Abstract
Purpose:
Vitamin K (phytonadione) is a commonly used first-line reversal agent for vitamin K antagonist (VKA) therapy in patients presenting with a supratherapeutic international normalized ratio (INR) with or without significant bleeding or in patients with a therapeutic INR in need of surgery. The purpose of this study was to determine the impact of education on the appropriate use of vitamin K for VKA reversal.
Methods:
Data were collected on patients admitted to a community teaching hospital during February 2010 (pre-education group). These data were analyzed to determine the most common guideline deviations in vitamin K use. Following this analysis, pharmacist education took place in the form of in-service presentations; a protocol, including a guideline-based dosing table, was developed to assist pharmacists in evaluating vitamin K therapy. Data were then collected on patients admitted during February 2011 (post-education group).
Results:
Forty patients and 47 vitamin K administrations were included in the pre-education group, and 34 patients and 49 vitamin K administrations were included in the post-education group. The number of patients with appropriate vitamin K administrations improved after pharmacist education (25% pre-education vs 55.8% post-education; P = .01). Whereas 27.6% of individual vitamin K administrations were appropriate in the pre-education group, this increased to 63.2% in the post-education group (P = .04).
Conclusion:
Education techniques on the appropriate use of vitamin K for VKA reversal significantly improved compliance with standards of care for proper use of vitamin K. Additional education sessions are necessary to further increase compliance with standards of care and subsequently optimize patient care.
Keywords: anticoagulation; anticoagulation reversal; phytonadione; vitamin K, warfarin
Warfarin has become the most widely prescribed outpatient anticoagulant in the world since being approved in 1954. The US Food and Drug Administration (FDA) has estimated that 2 million patients initiate warfarin therapy annually in the United States.1,2 This high utilization rate is most likely due to its wide array of approved indications such as prophylaxis and/or treatment of venous thrombosis and its extension and pulmonary embolism; prophylaxis and/or treatment of thromboembolic complications associated with atrial fibrillation and/or cardiac valve replacement; and reduction of risk of death, recurrent myocardial infarction, and thromboembolic events such as stroke or systemic embolization after myocardial infarction.3
Warfarin, and other vitamin K antagonist (VKA) therapy, acts by inhibiting clotting factors II, VII, IX, and X and proteins C and S.3 Warfarin inhibits the enzyme vitamin K epoxide reductase (VKOR), preventing the reduction of vitamin K, a step necessary for activation of clotting factors II, VII, IX, and X. The administration of supplemental vitamin K can overcome the effects of VKOR inhibition and reverse the anticoagulation present, therefore making vitamin K (phytonadione) the agent of choice for patients requiring reversal of anticoagulation with warfarin.
For most indications, warfarin is dosed to achieve a therapeutic international normalized ratio (INR) of 2.0 to 3.0.4 Patients with an INR of <2.0 are subtherapeutic and are considered to be at an increased risk of clotting events, whereas patients with an INR of >3.0 are considered supratherapeutic and at an increased risk of bleeding events. Well-designed trials have established the effectiveness of VKAs; however, its use in clinical practice continues to be an arduous task due to multiple reasons including its narrow therapeutic window, considerable variability in dose response among patients due to genetic and other factors, and numerous dietary and drug-drug interactions and the difficulty in standardizing laboratory control. Therefore, maintenance of a therapeutic level of anticoagulation requires both a good understanding of the pharmacokinetics and pharmacodynamics of warfarin and good patient communication.4 These challenges associated with warfarin therapy and additional barriers in the inpatient setting, including various procedures and surgeries, still necessitate the use of vitamin K to reverse the anticoagulant effects from warfarin.
It has been estimated that there are over 2 million adverse drug reactions in hospitalized patients annually, including more than 100,000 fatal adverse drug reactions.5 Warfarin is the second most common medication, only behind insulin, requiring visits to emergency departments due to adverse effects.6 The previously discussed challenges associated with warfarin therapy may be significant contributors to these estimates. This high prevalence of adverse drug reactions and use of vitamin K for reversal of VKAs warrant guideline recommendations and an evaluation of adherence to those guidelines.
The American College of Chest Physicians (ACCP) released guidelines outlining recommendations for the use of vitamin K for reversal of VKAs in 2008 and 2012.4,7 These recommendations are summarized in Table 1. Additional recommendations indicate that oral administration of vitamin K is more appropriate than subcutaneous administration for patients with mild to moderately elevated INRs without major bleeding (1A evidence).4 Due to the lipophilic properties of vitamin K, subcutaneous administration results in erratic absorption and extended durations of action. This can result in difficulties obtaining a therapeutic INR when VKA therapy is re-initiated.
Table 1.
Summary of 2008 and 2012 vitamin K administration recommendations from the American College of Chest Physicians4,7
| INR range | Intervention | Level of evidence |
| 2008 recommendations | ||
| INR <5 and no significant bleed | Lower or omit dose | 1C |
| INR 5 to 8.9 and no significant bleed | Omit 1 to 2 doses OR omit dose and give 1 to 2.5 mg vitamin K PO if at increased risk of bleeding OR vitamin K ≤5 mg PO may be given if rapid reversal required due to surgery | 1C |
| INR >9 and no significant bleed | Omit dose and give 2.5 to 5 mg PO vitamin K | 1B |
| Serious bleed | Omit dose and give 10 mg vitamin K by slow IV infusion; may supplement with FFP, PCC, or rVIIa. Vitamin K may be repeated in 12 hours if satisfactory results are not yet obtained. | 1C |
| Life-threatening bleed | FFP, PCC, or rVIIa + vitamin K 10 mg by slow IV infusion | 1C |
| INR <5 and no significant bleed | Lower or omit dose | 1C |
| 2012 recommendations | ||
| INR 4.5-10 and no evidence of bleed | Suggest against the routine use of vitamin K | 2B |
| INR > 10 and no evidence of bleed | Suggest oral vitamin K be administered | 2C |
| VKA-associated major bleeding | Rapid reversal with 4-factor PCC rather than plasma | 2C |
| Suggest additional use of vitamin K 5 to 10 mg administered by slow IV injection rather than reversal with coagulation factors alone | 2C | |
Note: FFP = fresh frozen plasma; INR = international normalized ratio; IV = intravenous; PCC = prothrombin complex concentrate; PO = by mouth; rVIIa = recombinant factor VIIa; VKA = vitamin K antagonist.
In January 2011, a 979-bed community teaching hospital implemented a new system for pharmacists to verify orders for vitamin K. Education by a student pharmacist and clinical pharmacists on the guideline recommendations at the time, ACCP 2008 (Table 1),7 and appropriate vitamin K use took place 1 month prior and was an integral component to this newly employed system. Implementation of this system provided an opportunity to improve patient safety and overall patient care. The evaluation of data pre and post vitamin K reversal education provides information on the effectiveness of the system and adherence to the ACCP guideline recommendations.
Methods
Institutional review board approval was obtained prior to commencement of this study, which was performed at a large, suburban, academic medical center. The primary objective was to determine the difference between the proportion of patients with appropriate vitamin K use during hospitalization in the pre-education and post-education groups. Secondary outcomes included the difference between the proportion of individual appropriate vitamin K administrations during hospitalization in the pre- and post-education groups, including a subanalysis of the appropriateness of the dose and route. Inappropriate vitamin K administration was defined as an incorrect dose, route, or both. Appropriate dose and route were defined according to the algorithm outlined in the 2008 ACCP guidelines (Table 1). An intravenous route was determined to be appropriate if it was indicated by the 2008 ACCP guidelines or if the patient was not tolerating oral therapy (ie, due to a gastrointestinal bleed, NPO status, or vomiting). Additional recommendations indicate that oral administration of vitamin K is more appropriate than subcutaneous administration for patients with mild to moderately elevated INRs without major bleeding (1A evidence),7 therefore a subcutaneous administration was not considered appropriate.
The pre-education study population was defined as all patients admitted to the hospital who received at least 1 dose of vitamin K between February 1, 2010, and February 28, 2010. The post-education study population was defined as all patients who received at least 1 dose of vitamin K between February 1, 2011, and February 28, 2011. Patients were excluded if they were younger than 18 years of age, received vitamin K for reasons other than to reverse a supratherapeutic INR due to warfarin use, received vitamin K as part of total parenteral nutrition supplementation, or were pregnant. Data collected included age, gender, indication for warfarin use, warfarin dose, INR at time of warfarin reversal, signs/symptoms of bleeding, indication for vitamin K administration (including medical procedures and surgeries), vitamin K dose, vitamin K route of administration, and administration of other reversal agents. To maximize confidentiality, specific patient identifiers were not recorded and all data were collected by one investigator.
A retrospective analysis of pre-education data by patient electronic medical records (EMR) was conducted prior to the implementation of educational strategies. These data were then analyzed to determine the most common guideline deviations in vitamin K use so that common errors could be targeted during education. The primary component of pharmacist education was in the form of pharmacy in-service presentations that highlighted the mechanism of action of vitamin K when used for warfarin reversal, the 2008 ACCP guidelines on the use of vitamin K, and pre-education data demonstrating poor rates of appropriate administrations. A document also was distributed to pharmacy staff that described vitamin K dose appropriateness according to the ACCP guidelines. Additionally, pharmacists were required to complete a pharmacist-to-pharmacist communication in the EMR (specifically an i-vent in the EPIC health system) to describe whether the dose and route were appropriate for the most recently documented INR value and whether any action was taken (eg, a call to a physician). Primary and secondary outcomes were analyzed by Fisher exact test. Demographic data were analyzed using Fisher exact test and Student t test. Statistical significance was defined a priori as a P value <.05.
Results
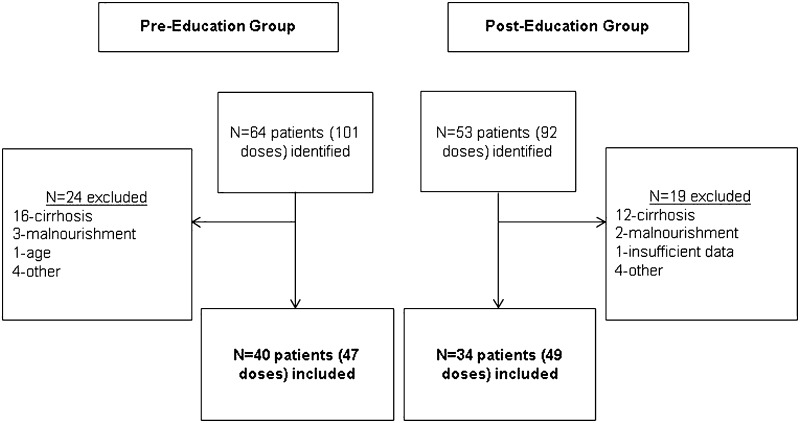
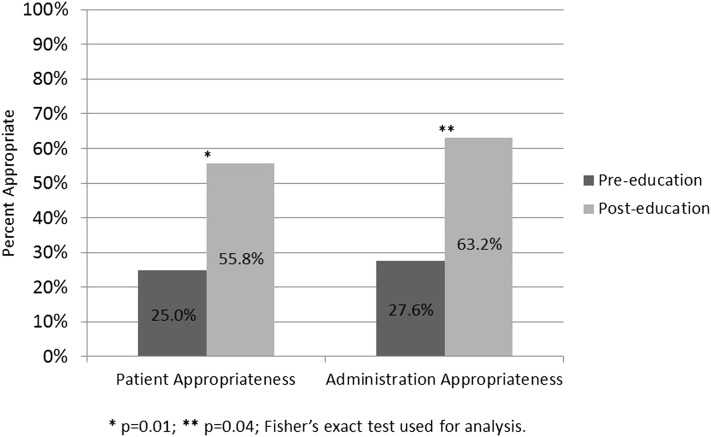
Forty patients and 47 doses were included in the pre-education group, and 34 patients and 49 doses were included in the post-education group (Figure 1). There was no difference in baseline characteristics between the pre- and post-education groups (Table 2). Twenty-five percent of patients in the pre-education group appropriately received vitamin K during hospitalization compared to 55.8% of patients in the post-education group (P = .009) (Figure 2). There was also an increase in the percentage of appropriate administrations in the post-education group compared to the pre-education group (63.2% vs 27.6%; P = .043) (Figure 2).
Figure 1.
Patient sample. <production: delete caption from figure file>
Table 2.
Baseline characteristics of the pre- and post-education groups
| Characteristics | Pre-education group (n=40) | Post-education group (n=34) | P value |
| Female, % | 45 | 47 | 1.0 |
| Mean age, years | 75 | 69 | 0.14 |
| Mean weekly warfarin dose, mg | 25.5 | 25.4 | 0.94 |
| Mean INR prior to vitamin K administration | 4.3 | 3.6 | 0.18 |
Note: Fisher exact test and Student t test were used for analysis. INR = international normalized ratio.
Figure 2.
Percentage of appropriate vitamin K use by patient or administration: pre- vs post-education. <production: delete caption from figure file>
In the subanalysis, the appropriateness of the doses administered and the combination of dose and route appropriateness improved in the post-education period (P = .002 and .021, respectively) (Table 3). There was no difference when route alone was compared between data collection periods (P = .06).
Table 3.
Inappropriate administrations of vitamin K in adult inpatients by route or dose
| Pre-education group (n=34) | Post-education group (n=18) | P value | |
| Dose | 19 | 6 | <.01 |
| Route | 1 | 7 | .06 |
| Both dose and route | 14 | 5 | .02 |
Note: Fisher exact test was used for analysis.
Discussion
The education techniques that were implemented in this project incorporated the standards of care for the appropriate use of vitamin K for VKA reversal. The overall compliance with the guidelines for the appropriate use of vitamin K for VKA reversal improved with this intervention. The significant consequences that can be associated with anticoagulant reversal still necessitate an overall higher rate of appropriateness. Insufficient anticoagulant reversal can result in prolonged anticoagulation and increased bleeding, whereas excessive reversal can increase difficulty in anticoagulating patients in the near future.
Poor compliance to guideline recommendations for the use of vitamin K for warfarin reversal has previously been observed.8 A retrospective evaluation performed in 2003 showed an overall compliance rate similar to the findings of this study at 17% when comparing the practices at one institution8 to the warfarin reversal guidelines set forth by the ACCP at the time. At our institution, we attempted to increase compliance through the implementation of educational techniques and improved order verification. In a similar study evaluating the value of vitamin K use education on the appropriateness of vitamin K administration to patients, researchers found that appropriate indication improved from 53% to 62% (P = .181) and appropriate dose improved from 44% to 46% (P = .876).9 With a dual (written and oral) mode of pharmacist education and implementation of a program for vitamin K order verification, we were able to significantly improve guideline compliance by 31%, with a 55.8% rate of compliance post education. This education strategy and clinical program implementation can be adapted to other institutions, which likely experience the same nonadherence with vitamin K use.
There are a few limitations to this study. First, data regarding complications of inappropriate reversal, either thromboembolism or persistent bleeding, were not collected. Also, information regarding inappropriate reversal necessitating a longer period of warfarin re-introduction and possibly longer lengths of stay was not collected. These data could have further added to the value of the education techniques. Second, the analysis occurred 2 months after the education. It is unclear whether the information that is provided, and therefore the compliance with the standards of care, can be maintained long-term and whether the effects that are seen may be confounded by the temporal relationship of the education. A repeat analysis may be able to demonstrate a change in compliance over time. As with any educational technique, routine repetition would assist with retention of information and implementation of the standards. Subsequent regularly scheduled education sessions that incorporate alternative educational techniques may be beneficial for furthering appropriateness. An alternative strategy would be providing physicians of the institution with the same materials as pharmacists. This would not only educate physicians on the guidelines for the use of vitamin K, but also allow for a more open communication should pharmacists have to contact the prescribing physicians. The physicians would then be more aware of the program. Third, due to the retrospective nature of this study, there is an increased risk of collection bias. No cause and effect relationship may be identified with this study design.
This study highlights options for future studies to examine VKA reversal. For example, there would most likely be benefit in determining whether agents such as recombinant activated factor VII, prothrombin complex concentrates, and fresh frozen plasma are utilized appropriately. As these agents are usually reserved for major bleeding cases and associated with high financial costs, the potential exists for a highly impactful program. A program designed specifically to improve appropriateness of these agents not only may have an impact on patient care, but also may have significant financial consequences. Also, as mentioned previously, an examination of the complications of inappropriate VKA reversal, thromboembolism, or persistent bleeding could assist institutions in determining their need for pharmacist and/or physician education. Additionally, the release of newer anticoagulants will create more situations that may require reversal. Agents such as rivaroxaban, apixaban, and dabigatran are becoming more widely available and approved for new indications. The reversal of some of these agents is controversial. For those agents with recognized reversal techniques, standards and guidelines could be developed, implemented, and assessed.
An important factor to consider with the results of this study is the release of the updated ACCP guidelines in 2012 (Table 1). Although many of the concepts for VKA reversal remained the same, there are slight differences in the recommendations that result in less aggressive prescribing habits of vitamin K. It would be interesting to determine whether physicians and pharmacists are appropriately adjusting their practice habits to fit the new standards or whether the former standards continue to be followed. Education on the new guidelines is necessary to ensure that patients are receiving care based on current evidence-based medicine. We believe that education on the new guidelines would assist compliance with standards of care, as was determined by this study.
Conclusion
Pharmacist education significantly increased compliance with standards of care for proper use of vitamin K for VKA reversal. The subanalysis revealed an improvement in appropriate dosing, but there was no significant improvement in appropriate route of administration of vitamin K for VKA reversal. Since the route of vitamin K administration was appropriate 98% of the time prior to implementation of the education techniques, it is unlikely that a significant difference would have been found. Appropriate utilization improved with the pharmacist educational techniques employed, but there is still room for improvement with overall appropriateness to optimize the care of patients requiring VKA reversal therapy. Routine education sessions utilizing innovative strategies are necessary to further increase compliance with standards of care and achieve this level of care.
REFERENCES
- 1.Kim MJ, Huang SM, Meyer UA, Rahman A, Lesko LJ. A regulatory science perspective on warfarin therapy: A pharmacogenetic opportunity. J Clin Pharmacol. 2009;49(2):138–146 [DOI] [PubMed] [Google Scholar]
- 2. US Food and Drug Administration. FDA approves updated warfarin (Coumadin) prescribing information. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108967.htm. Accessed on July 25, 2012.
- 3.Coumadin (warfarin) [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2011 [Google Scholar]
- 4.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (9th edition). Chest. 2012;141(2 Suppl):e152S-e184S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1205 [DOI] [PubMed] [Google Scholar]
- 6.Woodcock J. 2012 The critical path: Making medical products better, faster, and cheaper. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm061234.htm. Accessed on July 25,
- 7.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed.). Chest. 2008;133(suppl):160s-198s [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Armitstead JA, Adams AG, Davis GA. A retrospective evaluation of vitamin K1 therapy to reverse the anticoagulant effect of warfarin. Pharmacotherapy. 2003;23(10):1245–1250 [DOI] [PubMed] [Google Scholar]
- 9.Murphey LM, Byrd DC. Phytonadione education program in a community hospital. Hosp Pharm. 2003;38:458–462 [Google Scholar]