Abstract
BKM120, a pan class I PI3K inhibitor, was cytotoxic in the majority of primary B-chronic lymphocytic leukemia (CLL) lymphocytes, including samples from patients who have a high-risk for poor response to treatment (patient with del11 and del17) at clinically obtainable concentrations. The PI3Kδ inhibitor Cal-101 is cytotoxic in B-CLL lymphocytes in vitro and is active in the treatment of CLL in vivo. Interestingly, we demonstrated that BKM120 is 3.6 fold more toxic than Cal-101 in malignant B-CLL lymphocytes in vitro. BKM120 cytotoxicity correlated with the basal expression of proteins involved in the PI3K/Akt pathway. A protein signature of PI3K pathway proteins predicts the response to BKM120 treatment. In the primary B-CLL lymphocytes tested in vitro, BKM120 decreased the phosphorylation status of molecular biomarkers used as indicators of PI3K pathway inhibition in vivo. Also, BKM120 induced apoptosis in primary B-CLL cells culture in the presence and absence of stromal cell support. Our findings suggest that BKM120 should be tested clinically in CLL.
Keywords: chronic lymphocytic leukemia, BKM120, PI3K, leukemia therapy, CLL apoptosis
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of malignant B lymphocytes expressing the surface antigens CD19, CD20, CD23 and CD5.1 These B-CLL cells display altered apoptosis that is caused by both primary tumor features and co-dependent stromal elements.2 Current treatments for this disease include chemotherapeutic [alkylating agents (chlorambucil, cyclophosphamide, bendamustine)], purine analogs (Fludarabine) and immunotherapeutic agents (Rituximab, Alemtuzumab) or the combination of immunotherapy with chemotherapeutics drugs [the gold standard for CLL treatment is now fludarabine, cyclophosphamide and rituximab (FCR)]. However, none of these standard treatments result in curative therapy supporting the need for the investigation of new therapeutic targets and drug development in CLL as well as markers that can predict the clinical response to these treatments.3
The B-cell receptor (BCR) signaling has been considered critical for B-CLL survival and recent studies demonstrated that inhibition of the Bruton tyrosine kinase (BTK), a BCR associated kinase, blocked chemokines signaling leading to inhibition of CLL cell migration and survival resulting in CLL regression.4,5
The phosphoinositide 3-kinase (PI3K) cascade is also a critical component of survival signaling including PI3K-activated Akt (phosphorylated Akt) which inhibits cell death pathways by inactivating pro-apoptotic proteins such as Bad, procaspase-9 and members of the Forkhead transcription factor family. Overexpression of PI3K appears to play a critical role in B-CLL cell survival. Freshly isolated CLL lymphocytes have high levels of NF-κB activity and loss of NF-κB and Akt activity is associated with apoptosis.6–8 Cal-101 is a specific inhibitor of the delta isoform of phosphatidylinositol-3-kinase; the delta isoform is expressed primarily in hematopoietic lineages and is functionally important in B cells. Preclinical data suggest that Cal-101 is cytotoxic to CLL in vitro.8 At the 2010 ASH meeting, results were presented of a phase I study of 37 patients with CLL who were treated with Cal-101.9 Patients had a median of five prior regimens; 65% were refractory to their most recent prior regimen; 81% had bulky lymphadenopathy; and 36% of the patients had del17. All patients showed reduction in lymphadenopathy with 80% achieving a nodal response. However, concomitant with the decrease in lymphadenopathy there was an increase in lymphocytosis in about 60% of patients over the first 1–2 months of therapy. This lymphocytosis appeared to represent a redistribution of lymphocytes from lymph nodes and bone marrow to peripheral blood; it generally peaked by month 2, after which it slowly resolved. Patients generally experienced significant clinical benefit from the improvement in lymphadenopathy, with no negative consequences from the lymphocytosis. The lymphocytosis did limit the response rate, however, to 26%. Thus, Cal-101 is active in the treatment of refractory CLL. Currently, the pan class I PI3K inhibitor BKM120 developed by Novartis is under investigation in phase I and II clinical trials in advanced solid tumor patients8,10 (clinicaltrials.gov).
Material and Methods
Patients
Sixty-five patients with a diagnosis of B-CLL followed at the Jewish General Hospital of Montreal were enrolled in the study after informed consent. Patients were either clinically untreated (n = 36) or treated with chemotherapy (n = 29) for various time periods (Supporting Information Table 1).
Cell lines
The three B-CLL cell lines JVM2, EHEB and MEC2 (DSMZ, Braunschweig, Germany) were maintained in RPMI (Invitrogen, Carlsbad, CA), complemented with 10% fetal bovine serum (FBS). BMS2 stromal cell line were maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% FBS.
Cytotoxicity assay
Lymphocytes were isolated from the peripheral blood using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) as described.11 The isolated lymphocyte population was 97.85 ± 1.72% malignant B-lymphocytes (expressed as a mean % ± S.D.). The CLL lymphocytes (3 × 106 cells/ml) were treated with various concentrations of BKM120 (0.2–20 μM) (Novartis Pharma AG, Basel, Switzerland) or Cal-101 (0.4–50 μM) (LC Laboratories, Woburn, MA). Control samples were incubated with the greatest volume of DMSO. The MTT assay was performed 72 h after treatment as previously described12 and the cytotoxic effect of the drug presented as the IC50 (the drug concentration resulting in 50% of control).
Western blot analysis
Cell lysates (50 μg/sample) and protein migration were obtained as described before.13 The antibodies utilized were: 4E-BP1, 4E-BP1 (Thr37/46), Akt, Akt (Ser473), mTor, p70S6K, p70S6K (Thr389), PTEN, raptor and rictor (Cell signalling Technology, Danvers, MA) and actin, (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were developed using the appropriate HRP-secondary antibodies [anti-mouse (GE Healthcare, Piscataway, NJ), anti-rabbit (KPL, Gaithersburg, MD) or anti-goat (Santa Cruz)] and ECL (GE Healthcare). Protein levels were quantified by densitometry with Scion image software (Scion Corporation, Frederick, MA) and normalized to actin or the total protein expression for the phosphorylated form of the protein.
Apoptosis assay
For this assay, 3 × 106 cells were treated with the DMSO or BKM120 IC50 in the presence or absence of stromal cell for 24 hr. The induction of apoptosis was determined using the APC AnnexinV/Dead cell apoptosis kit (Invitrogen).
Statistical analysis
The Pearson Product Moment Correlation and t-testing were used for the correlative analysis of predictive biomarkers. The protein expression correlation with BKM120 cytotoxicty with the best p values were utilized to generate Figure 1c. Statistical analyses were performed by Friedman Repeated Measures Analysis of Variance on Ranks followed by Bonferroni t-test with SigmaStat software (Systat Software, San Jose, CA).
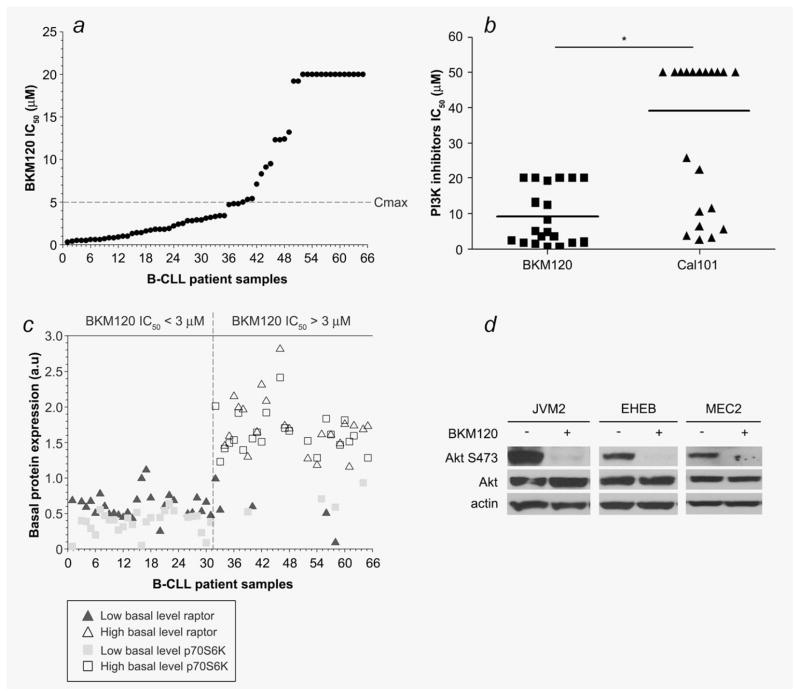
Figure 1.
The MTT assay was used (a) to determine the cytotoxicity of BKM120 in primary lymphocytes from 65 B-CLL patients and (b) compare the cytotoxic effect of the two PI3K inhibitors BKM120 and Cal-101 in cells from 20 B-CLL patients. (c) The basal protein expression of raptor and p70S6K were analyzed by western blot in 54 B-CLL samples. For each protein, samples were segregated in two groups (the mean expression value for each protein was used as cut-off) and correlated with the BKM120 IC50. (d) Akt phosphorylation (Akt S473) was assessed by western blot analysis in three B-CLL cell lines 24 hr after BKM120 treatment. *: p < 0.0001.
Results and Discussion
In view of the critical role of PI3K in CLL homeostasis, the activity of BKM120 was examined in primary B-CLL lymphocytes and in B-CLL cell lines. The in vitro cytotoxic effect of BKM120 was assessed in 3 B-CLL cell lines and in primary B-lymphocytes isolated from the 65 B-CLL patients enrolled in our study (Supporting Information Table 1) utilizing the MTT assay. The IC50 (drug concentration resulting in 50% cell death) obtained in the B-CLL cell lines JVM2, EHEB and MEC2 were 0.9 ± 0.1, 0.7 ± 0.1 and 0.7 ± 0.1 μM, respectively. BKM120 was cytotoxic (IC50 below the maximum concentration (20 μM) of BKM120 used in the MTT assay) in 78% of the primary B-CLL lymphocytes samples tested. There are subsets of patients such as those with 17p (del17) or 11q (del11) deletions, who have a high-risk for poor response to treatment.14 In our study, BKM120 is cytotoxic in patients’ samples harboring these deletions (Supporting Information Tables 1–2, Supporting Information Fig. 1). In the phase I clinical study, the maximum plasma concentration (Cmax) of BKM120 obtained after administration of the maximum tolerated dose of the drug was 5 μM.15 Interestingly, 60% of the B-CLL samples tested in our study have an IC50 below the Cmax. Furthermore, five of six patient samples with del11 or del17 have a clinically achievable IC50. These results indicated that BKM120 may be useful as a single agent in CLL therapy (Fig. 1a, Supporting Information Table 2). Previous studies have shown that the PI3Kδ inhibitor CAL-101 is cytotoxic in B-CLL lymphocytes in vitro.8,16 We therefore compared, by MTT assay, both PI3K inhibitors for their potency to induce cell death in 20 primary B-CLL cells samples and demonstrated that BKM120 is 3.6 fold more toxic than CAL-101 in malignant B-CLL lymphocytes in vitro (Fig. 1b, Supporting Information Table 2). Furthermore, BKM120 is cytotoxic (IC50 < 20 μM) in 80% of these 20 samples tested while CAL-101 is only cytotoxic (IC50 < 50 μM) in 45% of these samples. CLL has a widely variable response to therapy. Thus, we were interested in identifying molecular features that can predict response to BKM120 treatment. We analyzed our samples for somatic mutations in the immunoglobulin variable region (IgVH) genes and CD38 expression profiles as well as the basal expression of proteins involved in the PI3K/Akt pathway. Data Analysis with the Pearson product moment correlation test demonstrated positive correlations between BKM120 cytotoxicity and the basal expression of Akt (r = 0.592, p = 2.468E-06, n = 54), rictor (r = 0.418; p = 1.65E-03; n = 54), raptor (r = 0.463; p = 4.5E-03; n = 54), p70S6K (r = 0.584, p = 3.561E-06, n = 54) and 4E-BP1 (r = 0.371, p = 5.75E-03, n = 54) but not with PTEN, mTor, IgVH or CD38 expression. To further analyze these predictive markers, we used the mean expression value for each protein as a cut-off and, segregated the samples in two groups, samples with low level of basal protein expression (below the cut-off) and high level of basal protein expression (above the cut-off). We then simultaneously examine these different correlative markers together. We demonstrated that all patients with a BKM120 IC50 ≤ 3 μM expressed low level of raptor and p70S6K (Fig. 1c). Thereby, simultaneous expression of low basal level of these two proteins may be useful to predict response to BKM120.
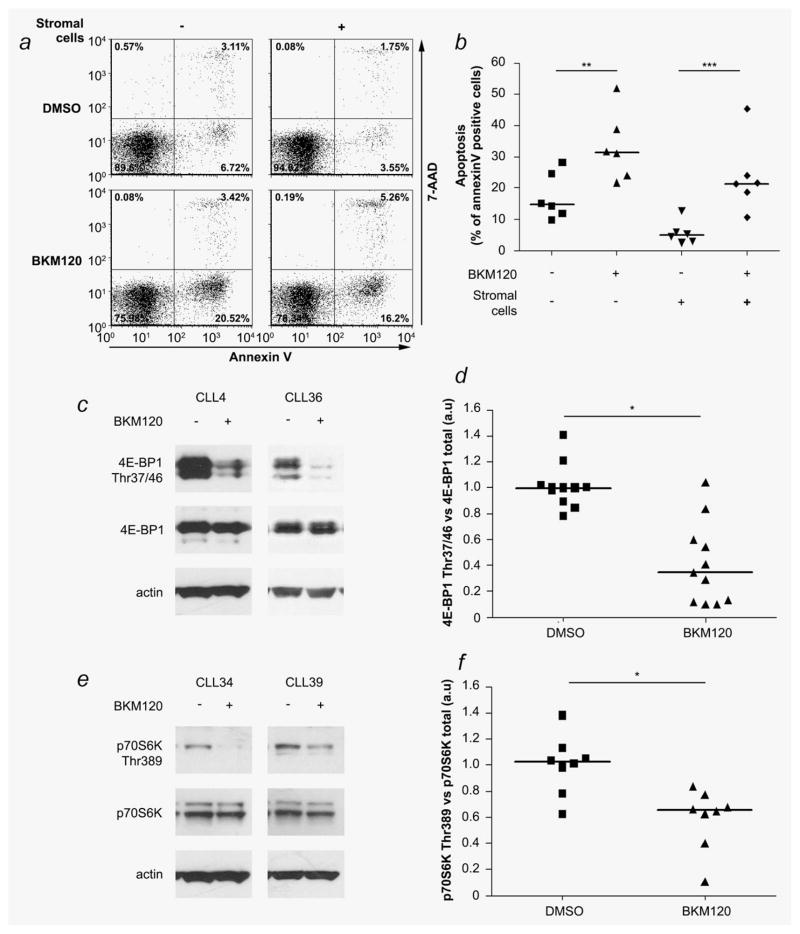
To determine whether the cytotoxic effect observed in our study was related to BKM120-induced PI3K inhibition, we investigated the phosphorylation status of Akt, a direct downstream target of PI3K in the three cell lines. In all cell lines, including the p53 mutated cell line MEC2, BKM120 inhibits Akt phosphorylation (Akt S473) 24 hr after treatment (Fig. 1d). In vitro studies have identified that stromal cells promoted cell survival and drug resistance of B-CLL lymphocytes by cell–cell interaction and secretion of chemokines.17 Furthermore, bone marrow microenvironment modulates the PI3K/Akt pathway and prevents apoptosis of primary CLL lymphocytes.18 To determine whether stromal cells can protect B-CLL against BKM120 activity, six primary B-CLL samples were tested for AnnexinV/7-AAD staining 24 hr after BKM120 treatment in the presence or absence of the murine stromal cells BMS2. In the absence of BMS2 stromal cell support, BKM120 induced apoptosis in the six primary B-CLL lymphocytes samples tested (mean AnnexinV positive cells: DMSO = 17.2 ± 3.7% vs. BKM = 33.2 ± 5.5%; p < 0.001). As previously described17 the stromal cells protected B-CLL lymphocytes from apoptosis (DMSO = 17.2 ± 3.7% vs. BMS2 + DMSO = 5.6 ± 1.1%; p = 0.004) but were not able to protect these malignant lymphocytes against BKM120 (BMS2 + DMSO = 5.6 ± 1.1% vs. BMS2 + BKM120 = 23.6 ± 5.8%; p = 0.005) (Figs. 2a and 2b). The same results were obtained when we assessed the cleavage of caspase-3 (Supporting Information Fig. 2). There is increasing evidence that the major cause of resistance to therapy in CLL patients is a consequence of a small number of leukemic cells that remain. This phenomenon, called minimal residual disease (MRD), is in part a consequence of protection conferred by the stromal microenvironment to the malignant lymphocytes and eradication of MRD by alemtuzumab treatment improved overall and treatment-free survival in CLL patients.17,19 Interestingly, our results demonstrated that the stromal cell microenvironment can not protect B-CLL lymphocytes from apoptosis induced by BKM120 treatment.
Figure 2.
The potency of BKM120 to induce apoptosis in primary B-CLL lymphocytes in the presence or absence of the BMS2 stromal cells support was analyzed by flow cytometry. (a) Dot plot example for patient CLL33. (b) Scatter plot analysis of the AnnexinV expression in six B-CLL samples. (c) Western blot analysis of the phosphorylation status of 4E-BP1 (Thr37/46) in B-lymphocytes (patients CLL4 and CLL36) 24 hr after BKM120 treatment. (d) Scatter plot of the 4E-BP1 Thr37/46 phosphorylation in 11 CLL samples. (e) Analysis of p70S6K Thr389 in B-lymphocytes (patients CLL34 and CLL39) after BKM120 treatment. (f) Scatter plot of the expression profile of p70S6K 24 hr after BKM treatment in eight B-CLL samples. *: p < 0.0001, **: p < 0.001, ***: p < 0.005.
Molecular biomarkers such as p70S6K and 4E-BP1 have been used as indicators of PI3K pathway inhibition in vivo and BKM120 inhibits p70S6K phosphorylation in rat fibro-blast cell lines in vitro.20,21 We demonstrated that the BKM120 IC50s correlated with basal p70S6K and 4E-BP1 protein expression in primary B-CLL lymphocytes. We then assessed in vitro the phosphorylation status of these two proteins used as biomarkers in vivo, in 8 (p70S6K T389) and 11 (4E-BP1 T37/46) primary B-CLL samples after treatment with BKM120. Results demonstrated that after 24 hr treatment, BKM120 decreased the phosphorylation status of 4E-BP1 (Figs. 2c and 2d) and p70S6K (Figs. 2e and 2f).
Although many patients are asymptomatic at diagnosis, CLL is a progressive disease and patients eventually require treatment. The main problems during the treatment of patients living with CLL are the side effects of therapies and the resistance developed by malignant cells after these treatments. Thereby, no current standard treatment option results in curative therapy, and all patients eventually die. Taken together, our results demonstrated that the class I PI3K inhibitor BKM120 may be useful as a single agent in CLL patients independently of their IgVh mutational status, CD38 expression or genomic deletions (del11 and del17). Furthermore, a combination of different biomarkers (expression of raptor and p70S6K) may predict the response to BKM120 treatment. Also, BKM120 abolished the protection against apoptosis and drug resistance conferred by the microenvironment to the primary B-CLL lymphocytes in vitro. Our preclinical study demonstrated that BKM120, a molecule with acceptable clinical toxicity,15 may be useful in the treatment of CLL.
Supplementary Material
What’s new?
None of the standard treatments for chronic lymphocytic leukemia (CLL) result in curative therapy. In view of the critical role of PI3K in CLL homeostasis, here the authors examine the activity of the pan class I PI3K inhibitor BKM120. They find that clinically achievable concentrations of BKM120 have antitumor activity in CLL lymphocytes associated with down-regulation of the PI3K pathway in vitro. They identify a protein signature predicting BKM120 sensitivity in CLL lymphocytes. Moreover, BKM120 presents significantly greater cytotoxicity than the PI3Kδ inhibitor Cal-101, which is especially relevant since Cal-101 has known antitumor activity in relapsed CLL patients.
Acknowledgments
The authors thank the Manitoba CLL tissue bank for the IgVH mutation analysis, Novartis for providing BKM120 and Dr Koren Mann for sharing the BMS2 cells.
Grant sponsor: Canadian Institutes of Health Research; Grant numbers: MOP-110991 to Lawrence Panasci and MOP-106528 to Raquel Aloyz
Abbreviations
- BCR
B-cell receptor
- BTK
Bruton tyrosine kinase
- CLL
chronic lymphocytic leukemia
- DMEM
Dulbecco’s modified eagle medium
- FBS
fetal bovine serum
- FCR
fludarabine, cyclophosphamide and rituximab
- MRD
minimal residual disease
- PI
phosphoinositide
References
- 1.Klein U, Tu Y, Stolovitzky GA, et al. Gene expression profiling of B cell chronic lymphocytic leukemia reveals a homogeneous phenotype related to memory B cells. J Exp Med. 2001;194:1625–38. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger JA, Tsukada N, Burger M, et al. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–63. [PubMed] [Google Scholar]
- 3.Gribben JG, O’Brien S. Update on therapy of chronic lymphocytic leukemia. J Clin Oncol. 2011;29:544–50. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Rooij MF, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119:2590–4. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 6.Ringshausen I, Schneller F, Bogner C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100:3741–8. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 7.Cuni S, Perez-Aciego P, Perez-Chacon G, et al. A sustained activation of PI3K/NF-kappaB pathway is critical for the survival of chronic lymphocytic leukemia B cells. Leukemia. 2004;18:1391–400. doi: 10.1038/sj.leu.2403398. [DOI] [PubMed] [Google Scholar]
- 8.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furman RR, Byrd JC, Brown JR, et al. CAL-101, An Isoform-Selective Inhibitor of Phosphatidylinositol 3-Kinase P110{delta}, Demonstrates Clinical Activity and Pharmacodynamic Effects In Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia. ASH Annual Meeting Abstracts. 2010;116:Abstract 55. [Google Scholar]
- 10.Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amrein L, Panasci L, Gibson SB, et al. Primary del 17 chronic lymphocytic leukaemia lymphocytes are hypersensitive to dasatinib in vitro. Br J Haematol. 2009;147:396–8. doi: 10.1111/j.1365-2141.2009.07814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amrein L, Loignon M, Goulet AC, et al. Chlorambucil cytotoxicity in malignant B lymphocytes is synergistically increased by 2-(morpholin-4-yl)-benzo[h]chomen-4-one (NU7026)-mediated inhibition of DNA double-strand break repair via inhibition of DNA-dependent protein kinase. J Pharmacol Exp Ther. 2007;321:848–55. doi: 10.1124/jpet.106.118356. [DOI] [PubMed] [Google Scholar]
- 13.Amrein L, Davidson D, Shawi M, et al. Dual inhibition of the homologous recombinational repair and the nonhomologous end-joining repair pathways in chronic lymphocytic leukemia therapy. Leuk Res. 2011;35:1080–6. doi: 10.1016/j.leukres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Grever MR, Lucas DM, Dewald GW, et al. Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: results from the US Intergroup Phase III Trial E2997. J Clin Oncol. 2007;25:799–804. doi: 10.1200/JCO.2006.08.3089. [DOI] [PubMed] [Google Scholar]
- 15.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–90. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 16.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayden RE, Pratt G, Roberts C, et al. Treatment of chronic lymphocytic leukemia requires targeting of the protective lymph node environment with novel therapeutic approaches. Leuk Lymphoma. 2012;53:537–49. doi: 10.3109/10428194.2011.610014. [DOI] [PubMed] [Google Scholar]
- 18.Shehata M, Schnabl S, Demirtas D, et al. Reconstitution of PTEN activity by CK2 inhibitors and interference with the PI3-K/Akt cascade counteract the antiapoptotic effect of human stromal cells in chronic lymphocytic leukemia. Blood. 2010;116:2513–21. doi: 10.1182/blood-2009-10-248054. [DOI] [PubMed] [Google Scholar]
- 19.Moreton P, Kennedy B, Lucas G, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23:2971–9. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Clarke PA, Workman P. Phosphatidylinositide-3-kinase inhibitors: addressing questions of isoform selectivity and pharmacodynamic/predictive biomarkers in early clinical trials. J Clin Oncol. 2012;30:331–3. doi: 10.1200/JCO.2011.38.7167. [DOI] [PubMed] [Google Scholar]
- 21.Maira SM, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–28. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.