Abstract
Many bacterial pathogens encode the cytolethal distending toxin (CDT), which causes host cells to arrest during their cell cycle by inflicting DNA damage. CDT is composed of three proteins, CdtA, CdtB, and CdtC. CdtB is the enzymatically active or A subunit, which possesses DNase I-like activity, whereas CdtA and CdtC function as heteromeric B subunits that mediate the delivery of CdtB into host cells. We show here that Salmonella enterica serovar Typhi encodes CDT activity, which depends on the function of a CdtB homologous protein. Remarkably, S. enterica serovar Typhi does not encode apparent homologs of CdtA or CdtC. Instead, we found that toxicity, as well as cdtB expression, requires bacterial internalization into host cells. We propose a pathway of toxin delivery in which bacterial internalization relieves the requirement for the functional equivalent of the B subunit of the CDT toxin.
Keywords: cell cycle progression, bacterial pathogenesis, host-pathogen interactions, typhoid fever
The cytolethal distending toxin (CDT) was first described as an activity in culture supernatants of Campylobacter spp. and certain enteropathogenic strains of Escherichia coli that caused eukaryotic cells to slowly distend over a period of 2-5 days, eventually leading to their death (1, 2). The cellular enlargement involved not only the cytoplasm but also the nucleus, which appeared as twice the normal size. Since then, this toxin has been identified in many other bacterial pathogens, including other strains of E. coli (3), Shigella dysenteriae (4), Haemophilus ducreyi (5), Actinobacillus actinomycetemcomitans (6), and Helicobacter hepaticus (7). The CDT is composed of three subunits, CdtA, CdtB, and CdtC, which form a tripartite complex (8). CdtA and CdtC form a heterologous B subunit that is necessary for the delivery of CdtB, the active or A subunit (9). The mechanism of action of this toxin is reasonably well understood. On delivery into host cells by CdtA and CdtC, the active subunit CdtB is transported to the nucleus where it causes DNA damage (10, 11). Indeed, CdtB exhibits limited amino acid sequence similarity with the DNase I family of proteins, and purified CdtB exhibits very low but measurable DNase activity. The cellular responses to DNA damage lead to the characteristic G2/M cell cycle arrest, cellular distention, and nuclear enlargement observed in intoxicated cells (3).
Although cytotoxicity by exogenously administered toxin requires all three CDT subunits, CdtB alone can recapitulate all of the CDT effects, provided that it is administered in very small quantities directly into the cytosol of target cells by either microinjection or transient expression (10). The mechanism by which CDT enters cells is not completely understood. However, results of experiments using a number of pharmacological inhibitors have suggested that the toxin enters cells by means of receptor-mediated endocytosis, traveling deep into the endocytic pathway (12) before CdtB is delivered first into the cytosol and then into the nucleus of the target cell.
The CDT is encoded by an operon composed of the cdtA, cdtB, and cdtC genes (13, 14). The recent determination of the entire nucleotide sequence of the genomes of two different strains of Salmonella enterica serovar Typhi (S. typhi), the cause of human typhoid fever, revealed an ORF capable of encoding a protein with significant amino acid sequence similarity to CdtB (15, 16). Surprisingly, however, no ORFs encoding proteins with amino acid sequence similarity to CdtA or CdtC were detected either in the vicinity of the putative cdtB gene or elsewhere in the S. typhi genome. In this article we show that S. typhi cdtB encodes a functional protein and that, despite the absence of CdtA and CdtC, this bacterium produces a cdtB-dependent CDT activity that requires bacterial internalization into host cells. We propose a mechanism for toxin delivery into target cells.
Materials and Methods
Bacterial Strains, Media, and Growth Conditions. All S. typhi strains were derived from the wild-type strain ISP2825 (17). Strains carrying loss-of-function mutations in asd (18), which encodes for the aspartate semialdehyde dehydrogenase, invA (19), which encodes for an essential component of the type III secretion system (TTSS) encoded within pathogenicity island 1 (SPI-1 TTSS), or spiA (20), which encodes for an essential component of the TTSS encoded within pathogenicity island 2 (SPI-2 TTSS), were constructed by P22 transduction and/or conjugation as previously described (17, 21). S. typhi strains were grown in LB broth containing 0.3 M sodium chloride at 37°C. When required, ampicillin (100 μg/ml), kanamycin (50 μg/ml), tetracycline (12 μg/ml), and diaminopimelic acid (DAP) (50 μg/ml) (Sigma) were added. When appropriate, 0.1% l-arabinose was added to cultures at the early logarithmic phase of growth (OD600 of 0.4) to induce expression of genes under the control of the paraBAD promoter (22).
Tissue Culture and Immunofluorescence. Growth, transfection, and immunofluorescence staining of Cos-2 and Henle-407 intestinal epithelial cells were conducted as previously described (10).
Construction of Plasmids and Bacterial Strains. Detailed description of plasmid and strain constructions is provided in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Bacterial Infections and Toxicity Assay. Overnight cultures of the different S. typhi strains were diluted 1:50 in LB broth containing 0.3 M NaCl and 50 μg/ml DAP and grown until they reached an OD600 of 1.0. Henle-407 intestinal epithelial cells were infected with the different strains of S. typhi at a multiplicity of infection of 50 in Hanks' balanced salt solution (HBSS) supplemented with 50 μg/ml DAP. Cells were washed three times with HBSS 90 min after infection and incubated in DMEM supplemented with DAP and gentamicin for 1 h to kill extracellular bacteria. Cells then were washed, and fresh DMEM supplemented with DAP was added. The medium was replaced 4 h later with DMEM (without DAP) for the remainder of the experiment. Cells were observed for up to 4 days and were processed for cell cycle analysis or observed by standard microscopy. Toxicity assays were carried out as previously described (9, 10).
Cell Cycle Analysis. Four days after bacterial infection or treatment with bacterial extracts, intestinal Henle-407 cells were processed for flow cytometry as previously described (9, 10).
Luciferase Reporter Assay. Henle-407 cells were infected with the different reporter strains of S. typhi as indicated above. At different times after infection, cells were washed and lysed in Passive Lysis Buffer (Dual-Luciferase Reporter Assay System, Promega), followed by sonication. Firefly luciferase activity in lysates was measured by using a luminometer as recommended by the manufacturer (Promega). Cells processed and infected in parallel and in identical fashion were lysed with 0.1% deoxycholic acid, and dilutions of lysates were plated onto LB agar plates to determine the number of intracellular bacteria.
Protein Secretion Assay. Western blot analysis of culture supernatant proteins and whole bacterial lysates was carried out as previously described (23).
Results
S. typhi Encodes a Functional CdtB. Examination of the recently completed genome sequence of two S. typhi strains reveals an ORF that encodes a putative polypeptide with a sec-dependent secretion signal and significant amino acid sequence similarity with the CdtB subunit of the CDT from other pathogenic bacteria (see Fig. 7, which is published as supporting information on the PNAS web site). The overall amino acid sequence identity is ≈50%, and all of the residues that have been shown to be essential for the catalytic activity of CdtB (10, 11), as well as other members of the DNase I family of proteins (24, 25), are conserved. The putative cdtB gene is encoded within a region of the chromosome that is absent from other S. enterica serovars and exhibits features consistent with horizontally acquired genetic material, such as remnants of insertion sequences (e.g., IS200) and a transposase gene. In both sequenced strains of S. typhi, the ORF is preceded by a canonical ribosome-binding site and does not contain any missense mutations, suggesting that this gene may encode a functional polypeptide. Surprisingly, close examination of the two available S. typhi genome sequences did not yield recognizable homologs of the cdtA and cdtC genes (data not shown). This observation is puzzling, because CdtA and CdtC are known to be essential components of the CDT, and cdtB has not been previously observed without cdtA and cdtC (9). In addition, CDT toxicity has in all cases been shown to be strictly dependent on the three subunits.
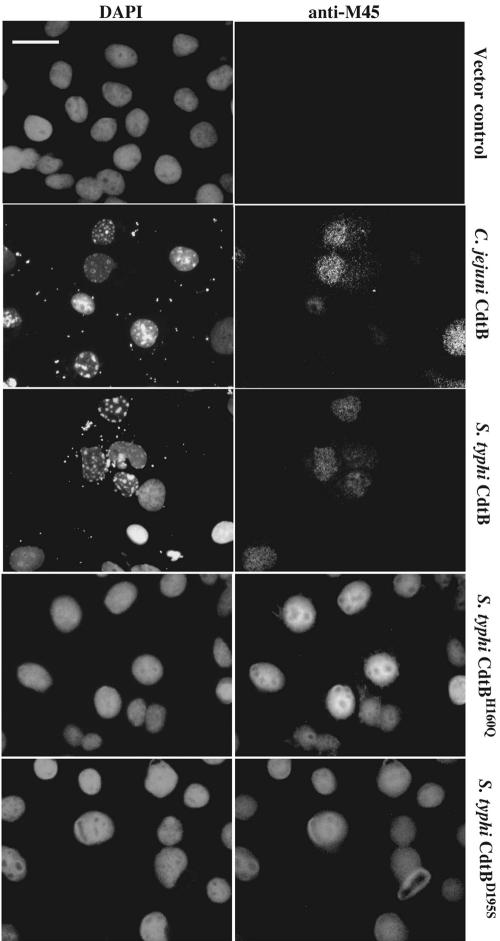
We therefore tested whether S. typhi cdtB encodes a protein functionally similar to the CdtB subunit of CDTs from other bacteria. To this end, we transiently expressed the epitope-tagged S. typhi cdtB gene in cultured Cos-2 cells, and, 48 h after transfection, the cells were stained with an antibody directed to the epitope tag and with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the chromatin structure. As shown in Fig. 1, expression of S. typhi CdtB in these cells resulted in drastic changes in the chromatin of transfected cells, characterized by severe fragmentation. The changes in the chromatin were indistinguishable from those induced by the expression of the CdtB subunit of Campylobacter jejuni (Fig. 1) (10) and entirely consistent with the reported DNase I-like activity of the CdtB protein family (10, 11). Consistent with its predicted site of activity and as shown for C. jejuni CdtB (10), S. typhi CdtB localized within the nucleus of transfected cells (Fig. 1). These results indicate that S. typhi CdtB not only shares amino acid sequence similarity with the CdtB protein family but also displays very similar activity when expressed in host cells.
Fig. 1.
Effect of transient expression of wild-type and catalytic mutants of S. typhi CdtB in cultured cells. Cos-2 cells were transfected with vectors encoding M45 epitope-tagged S. typhi CdtB, the catalytic mutants CdtBH160Q and CdtBD195S, or C. jejuni CdtB. Cells were stained 48 h after transfection with a monoclonal antibody directed to the M45 epitope tag (anti-M45) to visualize cells expressing the different CdtB proteins and with 4′,6-diamidino-2-phenylindole (DAPI) to visualize the chromatin. Images were obtained with a Nikon Eclipse inverted microscope fitted with a Princeton Instruments (Trenton, NJ) MicroMAX digital camera. (Scale bar, 50 μm.)
Previous studies have demonstrated that residues that are essential for the enzymatic activity of the DNase I protein family are also essential for CdtB function (10, 11). We therefore tested whether equivalent amino acid substitutions in S. typhi CdtB, which would affect its putative active (His-160) or Mg2+-binding (Asp-195) sites (see Supporting Materials and Methods), would have the same deleterious effect on its activity. Plasmids encoding epitope-tagged versions of S. typhi CdtBH160Q or CdtBD195S were transfected into Cos-2 cells, and 48 h after transfection the cells were observed by fluorescence microscopy. Cells transfected with either of these plasmids exhibited normal cellular and nuclear morphology, despite evidence of a high level of expression of the CdtB mutants, which appeared exclusively localized within the nucleus (Fig. 1). Consistent with its predicted site of activity, and as shown for C. jejuni CdtB (10), S. typhi CdtB mutants localized exclusively within the nucleus of transfected cells (Fig. 1). Taken together, these results indicate that S. typhi encodes a CdtB homolog that, when expressed in cultured mammalian cells, displays the same activities as its homologs of other bacterial pathogens.
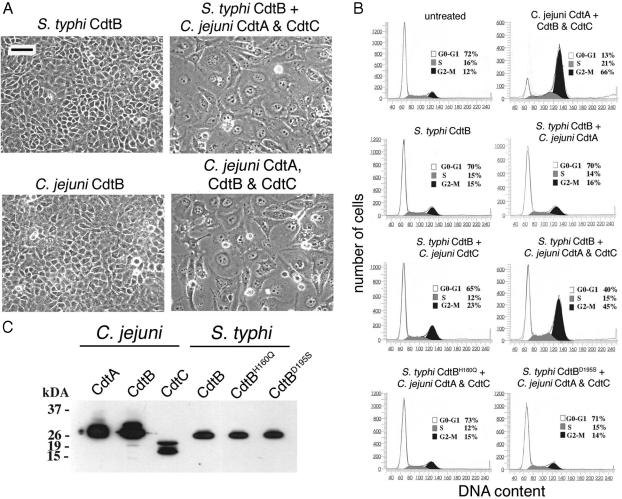
S. typhi CdtB Is Not Toxic When Exogenously Applied but Can Form a Functional Toxin with Heterologous CdtA and CdtC Subunits. Because S. typhi does not encode obvious homologs of CdtA and CdtC, we tested whether S. typhi CdtB alone could display CDT-like toxic activity when exogenously applied to cultured mammalian cells. Extracts from an E. coli DH5α strain expressing S. typhi cdtB from a paraBAD promoter (22) were applied to Henle-407 cells, which were then observed for signs of intoxication. As shown in Fig. 2A, S. typhi CdtB had no toxic activity when exogenously applied to cultured mammalian cells. These results indicate that S. typhi CdtB does not differ in this regard from CdtBs from other pathogens, which when exogenously administered are not sufficient to induce toxicity.
Fig. 2.
Effect of Cdt proteins on cultured intestinal epithelial cells. Extracts of E. coli strains (100 μg/ml) expressing different M45 epitope-tagged S. typhi or C. jejuni Cdt proteins as indicated, alone or in combination, were added to cultured Henle-407 cells. (A and B) Cells were examined 72 h after addition of the protein extracts under a phase microscope (A) or processed to measure DNA content by flow cytometry (B) as described in Materials and Methods. The peaks corresponding to cells in G0/G1 (G0-G1), S, or G2 (G2-M) are indicated. (C) The presence of the different Cdt proteins in the bacterial extracts was verified by Western blot analysis with an antibody directed to the M45 epitope tag. (Scale bar, 50 μm.)
It has been previously shown that C. jejuni CdtA, CdtB, and CdtC can form a complex in vitro that displays full toxicity (9). We therefore tested whether S. typhi CdtB could form an active CDT holotoxin when combined with the heterologous CdtA and CdtC subunits from C. jejuni. Extracts from E. coli DH5α strains expressing epitope-tagged versions of C. jejuni CdtA or CdtC or S. typhi CdtB were prepared (Fig. 2C), combined as indicated in the Fig. 2 legend, and evaluated for CDT activity as described in Materials and Methods. Henle-407 intestinal epithelial cells treated with a combination of extracts containing C. jejuni CdtA and CdtC and S. typhi CdtB showed clear signs of CDT intoxication characterized by dramatic cellular distention (Fig. 2A) and G2/M cell cycle arrest (Fig. 2B). The cytotoxic activity was indistinguishable from that induced by a combination of extracts containing C. jejuni CdtA, CdtB, and CdtC (Fig. 2 A and B). Furthermore, CDT activity was strictly dependent on the enzymatic activity of S. typhi CdtB, because introduction of single amino acid substitutions within the predicted catalytic (CdtBH160Q) or Mg-binding (CdtBD195S) sites completely abolished the cytotoxic activity of S. typhi CdtB (Fig. 2B). Taken together, these results indicate that S. typhi CdtB, when combined with the heterologous CdtA and CdtC subunits of C. jejuni, can form an active CDT holotoxin functionally indistinguishable from other CDTs. Furthermore, these results demonstrate that the amino acid sequence similarity between S. typhi CdtB and its homologs translates into equivalent activity.
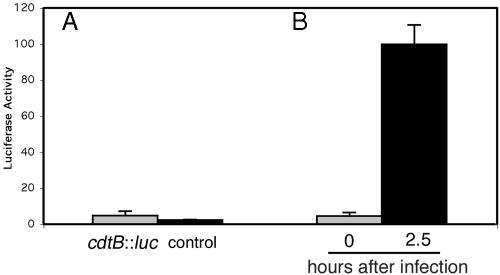
Expression of CdtB Is Induced Within Host Cells. Although the results described above indicated that the S. typhi cdtB ORF encodes a polypeptide that is functionally indistinguishable from CdtB proteins from other pathogens, those experiments did not demonstrate that cdtB is actually expressed by S. typhi. To address this issue, we constructed a S. typhi reporter strain in which the firefly luciferase gene is integrated at the cdtB locus so that its expression is driven by the native cdtB promoter. We first assayed for the luciferase activity of the S. typhi reporter strain grown in LB broth to late logarithmic growth phase (OD600 of 1.0). As shown in Fig. 3A, no luciferase activity was detected in whole-cell lysates of the reporter strain, indicating that the cdtB gene is not expressed when S. typhi is grown under these conditions.
Fig. 3.
Expression of S. typhi cdtB after growth in vitro or after infection of cultured cells. (A) A S. typhi strain (control) and an isogenic derivative encoding a cdtB::luc reporter fusion were grown in LB broth to an OD600 of 1.0, and the luciferase activity in total cell lysates was measured as described in Materials and Methods. (B) Alternatively, cultured intestinal Henle-407 cells were infected with the S. typhi cdtB::luc reporter strain, and the luciferase activity at different times after infection was measured as indicated in Materials and Methods. In all cases, values were standardized relative to the colony-forming units and the values obtained in cells 2.5 h after infection, which was considered 100%.
We reasoned that expression of cdtB might be induced on bacterial infection of host mammalian cells. We tested this possibility by infecting Henle-407 cells with the S. typhi reporter strain and assayed the luciferase activity in total cell lysates of infected cells at different times after infection as described in Materials and Methods. Similar to what we observed with LB-grown S. typhi, there was no expression of cdtB::luc immediately after infection (Fig. 3B). However, a drastic induction (≈50-fold) of the expression of cdtB::luc was observed 150 min after infection (Fig. 3B). These results indicate that S. typhi expresses cdtB only on infection of host cells.
S. typhi Exhibits a CDT Activity That Is Dependent on CdtB. The observation that S. typhi cdtB is expressed prompted us to examine whether S. typhi produces a CDT activity. We found no traces of CDT activity in culture supernatants of S. typhi (data not shown), and such toxic activity has never been reported for S. typhi. However, because cdtB is expressed only on infection of host cells, we tested the possibility that the CDT activity may be detectable only under similar conditions. The CDT activity is the result of the stimulation of the DNA damage response; therefore, observation of toxicity (i.e., cytoplasmic distention and cell cycle arrest) requires an incubation of 48-72 h after intoxication. S. typhi has the ability to efficiently enter culture cells (17), and such an extended incubation time subsequent to bacterial invasion results in cell death due to bacterial replication, which would effectively preclude the observation of a potential CDT activity by the cell cycle arrest and microscopy assays.
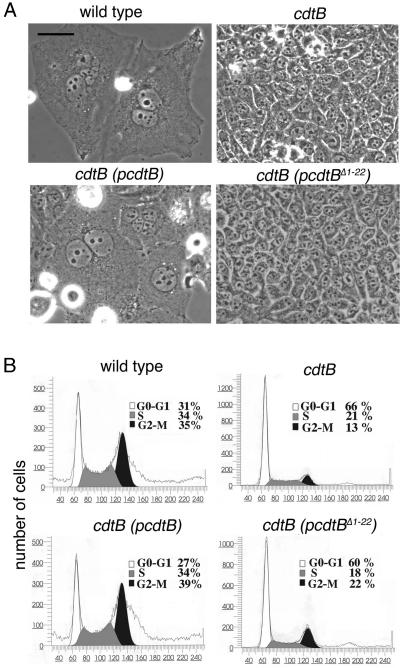
To circumvent this problem, we used an S. typhi mutant that carries a deletion in the gene for the aspartate semialdehyde dehydrogenase (asd) (18). This strain can grow only in the presence of DAP, which is absent from mammalian cells, affording the opportunity to restrict the growth of these bacteria by withdrawing DAP from the infection medium. Henle-407 cells were therefore infected with a S. typhi asd strain or an isogenic derivative carrying a loss-of-function mutation in cdtB. Ninety minutes after infection, cells were washed, and noninternalized bacteria were killed by adding gentamicin. DAP was kept in the medium for up to 5 h after bacterial infection and then removed for the duration of the experiment. Cells infected with the S. typhi asd strain showed marked signs of CDT intoxication, such as G2/M cell cycle arrest, pronounced cytoplasmic distention, and nuclear enlargement (Fig. 4) 72 h after infection. In contrast, cells infected with the S. typhi cdtB isogenic mutant showed no signs of intoxication (Fig. 4). Introduction into the cdtB mutant strain of a plasmid encoding wild-type CdtB fully restored the toxicity of this strain (Fig. 4). In contrast, a plasmid encoding a deletion mutant lacking the sec-dependent signal sequence failed to complement the cdtB mutations (Fig. 4). These results suggest that the secretion of CdtB through the sec pathway of S. typhi is necessary for this protein to exert its toxic activity, because expression of this same mutant form of CdtB directly into host cells resulted in toxicity (Fig. 1). Taken together, these results indicate that S. typhi encodes a CDT activity and that such activity is strictly dependent on cdtB.
Fig. 4.
Effect of S. typhi infection of cultured intestinal epithelial cells. Henle-407 cells were infected with S. typhi, its isogenic cdtB mutant, or the cdtB mutant carrying a plasmid encoding either wild-type CdtB or a mutant lacking the putative sec-dependent secretion signal (pcdtBΔ1-22). Cells were examined 72 h after transfection under a phase microscope (A) or processed to measure DNA content by flow cytometry (B) as indicated in Materials and Methods. The peaks corresponding to cells in G0/G1 (G0-G1), S, or G2 (G2-M) are indicated. (Scale bar, 50 μm.)
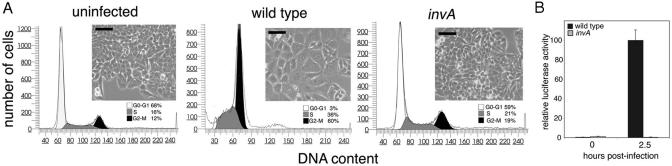
CDT Activity and cdtB Expression Require Bacterial Internalization. The experiments described above indicated that infection of cultured cells with S. typhi results in both induction of cdtB expression and CDT activity. However, as contact with the host cell alone could lead to the stimulation of gene expression, these experiments did not directly address whether actual bacterial internalization was required for any of these activities. To directly address this issue, we infected Henle-407 cells with a S. typhi asd derivative strain that carries a loss-of-function mutation in invA, a gene that encodes an essential component of the TTSS that mediates bacterial entry (19). This mutant strain is fully competent for attachment to host cells but completely defective for internalization. Cells infected with this strain did not show any signs of CDT intoxication even 5 days after infection (Fig. 5A). Because CdtB is not secreted through the invasion-associated TTSS (see Supporting Materials and Methods), these results indicate that bacterial entry is an essential requirement for CDT activity. We also examined the requirement of bacterial internalization for induction of cdtB expression. Cells were infected with an S. typhi invA mutant strain carrying the cdtB::luc reporter fusion, and the luciferase activity was measured at different times after infection. As shown in Fig. 5B, no luciferase activity was detected in extracts of cell infected with this strain, further demonstrating that bacterial internalization is essential for CDT activity and cdtB expression.
Fig. 5.
Display of S. typhi CDT activity and expression of cdtB require bacterial internalization. Henle-407 cells were infected with S. typhi or its isogenic invA mutant, which is unable to enter host cells. (A) Cells were examined 72 h after infection under a phase microscope for signs of intoxication. (Scale bar, 50 μm.) In addition, cells were infected with derivatives of S. typhi or its isogenic invA mutant encoding the cdtB::luc reporter fusion strain, and the luciferase activity at different times after infection was measured as indicated in Materials and Methods. (B) In all cases, values were standardized relative to the colony-forming units and the values obtained in cells 2.5 h after infection, which was considered 100%.
To gain further insight into the kinetics of induction of CDT activity during bacterial infection, we examined the effect of the addition of the bacterial protein-synthesis inhibitor chloramphenicol at different times after infection. Henle-407 cells were infected with S. typhi for 1 h, bacteria were washed, and gentamicin was added to kill extracellular bacteria. Chloramphenicol was added either during infection or at different times after infection. In all cases, the antibiotic was removed 6 h after infection, and cells were examined for signs of toxicity 72 h after infection. As shown in Fig. 6, no signs of toxicity were observed when chloramphenicol was added at the time of infection or 1 or 2 h after infection. However, signs of cytotoxicity were observed when chloramphenicol was added 3 h after infection, and full cytotoxicity was evident when chloramphenicol was added 6 h after infection (Fig. 6). These results indicate that production of CDT occurs within 2-3 h after infection, which is consistent with the observed pattern of induction of cdtB gene expression during bacterial infection (see Fig. 3). Because bacterial internalization occurs shortly (≈10 min) after infection (26), these experiments also indicate that Salmonella must traffic within the cell for at least ≈2 h to reach an intracellular compartment capable of providing the cues for cdtB expression. Taken together, these results indicate that both CDT activity and the expression of the cdtB gene require S. typhi internalization into host cells and that bacteria must presumably reach a specific compartment to produce CDT activity.
Fig. 6.
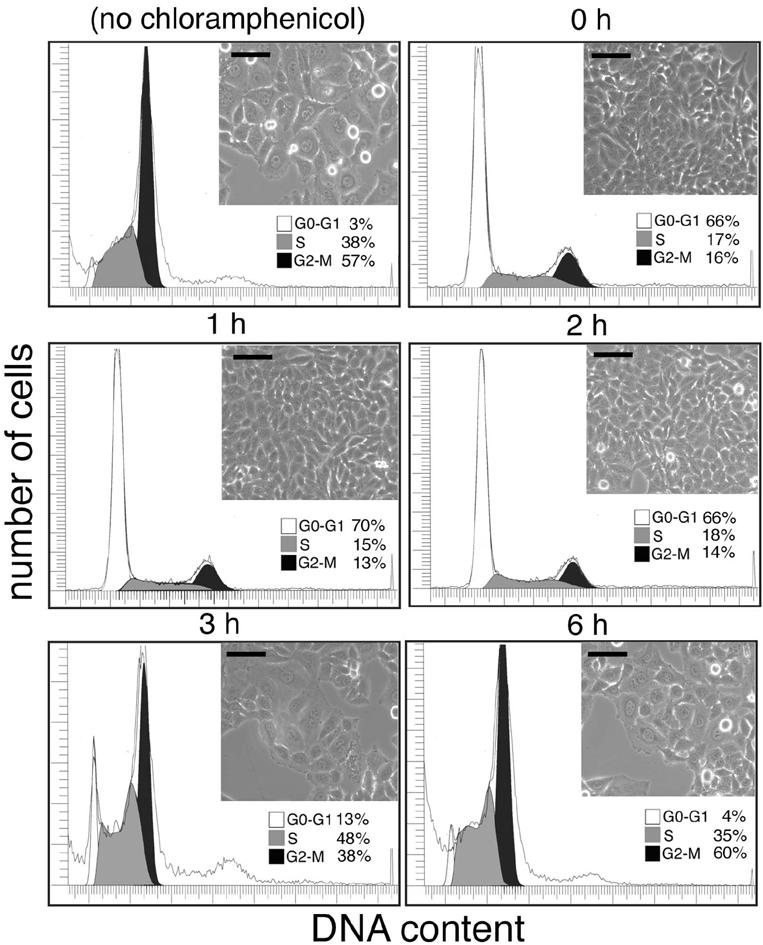
Effect of bacterial protein synthesis inhibition on the display of CDT activity by S. typhi. Cultured intestinal Henle-407 cells were infected with the S. typhi strain, and the bacterial protein synthesis inhibitor chloramphenicol was added at different times after infection as indicated. Cells were examined 72 h after infection under a phase microscope for signs of intoxication or processed to measure DNA content by flow cytometry as indicated in Materials and Methods. The peaks corresponding to cells in G0/G1 (G0-G1), S, or G2 (G2-M) are indicated. (Scale bar, 50 μm.)
Discussion
S. typhi is the causative agent of typhoid fever, a very serious disease that claims the lives of ≈500,000 people per year, the majority in developing countries (27). A distinguishing feature of this Salmonella enterica serovar is that humans are the only naturally susceptible species. The molecular bases for this remarkable host adaptation are not known. We have described here a cytotoxic activity in S. typhi that is identical to the CDT described in other important bacterial pathogens (8). The CDT activity was strictly dependent on the function of a protein with a high degree of sequence similarity to CdtB, the active or A subunit of the CDT. Remarkably, and unlike any other pathogens encoding CDT, S. typhi does not encode the two essential subunits of this toxin, CdtA and CdtC. These two subunits form the heteromeric B subunit that helps the delivery of the active subunit CdtB to its place of action (9). In other bacterial pathogens, it has been shown that the CDT holotoxin is internalized into target cells by means of receptor-mediated endocytosis. Experiments in other pathogens have suggested that after internalization CDT travels deep into the endocytic pathway and, similar to ricin or Shiga toxins, may reach the endoplasmic reticulum (ER) after retrograde transport (12). By analogy to other toxins (28), it is presumed that the CdtB subunit is delivered from the ER to the cytosol by retrotranslocation to finally reach the nucleus, its place of action.
In the absence of CdtA and CdtC, how does S. typhi deliver CdtB into the target cell? One potential mechanism by which CdtB could be delivered into the cytosol would be through a TTSS. Although S. typhi encodes two such systems (15, 16), our results indicate that the SPI-1 or SPI-2 TTSS does not mediate the delivery of CdtB into the cytosol (see Fig. 8, which is published as supporting information on the PNAS web site). This observation is consistent with the observation that, unlike TTSS-secreted proteins (29, 30), CdtB possesses at its amino terminus a canonical sec-dependent signal sequence, which is essential for its secretion and delivery (see Supporting Materials and Methods). The observation that cdtB expression and CDT activity are strictly dependent on bacterial internalization suggests a model of toxin delivery that would be consistent with the absence of CdtA and CdtC homologous toxin subunits in S. typhi. This model proposes that S. typhi synthesizes and secretes CdtB once it has reached an intracellular compartment where the toxin can be either retrotranslocated to the cytosol or transported to a compartment where retrotranslocation can take place (see Fig. 9, which is published as supporting information on the PNAS web site). The model would require that S. typhi traffic to an ER-like compartment or a compartment from where CdtB could be transported, directly or indirectly, to the ER. In addition, according to this model, CdtB would have to have all of the necessary information to be engaged by the retrotranslocation machinery. Subsequent to internalization, Salmonella deviates from the canonical endocytic pathway that leads to lysosomes, reaching an unusual membrane-bound compartment where it can survive and replicate (31, 32). Although it is not clear whether the Salmonella-containing vacuole (SCV) intersects with the ER at some stage during its maturation, recent studies have reported the presence of ER markers in this vacuole (33). Alternatively, the SCV may intersect with other compartments such as the Golgi apparatus, from where CdtB could be retrograde-transported into the ER. CdtA and CdtC, like other B subunits, mediate the initial stages of CdtB delivery, such as receptor-mediated endocytosis. Through bacterial internalization, S. typhi may bypass the requirements for CdtA and CdtC to deliver CdtB, providing an explanation for the absence of homologs of these proteins in this bacterial pathogen. More experiments are required to test the details and validity of this model.
Is it possible that other toxins may be delivered by this mechanism? Although no evidence substantiates this hypothesis, it is clear that many intracellular pathogens intersect with the ER or the Golgi apparatus (34), and therefore it is possible that some proteins secreted at those locations may find their way into the cytosol, provided that they can be engaged by the retrotranslocation machinery. It is also intriguing that the S. typhi genome contains an ORF capable of encoding a protein with significant similarity to the ADP-ribosylating active subunit of the pertussis toxin but does not seem to contain genes to encode the putative homologs of the B components of this toxin. Interestingly, this gene is encoded immediately adjacent to cdtB.
What is the function of CDT in S. typhi pathogenesis? It is intriguing that S. typhi is the only Salmonella serovar that encodes CdtB. Because S. typhi is an exclusively human pathogen, it is tempting to speculate that CDT plays a role in aspects of host-pathogen interactions that are unique to the human host. A unique feature of S. typhi is its ability to cause long, persistent infections in humans. It is possible that CDT may be involved in some aspects of the establishment of persistent infection, because, at least in other bacteria, this toxin has been shown to possess immunomodulatory activities (35). More experiments will be required to investigate this hypothesis.
Supplementary Material
Acknowledgments
We thank Marìa Lara-Tejero and members of the Galán laboratory for careful review of the manuscript. This work was supported by National Institutes of Health Public Health Service Grant GM52543.
Abbreviations: CDT, cytolethal distending toxin; S. typhi, Salmonella enterica serovar Typhi; TTSS, type III secretion system; DAP, diaminopimelic acid; ER, endoplasmic reticulum.
References
- 1.Johnson, W. M. & Lior, H. (1988) Microb. Pathog. 4, 103-113. [DOI] [PubMed] [Google Scholar]
- 2.Johnson, W. M. & Lior, H. (1988) Microb. Pathog. 4, 115-126. [DOI] [PubMed] [Google Scholar]
- 3.Peres, S., Marches, O., Daigle, F., Nougayrede, J., Herault, F., Tasca, C., De Rycke, J. & Oswald, E. (1997) Mol. Microbiol. 24, 1095-1107. [DOI] [PubMed] [Google Scholar]
- 4.Okuda, J., Kurazono, H. & Takeda, Y. (1995) Microb. Pathog. 18, 167-172. [DOI] [PubMed] [Google Scholar]
- 5.Cope, L., Lumbley, S., Latimer, J., Klesney-Tait, J., Stevens, M., Johnson, L., Purven, M., Munson, R. J., Lagergard, T., Radolf, J. & Hansen, E. (1997) Proc. Natl. Acad. Sci. USA 94, 4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugai, M., Kawamoto, T., Peres, S., Ueno, Y., Komatsuzawa, H., Fujiwara, T., Kurihara, H., Suginaka, H. & Oswald, E. (1998) Infect. Immun. 66, 5008-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young, V. B., Knox, K. A. & Schauer, D. B. (2000) Infect. Immun. 68, 184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lara-Tejero, M. & Galán, J. E. (2002) Trends Microbiol. 10, 147-152. [DOI] [PubMed] [Google Scholar]
- 9.Lara-Tejero, M. & Galán, J. E. (2001) Infect. Immun. 69, 4358-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara-Tejero, M. & Galán, J. E. (2000) Science 290, 354-357. [DOI] [PubMed] [Google Scholar]
- 11.Elwell, C. A. & Dreyfus, L. A. (2000) Mol. Microbiol. 37, 952-963. [DOI] [PubMed] [Google Scholar]
- 12.Cortes-Bratti, X., Chaves-Olarte, E., Lagergard, T. & Thelestam, M. (2000) Infect. Immun. 68, 6903-6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott, D. & Kaper, J. (1994) Infect. Immun. 62, 244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickett, C., Cottle, D., Pesci, E. & Bikah, G. (1994) Infect. Immun. 62, 1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkhill, J., Dougan, G., James, K. D., Thomson, N. R., Pickard, D., Wain, J., Churcher, C., Mungall, K. L., Bentley, S. D., Holden, M. T., et al. (2001) Nature 413, 848-852. [DOI] [PubMed] [Google Scholar]
- 16.Deng, W., Liou, S., Plunkett, G., III, Mayhew, G., Rose, D., Burland, V., Kodoyianni, V., Schwartz, D. & Blattner, F. (2003) J. Bacteriol. 185, 2330-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galán, J. E. & Curtiss, R., III (1991) Infect. Immun. 59, 2901-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galán, J. E., Nakayama, K. & Curtiss, R., III (1990) Gene 94, 29-35. [DOI] [PubMed] [Google Scholar]
- 19.Galán, J. E., Ginocchio, C. & Costeas, P. (1992) J. Bacteriol. 17, 4338-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochman, H., Soncini, F. C., Solomon, F. & Groisman, E. A. (1996) Proc. Natl. Acad. Sci. USA 93, 7800-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaniga, K., Bossio, J. C. & Galán, J. E. (1994) Mol. Microbiol. 13, 555-568. [DOI] [PubMed] [Google Scholar]
- 22.Guzman, L. M., Belin, D., Carson, M. J. & Beckwith, J. (1995) J. Bacteriol. 177, 4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaniga, K., Tucker, S. C., Trollinger, D. & Galán, J. E. (1995) J. Bacteriol. 177, 3965-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, S. J., Worrall, A. F. & Connolly, B. A. (1996) J. Mol. Biol. 264, 1154-1163. [DOI] [PubMed] [Google Scholar]
- 25.Evans, S. J., Shipstone, E. J., Maughan, W. N. & Connolly, B. A. (1999) Biochemistry 38, 3902-3909. [DOI] [PubMed] [Google Scholar]
- 26.Galán, J. E. (2001) Annu. Rev. Cell Dev. Biol. 17, 53-86. [DOI] [PubMed] [Google Scholar]
- 27.Pang, T., Bhutta, Z. A., Finlay, B. B. & Altwegg, M. (1995) Trends Microbiol. 3, 253-255. [DOI] [PubMed] [Google Scholar]
- 28.Sandvig, K. & van Deurs, B. (2002) Annu. Rev. Cell Dev. Biol. 18, 1-24. [DOI] [PubMed] [Google Scholar]
- 29.Galán, J. E. & Collmer, A. (1999) Science 284, 1322-1328. [DOI] [PubMed] [Google Scholar]
- 30.Cornelis, G. R. & Van Gijsegem, F. (2000) Annu. Rev. Microbiol. 54, 735-774. [DOI] [PubMed] [Google Scholar]
- 31.Holden, D. W. (2002) Traffic 3, 161-169. [DOI] [PubMed] [Google Scholar]
- 32.Gorvel, J. P. & Meresse, S. (2001) Microbes Infect. 3, 1299-1303. [DOI] [PubMed] [Google Scholar]
- 33.Gagnon, E., Duclos, S., Rondeau, C., Chevet, E., Cameron, P., Steele-Mortimer, O., Paiement, J., Bergeron, J. & Desjardins, M. (2002) Cell 110, 119-131. [DOI] [PubMed] [Google Scholar]
- 34.Roy, C. (2002) Trends Microbiol. 10, 418-424. [DOI] [PubMed] [Google Scholar]
- 35.Shenker, B., McKay, T., Datar, S., Miller, M., Chowhan, R. & Demuth, D. (1999) J. Immunol. 162, 4773-4780. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.