. After incubation at 37°C for 2 h, the cells were washed, and culture medium containing a 1:50 dilution of mouse antiserum was added to the cultures. Cells were fixed at 2 days with crystal violet.
Intracellular Cytokine Staining. P815 cells were incubated overnight without or with 10 pfu of MVA or vaccinia virus WR per cell, irradiated, and then incubated with fresh splenocytes. After 2 h at 37°C, brefeldin A (Sigma) was added to a concentration of 0.005 μg/μl. After overnight incubation, the cells were washed, blocked with purified anti-mouse CD16/32 (clone 2.4G2), and stained with fluorescein isothiocyanate-conjugated anti-mouse CD3e (clone 145-2C11) and allophycocyanin-conjugated anti-mouse CD8a (clone 53-6.7) (BD Pharmingen). After fixation and permeabilization, the cells were stained with phycoerythrin-conjugated anti-mouse IFN-γ, resuspended in 2% paraformaldehyde, and analyzed on a FACSCalibur flow cytometer (Becton Dickinson). flowjo software (Tree Star, Ashland, OR) was used to determine the percentage of CD3+ CD8+ splenocytes that expressed IFN-γ. The vaccinia virus-specific CD8+ T cells represent the difference between the values obtained by using infected and uninfected P815 cells.
Results
Isolation and Characterization of MVA Clones. We isolated five independent MVA clones by three successive terminal dilutions of virus that had been vialed in 1974 and obtained from A. Mayr. Restriction enzyme patterns were similar, and no significant differences were noted between the clones or the starting virus preparation with regard to cytopathic effect in CEF. The replication of each clone, the parent virus, and MVA derived from a 1983 stock, were similarly restricted in human HeLa and MRC-5 cells. In addition, the limited ability of each of the MVA clones to replicate in monkey VERO cells was maintained through 12 successive passages. In the absence of differences, we chose clone 1 for further experiments because of its slightly higher titer.
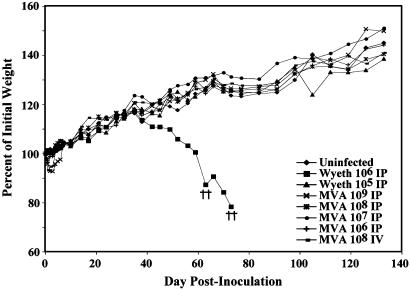
To assess the safety of MVA in immune-deficient animals, we inoculated SCID mice with 106 to109 pfu of MVA i.p. or 108 pfu i.v. Some mice lost a little weight during the first few days but rapidly regained it, and all remained healthy through the 133 days of observation (Fig. 1). In parallel, a sterile passage of the Wyeth seed strain of the licensed Dryvax vaccine was inoculated at 105 or 106 pfu i.p. After 35 days, mice that received the latter vaccine virus began losing weight and developed typical pox lesions on their tail, paws, and mouth. Ultimately, all mice in this group were killed at 30% weight loss (Fig. 1). Thus, MVA was safe in SCID mice even when given at 1,000 times the lethal dose of the standard vaccine virus.
Fig. 1.
Safety of MVA. SCID mice were uninfected, infected i.p. with MVA (106 to 109 pfu) or Wyeth strain of vaccinia virus (105 to 106 pfu), or infected i.v. with 108 pfu of MVA. Mice were weighed individually, and the averages were plotted. †, died naturally or were killed because of 30% weight loss. Only mice inoculated with 106 pfu of the Wyeth strain of vaccinia virus showed persistent weight loss, disease, and death.
Binding and Neutralizing Antibody Responses. BALB/c mice were inoculated i.m. with 106 to 108 pfu of MVA or percutaneously by multiple needle scratches at the base of the tail, with 5 × 105 pfu of Dryvax to mimic the dose and route given to humans. ELISA titers were measured by using purified IMV, recombinant L1 protein, or recombinant A33 protein. The L1 and A33 proteins are components of the IMV and CEV/EEV membrane, respectively, and are targets of protective antibodies. The IMV and recombinant proteins were derived from the WR strain of vaccinia virus, which, like Dryvax, was developed from the New York City Board of Health strain. By using IMV as capture antigen, ELISA titers were proportional to the inoculum of MVA and increased after a second immunization (Table 1). At 3 weeks after the first immunization, similar ELISA titers were obtained with the highest dose of MVA and with Dryvax. At 2 weeks after the second inoculation with 107 or 108 pfu of MVA, the IMV ELISA titers were equal or higher than those of mice that had received Dryvax once (Table 1). Note that Dryvax antibody titers continued to rise after 3 weeks because the virus replicates, whereas MVA is rapidly cleared in mice (29). ELISA titers obtained with the recombinant proteins as capture antigens showed the same pattern as obtained with IMV (Table 1). The L1 and A33 titers induced by two vaccinations with 107 or 108 pfu of MVA equaled or surpassed those achieved by the single Dryvax immunization.
Table 1. ELISA and neutralization titers.
| Reciprocal endpoint ELISA titer
|
IC50 neutralization titer
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IMV
|
L1
|
A33
|
||||||
| Immunization | 3 wk | 6 wk | 3 wk | 6 wk | 3 wk | 6 wk | 3 wk | 6 wk |
| None | < 100 | < 100 | < 100 | < 100 | < 100 | < 100 | < 50 | < 50 |
| 108 pfu MVA* | 6,400 | 102,400 | 6,400 | 51,200 | 1,600 | 51,200 | 583 | 12,130 |
| 107 pfu MVA* | 800 | 25,600 | 1,600 | 12,800 | 800 | 25,600 | 139 | 2,835 |
| 106 pfu MVA* | 200 | 6,400 | 200 | 3,200 | < 100 | 3,200 | < 50 | 1,046 |
| 5 × 105 pfu Dryvax† | 6,400 | 25,600 | 1,600 | 12,800 | 1,600 | 3,200 | 1,181 | 6,323 |
Eight mice were immunized at 0 time and bled on week 3; four mice were reimmunized on week 4 and bled on week 6.
Eight mice were immunized at 0 time and bled on week 3; four mice were bled on week 6.
Protective antibodies to the IMV and CEV/EEV forms of vaccinia virus were measured by using different assays. For the former, we used a recently described method in which serum is incubated with purified recombinant vaccinia virus WR that expresses enhanced GFP (28). After the neutralization step, cells were infected and the percent that expressed the fluorescent protein was determined by flow cytometry. Vaccinia virus neutralization titers elicited by MVA were proportional to the inoculum dose and increased after the second injection (Table 1). At the highest dose of MVA, the neutralization titer was similar to that attained with one Dryvax immunization.
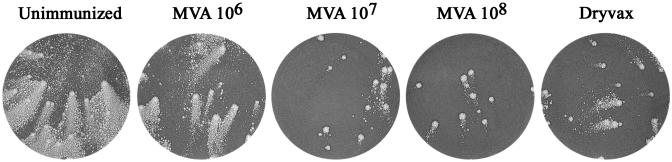
The second assay, known as the anti-comet test (30, 31), measures the inhibition of satellite plaque formation by the released EEV of the IHD strain of vaccinia virus, which was also derived from the New York City Board of Health strain. In the presence of control serum from unimmunized animals, the comet-like secondary plaques almost destroyed the cell monolayer in 48 h (Fig. 2). In contrast, comet sizes were greatly reduced by sera from immunized animals (Fig. 2). After two inoculations with 107 or 108 pfu of MVA, the sera exhibited greater comet-reducing activity than the sera from animals immunized once with Dryvax.
Fig. 2.
Anti-comet test. After BS-C-1 cells were infected with the IHD strain of vaccinia virus, pooled serum diluted 1:50 from mice immunized twice with MVA (106 to 108 pfu) or once with Dryvax was added to the liquid overlay medium. After 48 h, the monolayers were stained with crystal violet.
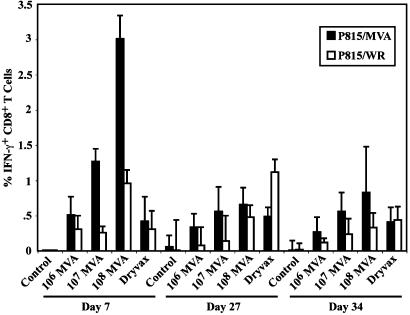
CD8+ T Cell Responses of BALB/c Mice. Spleen cells from immunized animals were stimulated in vitro with vaccinia virus MVA or WR and the numbers of CD8+ T cells that express IFN-γ were determined by intracellular cytokine staining. With splenocytes from MVA-immunized animals, the stimulation was usually better with MVA than with WR (Fig. 3), possibly because MVA is less cytopathic in vitro. This difference was not seen with spleen cells from animals immunized with Dryvax, however, raising the possibility of different immunodominant epitopes. At 7 days after immunization of mice with 106 pfu of MVA, the percentages of positive cells were similar to those obtained with Dryvax. Higher doses of MVA induced higher percentages of activated CD8+ T cells on day 7. On day 27 the percentages of positive cells in the MVA groups had decreased, whereas they had increased in the Dryvax-immunized animals, suggesting different kinetics that could have resulted from continued replication of Dryvax. After an additional week, however, the CD8+ IFN-γ+ T cells of Dryvax-immunized animals declined. The latter determination was made at 4 days after challenge with 106 pfu of vaccinia virus WR before the occurrence of significant signs of disease as shown below.
Fig. 3.
CD8+ T cell responses. BALB/c mice were unimmunized (controls) or immunized i.m. with MVA at 106, 107, or 108 pfu or percutaneously with Dryvax on day 0. On the indicated days, spleens were removed and mixed with P815 cells that had been stimulated overnight with MVA or WR. The percentage of vaccinia virus-specific IFN-γ expressing CD3+ CD8+ cells were determined for four animals in each group, and the values were plotted with standard deviations. On day 30, mice were challenged i.n. with 106 pfu of vaccinia virus WR.
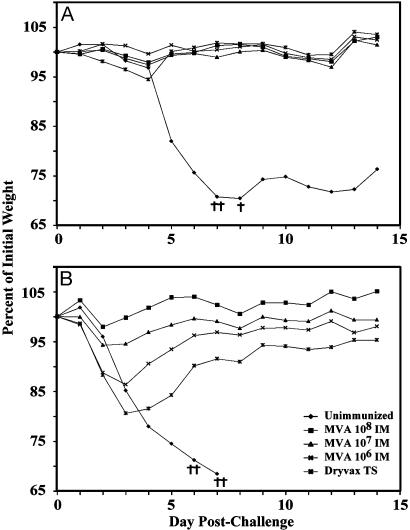
Protection of Mice Against i.n. Challenge with Pathogenic Vaccinia Virus. The WR strain of vaccinia virus provides a model for evaluating protective immunogenicity in mice. i.n. inoculation is followed by pneumonia, systemic dissemination, weight loss, and death (32-34). BALB/c mice were injected i.m. with a single dose of 106 to 108 pfu of MVA or percutaneously with Dryvax and then challenged 4 weeks later with 106 pfu of WR. Control mice lost weight continuously and 3 of the 4 mice were terminated when their weight loss reached 30% (Fig. 4A). In contrast, immunized mice lost little weight and survived (Fig. 4A), similar to previous results with a different passage stock of MVA (25). The difference in weight loss between each of the immunized groups and the control group was highly significant on day 5 (P < 0.0001), but no significant difference occurred between the immunized groups. Because CD8+ T cell and neutralizing antibody responses were detected after immunization with 107 or 108 pfu of MVA, protection could have resulted from either or both arms of the immune system. Mice immunized with 106 pfu of MVA, however, had vaccinia virus-specific CD8+ T cells at the time of challenge (Fig. 3) but only a low level of binding antibody and no detectable neutralizing antibody (Table 1). Nevertheless, they were also protected. After challenge, serum binding and neutralizing antibodies were still undetectable on day 5, even though enhanced-sensitivity assays were used (data not shown), a time at which the animals immunized with 106 pfu of MVA were already protected against weight loss compared with controls (Fig. 4A). Low levels of binding and neutralizing antibody were measured on day 7 and increased thereafter (data not shown), perhaps contributing to protection.
Fig. 4.
Protective immunization of mice. (A) Groups of four BALB/c mice were inoculated i.m. with MVA (106 to 108 pfu) or percutaneously with Dryvax and challenged 4 weeks later by i.n. inoculation of 106 pfu of vaccinia virus WR. (B) BALB/c mice vaccinated with MVA as in A were revaccinated after 4 weeks and challenged 3 weeks after that. Dryvax-vaccinated mice were challenged at 7 weeks after the single vaccination. Mice were weighed individually and averages were plotted. †, Died naturally or were killed because of 30% weight loss. The mice used here were the same as those in Table 1.
A 10-fold higher dose of WR was used to challenge mice that had received two inoculations of MVA. Under these conditions the control mice died or were terminated within 7 days, whereas all immunized mice survived. The differences in weight loss between each of the immunized groups and the control group were highly significant (P < 0.0001, days 5-7). Furthermore, weight losses were inversely proportional to the MVA dose, and the mice immunized with Dryvax lost the most weight (Fig. 4B). The differences between the two highest doses of MVA and Dryvax were highly significant on days 3, 4, and 5 (P ≤ 0.0003).
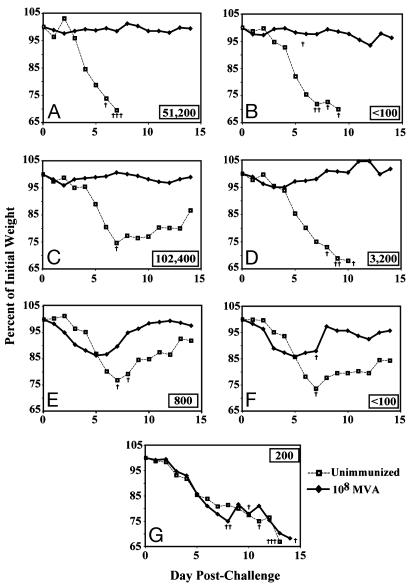
Protective Efficacy of MVA in B Cell-Deficient Mice. Further experiments were carried out by vaccinating genetically immune-deficient mice. To assess the requirement for antibody, BALB/c mice and B cell-deficient BALB/c mice (Jh) were compared. The Jh knockout mouse has a targeted deletion of the JH locus, resulting in inability to produce a complete, recombined version of the variable region of the heavy chain and no detectable IgM or IgG (35). T cell development, however, proceeds normally. An enhanced sensitivity ELISA was used to confirm the presence of induced antibody in BALB/c mice and its absence in Jh mice after immunization with 108 pfu of MVA (Fig. 5 A and B). In contrast, BALB/c and Jh mice made comparable antigen-specific CD8 cell responses (2.4 and 3.5% IFN-γ-secreting CD8+ T cells, respectively). After i.n. challenge with 106 pfu of WR virus, immunized Jh mice were protected as well as BALB/c mice, whereas unimmunized mice of both strains died (Fig. 5 A and B). The absence of weight loss indicated that antibody was not required to protect against disease or death in mice that received an immunizing dose of 108 pfu of MVA. In a previous study (25), in which the dose of MVA was only 106 pfu, considerable weight loss occurred.
Fig. 5.
Protective immunization of immune-deficient mice. Mice (n = 4) were vaccinated once i.m. with 108 pfu of MVA and challenged 3 weeks later with 106 pfu of vaccinia virus WR by the i.n. route. (A) BALB/c mice. (B) B cell-deficient mice. (C) C57BL/6 mice. (D) β2-microglobulin-deficient mice. (E) CD4-deficient mice. (F) MHC class II-deficient mice. (G) Double-knockout mice deficient in MHC class I and II. Inset numbers represent the IMV reciprocal endpoint ELISA titers at 3 weeks.
Protective Efficacy of MVA in T Cell-Deficient Mice. Four knockout strains of mice in a C57BL/6 background were used to determine the role of T cells in the development of a protective response. C57BL/6 mice (Fig. 5C) and β2m mice (Fig. 5D) were similarly protected by vaccination with MVA. β2m mice are β2-microglobulin-deficient; consequently, they are unable to express MHC class I molecules on the cell surface and therefore lack most CD8+ T cells (36). These mice also lack NK1.1+ CD4+ T cells, which depend on the MHC class 1b molecule CD1 for development. In addition to a deficiency in CD8+ T cells, β2m mice made relatively low antibody responses compared with C57BL/6 mice (Fig. 5 C and D), consistent with previous observations (37, 38). Nevertheless, this response appeared sufficient because vaccinated β2m mice were well protected against the i.n. WR challenge (P ≤ 0.005 on days 6-8).
We also immunized CD4-deficient mice (39). In this strain ≈90% of the α β T cells in the periphery are CD8+; primary class I responses are normal, but a decrease occurs in helper T cell and other class II-restricted activities. These mice made poor antibody responses and, although none died, exhibited weight losses that were only slightly less severe than controls (Fig. 5E). Similar results were obtained with Abb mice, which have a depletion of CD4+ T cells because of disruption of the H2-Ab1 gene (40). The antibody response to MVA was below detection and, on WR challenge, one mouse died and the others exhibited weight losses only slightly less than controls (Fig. 5F). Abb/β2m are double-knockout mice generated by mating β2-microglobulin-deficient, class I-deficient mice with MHC class II-deficient mice (41). Phenotypically, these mice are depleted of CD4+ and CD8+ T cells in peripheral lymphoid organs. Although the B cell compartments of these animals appear intact, only a barely detectable antibody response was detected and the animals did not survive the i.n. WR challenge (Fig. 5G).
Discussion
We isolated several clones of MVA from a stock that had last been propagated in 1974, a date that preceded known cases of bovine spongiform encephalitis. As expected from previous studies (13-15), the MVA clones replicated to high titers in CEF but exhibited a severe host restriction in monkey and human cell lines. Furthermore, the cloned virus was not pathogenic in SCID mice at 1,000 times the lethal dose of the standard Wyeth vaccine. Thus, the MVA clone described here may be suitable for development of smallpox and recombinant vaccines.
An important aspect of this study was the demonstration that antibody responses to MVA were directed toward the two infectious forms of orthopoxviruses and inhibited virus infectivity and spread in tissue culture. Antibody responses to MVA administered i.m. were dose-dependent and increased after a subsequent inoculation. A single injection of 108 pfu or two injections of 107 pfu induced antibody responses similar to those of Dryvax administered by the conventional percutaneous route. MVA-induced CD8 T cell responses were also dose-dependent. Presumably, only a low inoculum of Dryvax is required because it replicates well in the skin. Our studies suggest that the dose of 106 infectious units of MVA used in the early clinical trials in Germany may not have been optimal and that higher doses and boosting with a second inoculation should be investigated in new trials.
Smallpox is believed to spread mainly by the upper respiratory route, and we therefore used an i.n. infection model. Previous studies demonstrated the usefulness of the vaccinia virus WR challenge model for evaluating pathogenesis and therapeutic drugs (32-34). Prevention of weight loss and death were used as indicators of vaccine efficacy. At the lowest challenge dose of 106 pfu of WR, in which all control mice lost weight and most died, none of the MVA-immunized mice exhibited significant weight loss or death. Thus, the immune responses of animals receiving even the lowest dose of MVA, 106 pfu, were sufficient for protection. It is tempting to attribute the protection of the latter group of animals to their CD8+ T cell responses, because they exhibited low or no measured antibody to vaccinia virus. However, antibody titers were only determined after 1:50 or 1:100 dilutions of sera, so that biologically significant amounts of antibody might have been present in vivo. Furthermore, under similar conditions of MVA immunization, Belyakov and coworkers (25) found that mice were still protected when CD8+ T cells or CD4+ T cells were depleted with antibody at the time of challenge. We found that with the higher challenge dose of 107 pfu of WR, all the control animals died and the immunized animals lived. Nevertheless, with this stringent challenge, significant differences existed in the weight losses between the groups as follows: control > Dryvax > 106 pfu MVA > 107 pfu MVA > 108 pfu MVA in which all the MVA-immunized animals received two inoculations. Of the immune parameters measured, the antibody response to the A33R CEV/EEV envelope protein and the acute CD8+ T cell response most closely correlated with the degree of protection. Further studies are needed to compare the duration of immunity to MVA and Dryvax.
The high-risk groups, for which an alternative to the standard smallpox vaccine is most important, include groups with partially suppressed immune systems. It was of interest to determine whether mice with specific immune deficiencies could be protectively vaccinated. For these studies, we used a single MVA inoculation of 108 pfu and found that mice deficient in B cells or CD8 T cells were well protected against lethal i.n. inoculations with a pathogenic vaccinia virus, consistent with overlapping roles for both antibody and T cells. CD4 or MHC class II knockout mice, however, were poorly protected, confirming the important roles of CD4 cells and MHC class II for generating effective antibody and CD8 responses. Note, however, that MVA-immunized BALB/c mice were still protected when their CD4 cells were depleted at the time of challenge rather than at the time of immunization (25). In this context, recent reports state that memory CD8+ T cells that are generated without CD4 help are defective in their ability to respond to secondary encounters with antigen (42, 43). Double-knockout mice with diminished CD4 and CD8 cells were entirely unprotected.
Current thinking is that new vaccines, which cannot be field-tested because of the absence of disease, should be examined in at least two relevant animal models. Elsewhere, we reported that two inoculations of MVA or one inoculation of MVA followed by Dryvax substantially protected monkeys against a severe i.v. monkeypox virus challenge (44). Our findings of similar humoral and cellular immune responses to MVA and Dryvax in both rodent and non-human primate systems are important steps in the evaluation of MVA as a replacement vaccine for those with increased risk of severe side effects from the standard live vaccine or as a prevaccine. One scenario would be to vaccinate with MVA before any immediate smallpox threat, with the expectation that a standard vaccine or a second MVA (depending on individual risk factors) would be given as a boost in the event of documented smallpox.
Acknowledgments
We thank Jeffery Americo and Jennifer Dowd for excellent technical assistance, David Tscharke for advice on the CD8+ CTL assay, Jack Bennink for helpful discussions and critical reading of the manuscript, and Chuck Whitbeck, Rosalyn Eisenberg, and Gary Cohen for generously providing recombinant proteins.
Abbreviations: MVA, modified vaccinia virus Ankara; CEF, chicken embryo fibroblast; EEV, extracellular enveloped virion; SCID, severe combined immunodeficiency disease; pfu, plaque-forming unit; WR, Western Reserve; IMV, intracellular mature virion; i.n., intranasal; CEV, cell-associated enveloped virion.
References
- 1.Henderson, D. A. (1999) Science 283, 1279-1282. [DOI] [PubMed] [Google Scholar]
- 2.Fenner, F., Henderson, D. A., Arita, I., Jezek, Z. & Ladnyi, I. D. (1988) Smallpox and Its Eradication (World Health Organization, Geneva).
- 3.Fulginiti, V. A., Papier, A., Lane, J. M., Neff, J. M. & Henderson, D. A. (2003) Clin. Infect. Dis. 37, 251-271. [DOI] [PubMed] [Google Scholar]
- 4.Mayr, A., Hochstein-Mintzel, V. & Stickl, H. (1975) Infection 3, 6-14. [Google Scholar]
- 5.Stickl, H., Hochstein-Mintzel, V., Mayr, A., Huber, H. C., Schäfer, H. & Holzner, A. (1974) Dtsch. Med. Wochenschr. 99, 2386-2392. [DOI] [PubMed] [Google Scholar]
- 6.Sutter, G. & Moss, B. (1992) Proc. Natl. Acad. Sci. USA 89, 10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutter, G., Wyatt, L. S., Foley, P. L., Bennink, J. R. & Moss, B. (1994) Vaccine 12, 1032-1040. [DOI] [PubMed] [Google Scholar]
- 8.Durbin, A. P., Cho, C. J., Elkins, W. R., Wyatt, L. S., Moss, B. & Murphy, B. R. (1999) J. Infect. Dis. 179, 1345-1351. [DOI] [PubMed] [Google Scholar]
- 9.Amara, R. R., Villinger, F., Altman, J. D., Lydy, S. L., O'Neil, S. P., Staprans, S. I., Montefiori, D. C., Xu, Y., Herndon, J. G., Wyatt, L. S., et al. (2001) Science 292, 69-74. [DOI] [PubMed] [Google Scholar]
- 10.Mayr, A. (1967) Zentralbl. Bakteriol. Mikrobiol. Hyg. Abt. 1 Orig. B 183-189.
- 11.Meyer, H., Sutter, G. & Mayr, A. (1991) J. Gen. Virol. 72, 1031-1038. [DOI] [PubMed] [Google Scholar]
- 12.Antoine, G., Scheiflinger, F., Dorner, F. & Falkner, F. G. (1998) Virology 244, 365-396. [DOI] [PubMed] [Google Scholar]
- 13.Carroll, M. & Moss, B. (1997) Virology 238, 198-211. [DOI] [PubMed] [Google Scholar]
- 14.Drexler, I., Heller, K., Wahren, B., Erfle, V. & Sutter, G. (1998) J. Gen. Virol. 79, 347-352. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard, T. J., Alcami, A., Andrea, P. & Smith, G. L. (1998) J. Gen. Virol. 79, 1159-1167. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt, L. S., Carroll, M. W., Czerny, C.-P., Merchlinsky, M., Sisler, J. R. & Moss, B. (1998) Virology 251, 334-342. [DOI] [PubMed] [Google Scholar]
- 17.Stittelaar, K. J., Kuiken, T., de Swart, R. L., van Amerongen, G., Vos, H. W., Niesters, H. G., van Schalkwijk, P., van der Kwast, T., Wyatt, L. S., Moss, B. & Osterhaus, A. D. (2001) Vaccine 19, 3700-3709. [DOI] [PubMed] [Google Scholar]
- 18.Moss, B. (2001) in Fields Virology, eds. Knipe, D. M. & Howley, P. M. (Lippincott Williams & Wilkins, Philadelphia), Vol. 2, pp. 2849-2883. [Google Scholar]
- 19.Boulter, E. A. & Appleyard, G. (1973) Prog. Med. Virol. 16, 86-108. [PubMed] [Google Scholar]
- 20.Hooper, J. W., Custer, D. M., Schmaljohn, C. S. & Schmaljohn, A. L. (2000) Virology 266, 329-339. [DOI] [PubMed] [Google Scholar]
- 21.Blanden, R. V. (1971) J. Exp. Med. 133, 1074-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karupiah, G., Buller, R. M., Van Rooijen, N., Duarte, C. J. & Chen, J. (1996) J. Virol. 70, 8301-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulter, E. A., Zwartouw, H. T., Titmuss, D. H. J. & Maber, H. B. (1971) Am. J. Epidemiol. 94, 612-620. [DOI] [PubMed] [Google Scholar]
- 24.Galmiche, M. C., Goenaga, J., Wittek, R. & Rindisbacher, L. (1999) Virology 254, 71-80. [DOI] [PubMed] [Google Scholar]
- 25.Belyakov, I. M., Earl, P., Dzutsev, A., Kuznetsov, V. A., Lemon, M., Wyatt, L. S., Snyder, J. T., Ahlers, J. D., Franchini, G., Moss, B. & Berzofsky, J. A. (2003) Proc. Natl. Acad. Sci. USA 100, 9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drexler, I., Staib, C., Kastenmuller, W., Stevanovic, S., Schmidt, B., Lemonnier, F. A., Rammensee, H. G., Busch, D. H., Bernhard, H., Erfle, V. & Sutter, G. (2003) Proc. Natl. Acad. Sci. USA 100, 217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earl, P. L., Moss, B., Wyatt, L. S. & Carroll, M. W. (1998) in Current Protocols in Molecular Biology, eds. Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A. & Struhl, K. (Greene & Wiley, New York), Vol. 2, pp. 16.17.1-16.17.19. [Google Scholar]
- 28.Earl, P. L., Americo, J. L. & Moss, B. (2003) J. Virol. 77, 10684-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez, J. C., Finke, D., Esteban, M., Kraehenbuhl, J. P. & Acha-Orbea, H. (2003) Arch. Virol. 148, 827-839. [DOI] [PubMed] [Google Scholar]
- 30.Appleyard, G., Hapel, A. J. & Boulter, E. A. (1971) J. Gen. Virol. 13, 9-17. [DOI] [PubMed] [Google Scholar]
- 31.Law, M., Hollinshead, R. & Smith, G. L. (2002) J. Gen. Virol. 83, 209-222. [DOI] [PubMed] [Google Scholar]
- 32.Turner, G. S. (1967) J. Gen. Virol. 1, 399-402. [DOI] [PubMed] [Google Scholar]
- 33.Williamson, J. D., Reith, R. W., Jeffrey, L. J., Arrand, J. R. & Mackett, M. (1990) J. Gen. Virol. 71, 2761-2767. [DOI] [PubMed] [Google Scholar]
- 34.Smee, D. F., Bailey, K. W., Wong, M. & Sidwell, R. W. (2001) Antiviral Res. 52, 55-62. [DOI] [PubMed] [Google Scholar]
- 35.Chen, J., Trounstine, M., Alt, F. W., Young, F., Kurahara, C., Loring, J. F. & Huszar, D. (1993) Int. Immunol. 5, 647-656. [DOI] [PubMed] [Google Scholar]
- 36.Zijlstra, M., Bix, M., Simister, N. E., Loring, J. M., Raulet, D. H. & Jaenisch, R. (1990) Nature 344, 742-746. [DOI] [PubMed] [Google Scholar]
- 37.Spriggs, M. K., Koller, B. H., Sato, T., Morrissey, P. J., Fanslow, W. C., Smithies, O., Voice, R. F., Widmer, M. B. & Maliszewski, C. R. (1992) Proc. Natl. Acad. Sci. USA 89, 6070-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christianson, G. J., Brooks, W., Vekasi, S., Manolfi, E. A., Niles, J., Roopenian, S. L., Roths, J. B., Rothlein, R. & Roopenian, D. C. (1997) J. Immunol. 159, 4781-4792. [PubMed] [Google Scholar]
- 39.Killeen, N. & Littman, D. R. (1993) Nature 364, 729-732. [DOI] [PubMed] [Google Scholar]
- 40.Grusby, M. J., Johnson, R. S., Papaioannou, V. E. & Glimcher, L. H. (1991) Science 253, 1417-1420. [DOI] [PubMed] [Google Scholar]
- 41.Grusby, M. J., Auchincloss, H., Jr., Lee, R., Johnson, R. S., Spencer, J. P., Zijlstra, M., Jaenisch, R., Papaioannou, V. E. & Glimcher, L. H. (1993) Proc. Natl. Acad. Sci. USA 90, 3913-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun, J. C. & Bevan, M. J. (2003) Science 300, 339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shedlock, D. J. & Shen, H. (2003) Science 300, 337-339. [DOI] [PubMed] [Google Scholar]
- 44.Earl, P. L., Americo, J. L., Wyatt, L. S., Eller, L. A., Whitbeck, J. C., Cohen, G. H., Eisenberg, R. J., Hartmann, C. J., Jackson, D. L., Kulesh, D. A., et al. (2004) Nature 428, 182-185. [DOI] [PubMed] [Google Scholar]