Abstract
Late infantile neuronal ceroid lipofuscinosis (LINCL), a fatal, lysosomal storage disorder caused by mutations in the CLN2 gene, results in a deficiency of tripeptidyl-peptidase I (TPP-I) activity in neurons. Our prior studies showed that delivery of the human CLN2 cDNA directly to the CNS, using an adeno-associated virus serotype 2 (AAV2) vector, is safe in children with LINCL. As a second-generation strategy, we have demonstrated that AAVrh.10hCLN2, a rhesus-derived AAV vector, mediates wide distribution of TPP-I through the CNS in a murine model. This study tests the hypothesis that direct administration of AAVrh.10hCLN2 to the CNS of rats and nonhuman primates at doses scalable to humans has an acceptable safety profile and mediates significant CLN2 expression in the CNS. A dose of 1011 genome copies (GC) was administered bilaterally to the striatum of Sprague Dawley rats with sacrifice at 7 and 90 days with no significant impact except for mild vector-related histopathological changes at the site of vector administration. A dose of 1.8×1012 GC of AAVrh.10hCLN2 was administered to the CNS of 8 African green monkeys. The vector-treated monkeys did not differ from controls in any safety parameter except for mild to moderate white matter edema and inflammation localized to the administration sites of the vector. There were no clinical sequelae to these localized findings. TPP-I activity was >2 SD over background in 31.7±8.1% of brain at 90 days. These findings establish the dose and safety profile for human clinical studies for the treatment of LINCL with AAVrh.10hCLN2.
Sondhi and colleagues demonstrate that administration of Adeno-Associated Viral vector serotype rh10 encoding the human CLN2 gene into the striatum of rats and the CNS of African Green Monkeys results in efficient expression of tripeptidyl peptidase I (TPP-I), with minimal adverse effects.
Introduction
Late infantile neuronal ceroid lipofuscinosis (LINCL) is a fatal childhood autosomal recessive disorder resulting from mutations in the CLN2 gene and the consequent deficiency in its product, tripeptidyl-peptidase I (TPP-I) (Boustany, 1996; Sleat et al., 1997; Rawlings and Barrett, 1999; Williams et al., 1999; Haltia, 2003). Loss of this lysosomal storage enzyme results in neurodegeneration throughout the CNS, marked by high levels of autofluorescent material in lysosomes (Ezaki et al., 1999; Vines and Warburton, 1999; Williams et al., 1999). In humans the disorder is rare, with approximately 350 to 800 known cases worldwide (Wisniewski et al., 2001). It typically presents by 2 to 4 years of age, and patients have a life expectancy of 8 to 12 years (Williams et al., 1999; Haltia, 2003; Worgall et al., 2007).
There is no specific treatment for LINCL other than symptomatic management (Williams et al., 1999; Wisniewski et al., 2001). Because the CNS manifestations of LINCL are global, a treatment strategy must restore enzymatic function in a large portion of the brain. We have developed a strategy using adeno-associated virus (AAV) gene transfer vectors to deliver the normal human CLN2 cDNA to neurons to provide persistent TPP-I expression in the CNS. Previously, we assessed adeno-associated virus serotype 2 (AAV2) to deliver functional human CLN2 cDNA to the CNS of children with LINCL (Crystal et al., 2004; Worgall et al., 2008). Although this therapy suggested a slowing of the progression of the disease as assessed by a neurological rating scale (Steinfeld et al., 2002; Worgall et al., 2008), there remains significant room for improvement, primarily in increasing the distribution of the transgene product and increasing its expression levels in the brain. To this end, our laboratory focused on identifying a gene therapy vector with a high capacity for broad distribution in the CNS, allowing for sustained, widespread expression of the transgene. These studies led to the identification of AAVrh.10, an AAV isolated from rhesus monkeys, as possessing the characteristics of broad CNS distribution and with the added advantage that it is nonhuman in origin, allowing it to bypass the anti-human AAV immunity that is common in the population (Gao et al., 2003; Cearley and Wolfe, 2006; Sondhi et al., 2007; Hu et al., 2010). When an AAVrh.10 vector encoding the human CLN2 cDNA is administered to the CNS of a mouse deficient in CLN2, there is a marked increase in survival compared with the control CLN2-deficient mice (Sleat et al., 2004; Sondhi et al., 2007, 2008).
The objective of the present study, designed with input from the Center for Biologics Evaluation and Research, Food and Drug Administration (Rockville, MD), was to move the AAVrh.10hCLN2 vector to human trials by determining the safety and efficacy of administration of AAVrh.10hCLN2 directly to the CNS of rats and nonhuman primates (NHPs). On the basis of the parameters assessed, including behavioral analysis, the data show no significant adverse effects of treatment, and widespread expression of the human TPP-I throughout the CNS of nonhuman primates.
Materials and Methods
Vector production
The genome of AAVrh.10hCLN2 includes the inverted terminal repeats (ITR) from AAV2 surrounding the expression cassette (Sondhi et al., 2007). The expression cassette consists of a cytomegalovirus (CMV)/β-actin hybrid promoter (Daly et al., 1999; Sondhi et al., 2005), the human CLN2 cDNA with an optimized Kozak translational initiation signal before the start codon, and a rabbit β-globin poly(A) sequence. The vector was produced under Good Manufacturing Practice (GMP) conditions by cotransfection of forty 150-mm plates of 293T cells (80% confluent) with 500 μg of an expression cassette plasmid (pAAV2-CAG-hCLN2) and 1 mg of an adenovirus/AAVrh.10 helper plasmid (pPAK-MArh.10), using PolyFect reagent (Qiagen Sciences, Germantown, MD). The helper plasmid included the AAVrh.10 cap gene and AAV2 rep gene necessary for viral reproduction and capsid production.
Cells were harvested (centrifugation at 1150×g, 15 min) 72 hr posttransfection and a crude viral lysate (CVL) was made by three cycles of freeze–thawing. Benzonase (50 U/ml; Sigma-Aldrich, St. Louis, MO) was used to remove any contaminant genomic DNA. The remaining CVL was centrifuged at 3300×g for 20 min and the supernatant was applied to a discontinuous iodixanol gradient. It was then purified by Q-HP ion-exchange chromatography and centrifugally concentrated into phosphate-buffered saline (PBS). Vector concentration, expressed as genome copies, was determined by TaqMan real-time PCR with absolute quantitation. To confirm functionality, 293-ORF6 cells were infected with AAVrh.10hCLN2, and TPP-I enzymatic activities were assessed in the cell supernatant 72 hr postinfection (Lin and Lobel, 2001; Sondhi et al., 2005).
All vectors used in the toxicology studies were shown to be sterile by growth for 14 days on medium supporting the growth of aerobic bacteria, anaerobic bacteria, or fungi; endotoxin free to a level of <200 U of endotoxin per milliliter by the Endosafe method (Charles River, Charleston, SC); and uncontaminated by mycoplasma to a level of <1000/ml, using a quantitative polymerase chain reaction assay (WuXi AppTec Pharmaceutical, Philadelphia, PA).
Rat toxicology study
The overall design involved administration of AAVrh.10hCLN2 at the maximal achievable dose (1011 genome copies [GC] divided into two equal, 5-μl aliquots) bilaterally into the striatum of rats. The control group received PBS instead of AAVrh.10hCLN2. Both groups were assessed on the basis of clinical, hematological, and chemical parameters and brain histopathology at acute (7-day) and medium (90-day) time points (Supplementary Table S1; supplementary data are available online at http://www.liebertpub.com/hgtb) was determined. Each group included n=10 rats, 5 males and 5 females.
All surgical procedures in rats were performed in accordance with National Institutes of Health (NIH, Bethesda, MD) biosafety guidelines and with prior institutional approval. For direct administration into the brain, male and female Sprague Dawley rats (160–200 g; Taconic, Germantown, NY) were placed in a rat stereotaxic frame. Administration coordinates were anteroposterior (AP), +0.6 mm; mediolateral (ML), +2.8 mm; and dorsoventral (DV), −5.2 mm relative to bregma for left striatum and AP, +0.6 mm; ML, −2.8 mm; and DV, −5.2 mm relative to bregma for right striatum. The AAV vectors, formulated in PBS (5.0 μl per site), were administered on day 0 through burr holes placed at the vector administration location via a fine-gauge needle at a rate of 0.2 μl/min, using a microprocessor-controlled infusion pump. The vector administration needle was left in position for 1 min before, and for 2 min after, delivery before being slowly withdrawn. Animals received either AAVrh.10hCLN2 (1011 GC divided equally between each striatal site) or PBS control. After vector administration, the skin incision was sutured. All rats were individually monitored after intraparenchymal administration and evaluated at least three times per week for the following clinical parameters: (1) breathing, (2) alertness, (3) reflex responsiveness, (4) excretory system function (presence of urine and fecal matter), (5) healing of incision site, (6) suture condition and absorption, and (7) overall health.
The animals were killed by CO2 inhalation 7 or 90 days after vector administration, immediately followed by intracardiac blood sample collection and tissue collection (Supplementary Table S1). The complete blood count (CBC) and blood chemistry analysis were performed by ALX Laboratories (New York, NY). The data were entered into spreadsheets, audited, and then plotted and evaluated to determine whether there were significant differences between test and control groups for the toxicology parameters. Gross observations of the surgical area were recorded. Routine histological assessment was done for the brain and distant organs, including skeletal muscle (quadriceps), heart, liver, spleen, pancreas, kidney, lung, urinary bladder, male and female reproductive organs, stomach and intestinal tract, fat, thymus and lymph nodes, skin, sciatic nerve, and bone, taken at the time of sacrifice from both vector-treated and control animals. All tissues were immersion fixed in 10% buffered neutral formalin, embedded in paraffin, and sectioned once at a thickness of 5 μm. In the CNS, the area of the striatum was contained in a single tissue block and four areas of the remaining brain, both proximal and distal to the striatum, were examined within separate blocks. All slides were stained with hematoxylin and eosin (H&E). The slides were assessed in a blinded fashion by a board-certified veterinary pathologist at the Laboratory of Comparative Pathology (Memorial Sloan-Kettering Cancer Center [MSKCC], New York, NY) for inflammation and neuropathology-related morphological characterization.
Nonhuman primate toxicology and efficacy study
The overall design involved administration of AAVrh.10hCLN2 into the CNS of eight medium-sized African green monkeys with assessment of hematological parameters and histopathology at acute (7-day, n=4) and medium (90-day, n=4) time points (Supplementary Table S2). The vector AAVrh.10hCLN2 (1.8×1012 GC) was delivered in a total of 180 μl divided equally among 12 loci, through 6 burr holes (3 per hemisphere). As a control, an additional animal received PBS instead of AAVrh.10hCLN2; all criteria, assessments, and timeline were the same as for the treated animals. This control animal was killed at the 7-day time point. In addition to the toxicology assessments at sacrifice, one-half of each NHP brain was used for assessment of TPP-I enzyme activity to determine the extent of vector-induced protein distribution.
Each nonhuman primate received intraparenchymal administration of a total dose of 1.8×1012 GC of the AAVrh.10hCLN2 vector. This dose was administered in 15 μl at each of 12 locations, for a total volume of 180 μl. Vector and control PBS administration was performed at predetermined sites (Supplementary Table S3). Targeted locations were determined by magnetic resonance imaging (MRI) and computed tomography (CT) scan imaging, and were chosen to include areas of both white and gray matter. In total, eight rostral sites and four caudal sites were used.
Monkeys were initially anesthetized with tiletamine and zolazepam (Telazol, 3.7 mg/kg; intramuscularly) and their heads were shaved. Preoperatively, each monkey received glycol pyrrolate intramuscularly and 2 cm3 of bupivacaine (0.25%) at the incision site. An arterial line was percutaneously inserted to monitor blood pressure and blood gas levels. An endotracheal tube was inserted and monkeys were monitored continually and anesthesia was maintained throughout the duration of the procedure, using isoflurane administered via a pediatric ventilator. After a sterile field was established, the animal was first set up in the stereotaxic head holder. Next, the midline incision was made and the skeletal muscles retracted to each side to expose the administration areas. Six burr holes (three symmetrical holes per hemisphere) were made corresponding to the administration sites. A stereotaxic carrier containing a Stoelting microsyringe injector with a Hamilton 710 series 100-μl syringe and a replaceable 2-inch, 26-gauge beveled needle was attached to the manipulator arm of the stereotaxic frame. Ninety microliters of the appropriate vector or PBS was then drawn up into the chamber. Gray matter was targeted first and then the needle was lowered to the site of the white matter. Once the needle was lowered to the target site, it was left in place for 1 min. Each administration then proceeded at a rate of 1 μl/min followed by 2-min wait. The needle was then withdrawn at a rate of 0.5 mm/min. To minimize time under anesthesia, administrations were performed simultaneously at two sites, using two manipulator arms. After the second administration, the burr holes were sealed with bone wax and BIOPATCH (2.5 cm/1 inch; Ethicon/Johnson & Johnson, Somerville, NJ) was applied to minimize infection, and the skin wound was closed. The monkey was removed from the stereotaxic holder and observed in the recovery area until it was awake, at which time it was returned to its cage. Monkeys were assessed three times per day during recovery from surgery and daily thereafter.
Before surgery, on the day of administration, and on days 3, 7, 15, 30, 60, and 90 postadministration (Supplementary Table S2), animals were sedated for general assessment (weight, temperature, pulse, respiratory rate) and for blood draw for CBC, serum chemistry, and anti-AAV neutralizing antibody titers (this was performed on a subset of the days cited above: presurgery, and days 7, 15, 30, 60 and 90 postsurgery). General safety assessment was made by the veterinary staff at Memorial Sloan-Kettering Cancer Center and routine CBC and serum chemistry analyses were performed by the Laboratory of Comparative Pathology (MSKCC). The toxicology assessments included behavioral assessments, determination of anti-AAVrh.10 neutralizing antibody titers, determination of blood safety parameters, and histopathology.
Behavioral assessment in nonhuman primates
Before surgery and 7, 15, 30, 60, and 90 days postsurgery, monkeys were assessed for behavior. On these days, animals were videotaped in the absence of stimuli and with specific food and threat scenarios (Supplementary Table S4) (Hackett et al., 2005). Behavioral assessment based on these recordings was done at the conclusion of the study with analysis of various behaviors, by an observer blinded to the treatment group and time of assessment (presurgery or days postsurgery).
Anti-AAVrh.10 neutralizing antibody titers
Anti-AAVrh.10 neutralizing antibody titers were assessed by mixing serial 2-fold dilutions of serum, starting at a 1:10 dilution, with 5×108 genome copies of AAVrh.10Luciferase for 30 min, 37°C. The mixture was then used to infect 293-ORF6 cells in a 96-well plate, as previously described (De et al., 2006). After growth for 48 hr, cells were lysed and assayed for luciferase activity. The reciprocal dilution required for 50% inhibition of infection was interpolated. In all assays, human serum with neutralizing titer cross-reactive to AAVrh.10 was included as standard.
Assessment of safety and histopathology
At the time of scheduled sacrifice, a board-certified veterinary pathologist performed the necropsy and organ extraction (Supplementary Table S2). The monkeys were first anesthetized with Telazol (3.7 mg/kg, intramuscularly) in order to make a general health assessment and collect blood. The monkey was then killed with a lethal dose of sodium pentobarbital, administered intravenously. The necropsy proceeded with collection of two small sections of each of the following organs immediately after euthanasia: liver, lung, biceps, kidney, gonads, seminal vesicles, testes, epididymides, skin, cervical spinal cord, spleen, trachea, esophagus, stomach, duodenum, jejunum, ileum, pancreas, gall bladder, adrenal gland, lymph node, heart, eyes, bone marrow, and any gross abnormalities. The sections were fixed in 10% neutral buffered formalin at room temperature for histopathology. In addition, the brain of each monkey was collected and bisected. The left half of the brain was sliced into 1-cm3 sections (referred to as cubes) for TPP-I enzyme activity assessment. Routine histological assessment was done on the remaining half of the brain. This half was fixed in 10% neutral buffered formalin at room temperature, as done for the other organs.
Histological assessments were carried out by the Laboratory of Comparative Pathology (MSKCC). Tissues were routinely processed and embedded in paraffin wax. Sections (5 μm) were stained with H&E and evaluated by light microscopy in a blinded fashion by a board-certified veterinary pathologist. The stained sections were carefully examined for evidence of neuronal death, axonal degeneration, or abnormal glial cell reactions. For the brain, the right half of the brain was sliced into coronal slabs (approximately 4 mm thick) along the rostral–caudal axis after fixation. These 4-mm hemicoronal sections were examined macroscopically for any abnormalities. The areas around the administration sites (rostral and caudal) and an area distal from these sites (cerebellum and brainstem) were paraffin embedded, sectioned, and stained with H&E. Brain sections were examined for lesions such as neuronal death, axonal degeneration, and abnormal glial cell reactions.
Efficacy assessment in nonhuman primates
To assess the CNS for TPP-I enzymatic activity, the left half of each brain was sliced into 1-cm coronal slabs immediately after extraction. Each hemicoronal slab was further sectioned into 1-cm3 cubes and stored at −80°C. Later, each cube was thawed and homogenized in 1.5 ml of lysis buffer (0.15 M NaCl, Triton X-100 [1 g/liter]), using a disposable pellet pestle and matching tube (Kimble-Kontes, Vineland, NJ). The homogenate was clarified by centrifugation and the supernatant was diluted 10-fold in PBS, pH 7.4, for TPP-I enzymatic activity assessment as described previously (Sondhi et al., 2007). TPP-I activity was adjusted to total protein concentration, using a bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL) and calculated as change in fluorescence units per minute per milligram protein.
Statistical analyses
In the rat study, p values were assessed by a three-way analysis of variance (ANOVA), using sex and treatment group as factors and time (7 and 90 days) as an independent variable. The reported p value is a test of the null hypothesis that there was no difference between the AAVrh.10hCLN2 and PBS groups for that parameter. A p<0.01 cutoff was chosen for significance to reduce the incidence of false positives. Because the rat toxicology study involved a total of 39 parameters, accepting this low cutoff value of significance greatly reduces the chance of incorrectly rejecting the null hypothesis.
In the design of the nonhuman primate study, one group (n=4) was killed 7 days after vector administration, whereas the other group (n=4) was killed at 90 days, allowing assessment of the acute and medium time point effects of treatment, respectively. As a control, one monkey was administered an equivalent volume of PBS at the same locations, with sacrifice 7 days postsurgery. Because there was only one control for each parameter assessed, reference ranges were established by calculating the range inclusive of 95% of the values from all of the nonhuman primates presurgery.
Results
Rat toxicology
The overall design of the rat toxicology study involved administration of AAVrh.10hCLN2 at maximal achievable dose bilaterally into the striatum of rats with assessment of hematological parameters and histopathology at acute (7-day) and medium (90-day) time points (Supplementary Table S1).
Overall health
No vector-related abnormalities were noted in triweekly health checks of clinical parameters including breathing rates, alert response, reflex, excretory system (urine and fecal matter), and suture absorption. All 7-day PBS- and vector-administered rats had normal incision healing at the surgical site. Two rats each for the 90-day PBS and 90-day vector time points had slow healing with some swelling, redness, and formation of crusts at the incision site, due to scratching, but this did not correlate with administration of vector.
General safety and routine blood parameters
At sacrifice, blood was collected for CBC and serum chemistry and multiple organs were collected, weighed, and prepared for histopathological examination. Comparison of samples from AAVrh.10hCLN2-administered rats and PBS-administered control rats showed no difference between the groups for any parameter. The total animal weight at sacrifice was independent of vector treatment (p>0.1). There was no difference between vector and vehicle groups for the weight of any organ relative to total body weight (p>0.2 for 11 organs examined; Table 1). Comparison of complete blood count between vector- and PBS-administered rats showed no difference between the groups (p>0.05 for 11 parameters; Table 1). Similarly, there was no difference between vector and control rats for comprehensive serum chemistry (p>0.04 for 16 parameters; Table 1).
Table 1.
Toxicology Parameters After AAVrh.10hCLN2 Administration to Rat Brain Compared with Phosphate-Buffered Saline Controls
|
General safety |
Complete blood count |
Serum chemistry |
|||
|---|---|---|---|---|---|
| Organ weight | p valuea | Parameter | p value | Parameter | p value |
| Total body weight | >0.1 | Hemoglobin | >0.5 | Glucose | >0.5 |
| Brain | >0.6 | Hematocrit | >0.6 | Urea nitrogen | >0.4 |
| Kidney | >0.4 | RBC | >0.6 | Creatinine | >0.7 |
| Gonads | >0.2 | WBC | >0.7 | Bilirubin | >0.6 |
| Lymph nodes | >0.4 | MCHC | >0.3 | Alkaline phosphatase | >0.3 |
| Spleen | >0.2 | Platelets | >0.1 | ALT | >0.9 |
| Pancreas | >0.9 | Neutrophils | >0.8 | AST | >0.8 |
| Thyroid gland | >0.7 | Lymphocytes | >0.3 | Cholesterol | >0.04 |
| Eye | >0.3 | Monocytes | >0.05 | Calcium | >0.5 |
| Adrenal gland | >0.8 | Eosinophils | >0.4 | Potassium | >0.3 |
| Salivary gland | >0.2 | Basophils | >0.3 | Sodium | >0.1 |
| Urinary bladder | >0.5 | Phosphorus | >0.3 | ||
| Chloride | >0.05 | ||||
| Total protein | >0.2 | ||||
| Albumin | >0.8 | ||||
| Globulin | >0.1 | ||||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; MCHC, mean corpuscular hemoglobin content; MCV, mean corpuscular volume; RBC, red blood cell count; WBC, white blood cell count.
p values were assessed by three-way ANOVA, using gender and treatment group as factors and time (7 and 90 days) as an independent variable. The reported p value is a test of the null hypothesis that there is no difference between the AAVrh.10hCLN2 and PBS groups for that parameter.
Brain histopathology
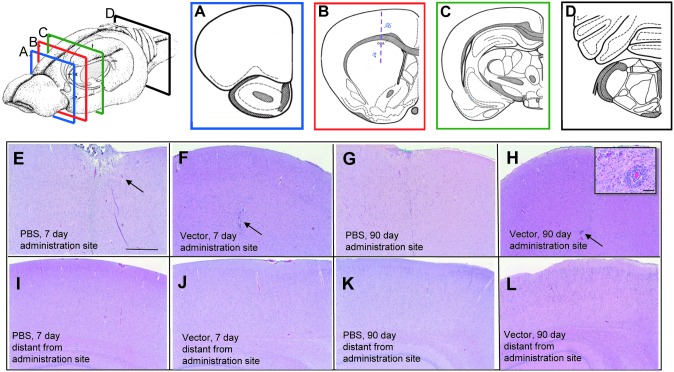
The proposed clinical strategy involves direct administration of the vector into the brain, the major site of LINCL pathology. Because a CNS inflammatory response to vector may be critical to tolerability of vector, H&E-stained coronal sections of the administration site in striatum were systematically examined for any abnormalities and analyzed quantitatively, using a severity rating scale (histopathology data not shown but quantified; Table 2). To determine the spatial limits of any histopathological response, coronal sections 3 mm distant encompassing either the striatum and frontal cortex in rostral direction or striatum and caudal diencephalon in the caudal directions were also examined. Finally, to detect any brainwide responses, a coronal section including the cerebellum was examined (Fig. 1).
Table 2.
Quantitation of Brain Pathology in Rats After CNS Administration of AAVrh.10hCLN2 or Phosphate-Buffered Salinea
| |
|
Abnormalities in various regions of the brainb |
|||
|---|---|---|---|---|---|
| Groupc | Parameters | Frontal cortexd | Striatum (injection site) | Caudal diencephalone | Cerebellum |
| 7-day PBS (n=10) |
Injection site gliosis | — | 2.3±1.1 (9/10) | — | — |
| Injection site hemosiderin | — | 1.1±1.3 (5/10) | — | — | |
| Lymphoplasmacytic perivascular cuffing | 0±0 | 0±0 | 0±0 | 0±0 | |
| Gliosis | 0±0 | 0±0 | 0±0 | 0±0 | |
| Swollen microvesiculated neurons | 0±0 | 0±0 | 0±0 | 0±0 | |
| 7-day vector (n=10) |
Injection site gliosis | — | 1.8±1.3 (9/10) | — | — |
| Injection site hemosiderin | — | 1.1±1.4 (5/10) | — | — | |
| Lymphoplasmacytic perivascular cuffing | 0±0 | 0±0 | 0±0 | 0±0 | |
| Gliosis | 0±0 | 0±0 | 0±0 | 0±0 | |
| Swollen microvesiculated neurons | 0±0 | 0±0 | 0±0 | 0±0 | |
| 90-day PBS (n=10) |
Injection site gliosis | — | 0±0 | — | — |
| Injection site hemosiderin | — | 0.6±1.3 (3/10) | — | — | |
| Lymphoplasmacytic perivascular cuffing | 0±0 | 0±0 | 0±0 | 0±0 | |
| Gliosis | 0±0 | 0±0 | 0±0 | 0±0 | |
| Swollen microvesiculated neurons | 0±0 | 0±0 | 0±0 | 0±0 | |
| 90-day vector (n=8) |
Injection site gliosis | — | 0.1±0.4 (1/8) | — | — |
| Injection site hemosiderin | — | 0±0 | — | — | |
| Lymphoplasmacytic perivascular cuffing | 0±0 | 1.9±1.0 (7/8) | 0.6±0.7 (4/8) | 0±0 | |
| Gliosis | 0±0 | 2.0±1.1 (8/8) | 0.3±0.5 (3/8) | 0±0 | |
| Swollen microvesiculated neurons | 0±0 | 2.4±0.9 (8/8) | 0.3±0.5 (2/8) | 0±0 | |
PBS, phosphate-buffered saline.
A total dose of 1011 genome copies (GC) of AAVrh.10hCLN2 was administered bilaterally into the striatum of rats in a volume of 5 μl at each site. The control group received PBS instead of AAVrh.10hCLN2.
Values represent average scores on a scale from 0 to 4±SD. Scale: 0, normal; 1, minimal; 2, mild; 3, moderate; 4, marked. When positive, it is followed by the number of animals with this abnormality/total assessed. See Fig. 1 for a schematic of the brain regions assessed. See Supplemental Fig. S1 for examples of scoring.
Both the vector and control groups were assessed for brain histopathology at acute (7-day) and medium (90-day) time points. Each group except the 90-day vector group had n=10 rats, 5 males and 5 females. The 90-day vector group had n=8 rats, 4 males and 4 females.
Section of the frontal cortex 3 mm rostral from the administration site.
Section of the caudal diencephalon 3 mm caudal from the administration site.
FIG. 1.
Histopathology of brain of AAVrh.10hCLN2- or PBS-treated rats. Rats were administered AAVrh.10hCLN2 (1011 genome copies, divided into two equal, 5-μl aliquots) or PBS in the right striatum and left striatum. Top left: Schematic of where brain was sectioned. (A–D) Schematic showing location of coronal sections of the rat brains that were assessed for histopathology. (A) Section of the brain rostral to the injection site. (B) Section of the brain containing the administration site, shown as a vertical line. (C) Section of the brain caudal to the site of administration. (D) Section of the brain distal from the site of administration, containing the cerebellum and the brainstem. Areas where perivascular cuffing was observed are shown by the blue stippling in (B) and (C). (E–H) Cerebral cortex near the injection site, represented in the schematic in (B). (E) PBS, 7 days postadministration. (F) AAVrh.10hCLN2, 7 days postadministration. For (E) and (H), the arrows indicate examples of surgery-related mechanical trauma. (G) PBS, 90 days postadministration. (H) AAVrh.10hCLN2, 90 days postadministration. The inset, which is a magnification of the region indicated by the arrow, shows some signs of inflammation as shown by the presence of perivascular cuffing, predominantly with accumulation of lymphocytes and plasma cells. (I–L) Caudal cerebral cortical regions distal from the administration site. (I) PBS, 7 days postadministration; (J) AAVrh.10hCLN2, 7 days postadministration; (K) PBS, 90 days postadministration; and (L) AAVrh.10hCLN2, 90 days postadministration. No abnormal changes were noted. All histological specimens are stained with H&E. Scale bars: (E–L) 500 μm; inset in (H), 20 μm.
In the rats killed 7 days after vector administration, there were focal inflammatory changes of moderate severity, at the sites of vector administration. These changes consisted of focal, circumscribed dorsoventral linear glial foci (mononuclear microglial cells and astrocytes), sometimes accompanied by pigment-laden macrophages (hemosiderin). These changes were confined to the immediate vicinity of the administration track. The incidence (9 of 10 rats for gliosis, 5 of 10 for hemosiderin) and severity were identical for PBS- and vector-administered rats. In addition, localized to the administration sites in the 7-day rats, there were areas of depressed neuropil (the dense network of axonal, dendritic, and glial branchings that form the bulk of the gray matter in which the nerve cell bodies are embedded) and meningeal fibrosis, cortical depression, and edema. These administration site-localized changes were present in equal frequency and intensity in the PBS- and vector-administered animals.
By 90 days, both the incidence and severity of the gliosis and hemosiderin had decreased. None of the PBS rats and only one of eight vector rats had gliosis of low severity at the immediate site of administration. Three of the PBS-administered and none of the vector-administered rats had hemosiderin-laden macrophages at the injection site. The 90-day rats had some additional histopathological changes in the vicinity of the injection track of the animals receiving the vector. These included perivascular cuffing, primarily with lymphocytes and plasma cells in seven of eight vector rats, gliosis in eight of eight rats, and swollen microvesiculated neurons (Supplementary Figs. S1 and S2) in eight of eight rats, all with moderate severity (Table 2). The 7-day PBS- and vector-administered rats and 90-day PBS-administered rats showed no such changes. To determine the spatial distribution of these changes, coronal sections 3 mm caudal and rostral from the sites of administration were assessed. No abnormalities were seen for any rat in the rostral section 3 mm away from site of administration that included the frontal cortex. In the section that was 3 mm caudal to the site of administration and included the caudal diencephalon, the 90-day vector-administered rats showed some changes of mild severity that included lymphocytic perivascular cuffing (four of eight rats), gliosis (three of eight rats), and swollen microvesiculated neurons (two of eight rats). The cerebellum sections were all normal for all rats for all time points. Thus the vector-related histopathological changes were limited to the area of administration and spread was not extensive.
Histopathology of visceral organs
Examination of the quadriceps, heart, liver, spleen, pancreas, kidney, lung, urinary bladder, male and female reproductive organs, stomach and intestinal tract, fat, thymus and lymph nodes, skin, sciatic nerve, and bone showed no vector-related histopathological changes. All abnormalities observed were of the type commonly observed in rats, and there were no trends suggesting treatment relationship. Some of the changes, that is, mineralization at the corticomedullary junction in female rats, or mononuclear infiltrates within the liver, were observed at comparable incidence in PBS controls and treated 7- and 90-day rats.
Nonhuman primates
The nonhuman primate study (Supplementary Table S2) included 9 NHPs, of which 8 received 1.8×1012 GC of AAVrh.10hCLN2 vector in 12 loci through 6 burr holes, similar to that proposed for the human study. Combined CNS CT/MRI scans were used to establish locations for administration (Supplementary Table S3). As in the rat study, the focus was on acute toxicity (n=4 vector NHPs and 1 PBS control at 7 days) and medium-term time frame toxicity (n=4 vector NHPs at 90 days). The parameters assessed during the study included immune parameters (Fig. 2), hematological parameters, behavior (see Fig. 3), and histopathology (see Fig. 4).
FIG. 2.
Anti-AAVrh.10 neutralizing antibodies evoked by CNS administration of AAVrh.10hCLN2 to nonhuman primates. Titers were assessed in serum by neutralization of in vitro gene transfer assay. Titers (average of two determinations) were evaluated presurgery (“presurgery” is defined as any time from 24 hr to 2 months before day 0, the day of surgery), and on days 7, 15, 30, and 60 postsurgery. Shown are data from treated animals killed on days 7 and 90 and the PBS control animal killed on day 7.
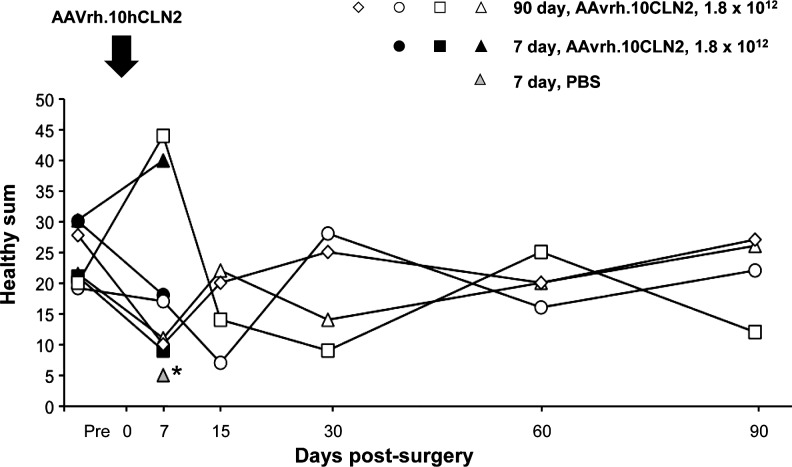
FIG. 3.
Behavioral evaluation before and after AAVrh.10hCLN2 administration in nonhuman primates. Nine African green monkeys were assessed for behavior (vector, n=8, of which 4 were killed at 7 days and 4 at 90 days; and PBS, n=1, which was killed at 7 days). The monkeys were videotaped for subsequent blinded behavioral analysis before surgery (pre-, defined as any time from 24 hr to 2 months before administration) and on days 7, 15, 30, 60, and 90 postadministration. The healthy sum, which is a sum of behaviors that relate to overall good health of the nonhuman primate and that includes anxiety, arousal, and quiet behavior (defined in detail in Supplementary Table S4), is plotted as a function of time. The data are shown for individual animals. The normal range for each parameter, calculated as the 5th to 95th percentile of measurement on all monkeys presurgery, is shown as the shaded area. *The PBS monkey was not evaluated at the presurgery time point. Behavior was evaluated only at the 7-day time point for this monkey.
FIG. 4.
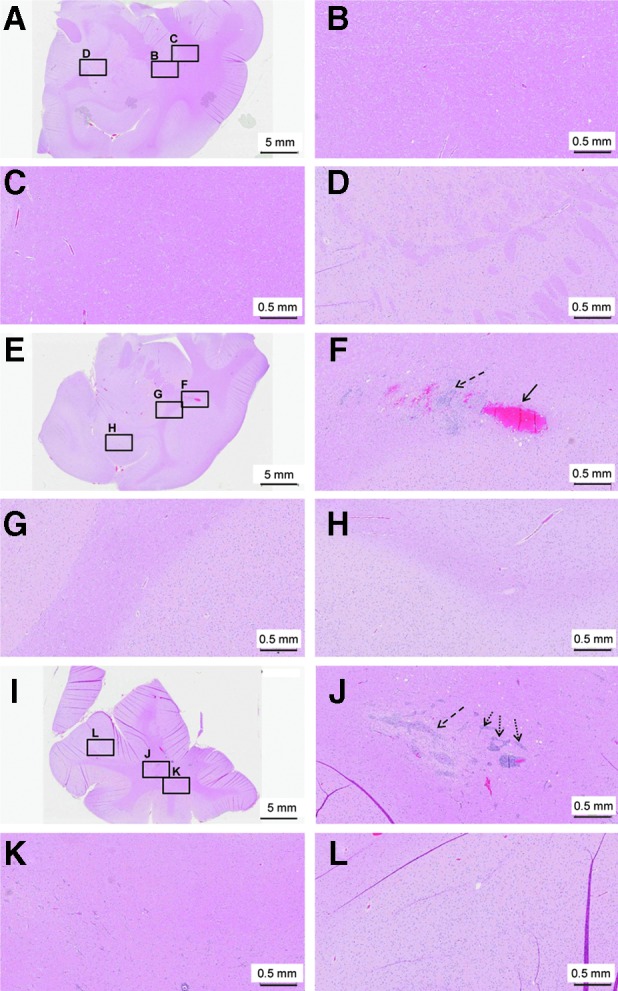
Histopathology of the brain of AAVrh.10hCLN2- or PBS-treated nonhuman primates. Brain sections from African green monkeys were administered 1.8×1012 genome copies of AAVrh.10hCLN2 in a total volume of 180 μl divided equally among 12 loci through 6 burr holes, or were administered PBS in the same volume and at the same locations. (A–D) PBS-administered animal killed 7 days postadministration. (A) Coronal section containing the administration site. (B–D) Higher magnification of the regions indicated by black boxes in (A): (B) site of administration; (C) region adjacent to site of administration; (D) region distant from the site of administration. (E–H) Representative AAVrh.10hCLN2-treated animal killed 7 days postadministration. (E) Coronal section containing the administration site. (F–H) Higher magnification of the regions indicated by black boxes in (E): (F) site of administration; (G) region adjacent to site of administration; (H) region distant from the site of administration. (I–L) A representative AAVrh.10hCLN2-treated animal killed 90 days postadministration. (I) Coronal section containing the administration site. (J–L) Higher magnification of the regions indicated by black boxes in (I): (J) site of administration; (K) region adjacent to site of administration; (L) region distant from the site of administration. In the 7- and 90-day vector-treated animals, various degrees of inflammatory response can be seen and includes gliosis [dashed arrows, (F) and (J)] hemorrhage [arrow, (F)] and perivascular cuffing predominantly by lymphocytes and histiocytes, which are types of tissue macrophage [dotted arrows, (J)]. No inflammatory changes were noted in regions adjacent to and distant from the administration site. All histological specimens are stained with H&E. Scale bars: (A, E, and I) 5 mm; 0.5 mm for all other panels.
Neutralizing anti-AAVrh.10 titers
Before vector administration, all NHPs showed undetectable to low levels of preexisting neutralizing anti-AAVrh.10 antibody titers (Fig. 2). By day 7 postsurgery, all vector-treated NHPs exhibited an increase in anti-AAVrh.10 neutralizing titers ranging from 5- to 100-fold. Of the eight vector-administered monkeys, four were killed at 7 days as per schedule and the remaining four had declined by the conclusion of the study at 90 days. No increase in titer was observed in the one PBS monkey assessed on day 7 before sacrifice.
General safety parameters
To assess general safety, periodic assessments included pulse, respiratory rate, temperature, and weight. For each parameter, reference ranges were established by calculating the range inclusive of 95% of the values from all of the NHPs presurgery. No vector-related changes were observed when comparing the NHPs pre- and postsurgery. Animals typically maintained weight levels throughout the course of the study, with a slight increase most likely due to sedentary conditions. All NHPs maintained a consistent pulse within the predetermined range throughout the study, with the exception of one 7-day NHP, which experienced a dip in pulse at a pre–vector administration time point. All NHPs stayed within the calculated acceptable range for respiratory rate. Six of the nine NHPs, including the PBS control, were below the range in temperature on the day of surgery, most likely related to the anesthesia.
Complete blood count
Assessment of complete blood count included 19 different parameters, including hematocrit, red blood cell count, hemoglobin, mean corpuscular hemoglobin, mean corpuscular volume, red blood cell distribution width, mean corpuscular hemoglobin concentration, platelets, white blood cell count, basophil count, basophil percent, eosinophil count, eosinophil percent, monocyte count, monocyte percent, lymphocyte count, lymphocyte percent, neutrophil count, and neutrophil percent. For each parameter, reference ranges were established by calculating the range inclusive of 95% of the values from all of the NHPs presurgery. Analysis of the data revealed that for majority of the parameters, across all time points, values fell within the limits of the reference range with exceptions of random isolated or transient abnormalities.
Serum chemistry
Assessment of serum chemistry included 26 different parameters, including total bilirubin, blood urea nitrogen, creatine kinase, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase, albumin, globulin, creatinine, total protein, cholesterol, amylase, lipase, glucose, calcium, phosphorus, bicarbonate, chloride, potassium, total sodium, albumin-to-globulin ratio, blood urea nitrogen-to-creatinine ratio, sodium-to-potassium ratio, osmolarity, and anion gap. For each parameter, reference ranges were established by calculating the range inclusive of 95% of the values from all of the subjects presurgery. Like the blood counts, analysis of the data revealed that for all parameters across all time points, values fell within the limits of the reference range, with exceptions of random isolated or transient abnormalities. Immediately after surgery, several animals experienced spikes in alanine aminotransferase (seven of nine NHPs) and aspartate aminotransferase (six of nine NHPs). The transient nature of this spike, as well as a similar observation in the present PBS control and previous controls from the AAV2 study (Hackett et al., 2005), suggest that this elevation was surgery related.
Behavioral assessment
All NHPs were videotaped for behavioral assessment presurgery and on days 7, 15, 30, 60, and 90 postsurgery, and a set of previously defined normal behaviors was quantitated (Fig. 3, and Supplementary Tables S2 and S4). The 95th and 5th percentiles were calculated from the prevector time points and these were used as the upper and lower limits for all subsequent behavioral analysis. Blinded videotape analysis of behavior at the conclusion of the study showed no discernible behavioral differences between the prevector and any postvector time points.
No NHP demonstrated any sign of sedation at any point during the trial. One 7-day vector-administered NHP demonstrated a high level of arousal on day 7, shifting frequently within its cage. All other NHPs stayed within the predetermined range of behavior throughout the study. In addition, NHPs were also assessed on the basis of their food and threat responses. In all cases, they responded without hesitation or delayed movement, and at no point were tremors or sedation observed.
Histopathology of the brain
As with the rats, H&E-stained coronal sections of the CNS were assessed and histopathological abnormalities tabulated with a quantitative severity scale (Fig. 4, and Table 3). The two rostral sections included the cortical injection sites and a third caudal section included the cerebellum to help circumscribe the extent of any abnormalities observed. In all monkeys at all time points, there was mild depression of the cerebral surface at the sites of administration, with fibrous thickening of the overlying leptomeninges (which include the pia mater and the arachnoid mater). On day 7 after vector administration, there was focal and linear gliosis of moderate severity at a majority of the vector injection sites surveyed (seven of eight), extending less than 1 mm from the injection track. Over the same domain, there were also hemosiderin-laden macrophages of mild severity in four of eight monkeys (Fig. 4F, and Table 3). Surveys of sections of brain removed from the immediate vicinity of the site of administration showed no such changes (Fig. 4G and H). These changes were not seen at the sites of administration of the single PBS monkey (Fig. 4A–D). By day 90, the severity and intensity of both gliosis and hemosiderin at the immediate sites of administration were significantly reduced. As with the rats, NHP brain examined 90 days after vector administration showed mild to moderate white matter edema (spongiosis), accompanied by glial cells (in seven of eight sections), and focal perivascular cuffing of congested vessels by primarily lymphocytes in seven of eight sections in the region of the administration sites. These changes radiated no more than 1 mm from the injection track (Fig. 4J). No such changes were seen in regions distant from the administration sites (Fig. 4K and L). Because there were no PBS controls assessed at 90 days, it cannot be established whether these histopathological changes were vector-related.
Table 3.
Quantitation of Brain Pathology in Nonhuman Primates After CNS Administration of AAVrh.10hCLN2 or Phosphate-Buffered Salinea
| |
|
Abnormalities in various regions of the brainb |
||
|---|---|---|---|---|
| Groupc | Parameters | Rostral sectiond | Caudal sectione | Cerebellum and brainstem |
| 7-day PBS (n=1)f |
Injection site gliosis | 0 | 0 | 0 |
| Injection site hemosiderin | 0 | 0 | 0 | |
| Lymphoplasmacytic perivascular cuffing | 0 | 0 | 0 | |
| Gliosis | 0 | 0 | 0 | |
| Swollen microvesiculated neurons | 0 | 0 | 0 | |
| 7-day vector (n=4) |
Injection site gliosis | 2.5±1.7 (3/4) | 2.0±0.8 (4/4) | 0±0 |
| Injection site hemosiderin | 1.3±0.9 (3/4) | 0.3±0.5 (1/4) | 0±0 | |
| Lymphoplasmacytic perivascular cuffing | 0±0 | 0±0 | 0±0 | |
| Gliosis | 0±0 | 0±0 | 0±0 | |
| Swollen microvesiculated neurons | 0±0 | 0±0 | 0±0 | |
| 90-day vector (n=4) |
Injection site gliosis | 0.8±0.5 (3/4) | 1.0±0.8 (3/4) | 0±0 |
| Injection site hemosiderin | 0.3±0.5 (1/4) | 0±0 | 0±0 | |
| Lymphoplasmacytic perivascular cuffing | 2.8±1.0 (4/4) | 1.5±1.0 (3/4) | 0±0 | |
| Gliosis | 2.5±0.6 (4/4) | 1.5±1.0 (3/4) | 0±0 | |
| Swollen microvesiculated neurons | 0±0 | 0±0 | 0±0 | |
PBS, phosphate-buffered saline.
Each nonhuman primate received an intraparenchymal administration of a total dose of 1.8×1012 genome copies (GC) of the AAVrh.10hCLN2 vector. This dose was administered in 15 μl at each of 12 locations, for a total volume of 180 μl (3 symmetrical burr holes per hemisphere, with depositions at 2 depths through each burr hole). Targeted locations were determined using MRI and CT scan imaging. A control monkey was administered an equivalent volume of PBS to the same locations.
Values represent average scores on a scale from 0 to 4±SD. Scale: 0, normal; 1, minimal; 2, mild; 3, moderate; 4, marked. When positive, it is followed by the number of animals with this abnormality/total assessed. See Fig. 4 for brain regions assessed.
One AAVrh.10hCLN2-treated group (n=4) was killed 7 days after administration, and the other group (n=4) was killed at 90 days. The control monkey administered PBS was killed 7 days post-surgery.
Rostral region including cerebrum, caudate, lateral ventricle, choroid plexus.
Caudal region including cerebrum, hippocampus, lateral ventricle, choroid plexus.
Only one nonhuman primate was injected with PBS, and therefore standard deviation not possible.
Histopathology of visceral organs
No tumors or visible abnormalities of any organ were found in any of the NHPs at sacrifice. Histopathological examination did not identify any vector-specific abnormalities. There were some microscopic changes, including bronchial-associated lymphoid tissue in the lungs of seven of eight treated NHPs, and multifocal small aggregates of lymphocytes in the kidneys in three of four treated NHPs on day 7. However, these abnormalities were also observed in the PBS control NHP.
TPP-I expression
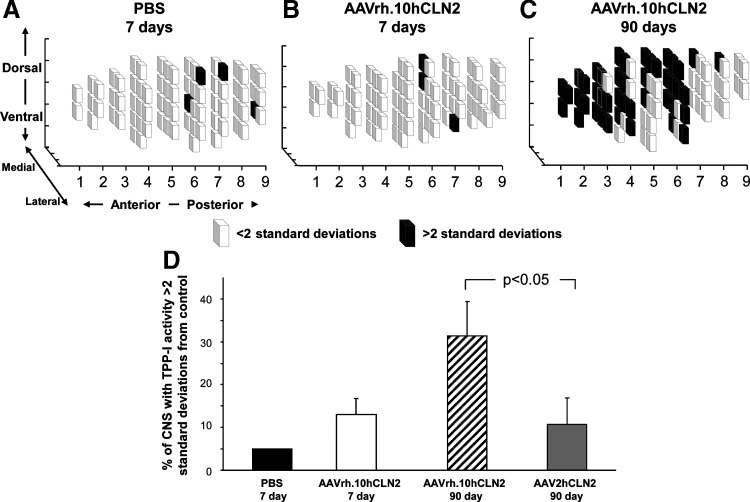
Prior studies done by our group have shown that direct administration of AAVrh.10hCLN2 to the CNS in mice and rats mediated a wide distribution of vector-derived TPP-I activity in the CNS (Sondhi et al., 2007, 2008). To assess whether this finding persisted in a brain size more comparable to that of humans, a similar analysis was conducted in the NHPs. All TPP-I activity in the PBS brain was assumed to be background (Fig. 5). In the four animals killed 90 days after vector administration, TPP-I activity in 32.8±16.2% of the cubes was >2 SD, and 17.7±8.6% was >4 SD, compared with the mean TPP-I level in the PBS control. Average total TPP-I activity in the 90-day animals was 160±18% (range, 136–214%) over the PBS control. Peaks in enzymatic activity corresponded to the sites of administration. In comparison, less dramatic results were seen in 7-day monkeys (2.9±1.4% of the cubes were >4 SD and 12.8±7.4% of the cubes were >2 SD); this follows known patterns of AAVrh.10 transgene expression. The distribution of TPP-I in 90-day post–AAVrh.10CLN2 vector-treated animals exceeded by more than 3-fold the percentage of the brain demonstrating measurable TPP-I activity resulting from AAV2CLN2 treatment in our prior study (Sondhi et al., 2007) (Fig. 5D).
FIG. 5.
Distribution of tripeptidyl-peptidase I (TPP-I) activity in the CNS of nonhuman primates after CNS administration of AAVrh.10hCLN2. African green monkeys were injected with AAVrh.10hCLN2 at a dose of 1.8×1012 genome copies in a total of 180 μl divided equally among 12 loci through 6 burr holes, or were injected with PBS in the same volume and at the same location. After sacrifice the nonhuman primate brains were hemisected. The left hemispheres were sectioned into 1-cm3 sections (referred to as cubes); these cubes were homogenized and assayed for TPP-I activity. The activity was measured as fluorescence units per minute per milligram of protein. TPP-I activity from all the cubes in the PBS control animal was averaged and this was taken to be the endogenous background. In (A–C), the lighter cubes represent regions that have an activity of TPP-I less than 2 SD above the background, and the darker cubes represent the regions that have an activity greater than 2 SD above endogenous background levels; the data are shown as a three-dimensional representation of distribution of TPP-I activity. (A) The data from the control PBS-injected animal. (B) Data from one representative animal administered AAVrh.10hCLN2 and killed at 7 days. (C) Data from one representative animal treated with AAVrh.10hCLN2 and killed at 90 days. (D) Percentage of CNS with TPP-I activity greater than 2 SD above endogenous background is shown as a bar graph for all groups in the study (n=1 PBS killed at 7 days, n=4 AAVrh.10hCLN2 killed at 7 days, n=AAVrh.10hCLN2 killed at 90 days) and is compared with the historical data obtained from our study using AAV2hCLN2 in the same setting.
Discussion
Because the disease globally affects the entire CNS, one of the biggest challenges in developing a successful gene therapy treatment for LINCL is the ability to achieve widespread transgene expression (Williams et al., 1999; Sondhi et al., 2001). With this in mind, we chose a rhesus-derived serotype, AAVrh.10, which results in high levels of widespread neuronal TPP-I expression in vivo in mice (Sondhi et al., 2007, 2008). Using a clinical-grade vector produced under GMP conditions, toxicology assessment in rats bilaterally administered a total of 1011 genome copies of AAVrh.10hCLN2 to the striatum showed no significant vector-related adverse changes in complete blood count, serum chemistry, overall health, or general safety parameters directly attributable to vector administration. At the 90-day time point in rats, some vector-related histopathological changes were observed that were limited to the area of administration. In nonhuman primates, administration of a total of 1.8×1012 genome copies of AAVrh.10hCLN2 to six sites in the cortex did not result in detectable changes in blood safety parameters or behavior compared with PBS-administered controls. Histopathological examination of the CNS demonstrated vector-related mild to moderate white matter edema, inflammation, and gliosis localized to the vector injection sites. All of the nonhuman primates receiving vector demonstrated an increase in systemic anti-AAVrh.10 antibody titers at the 7-day time point, which subsided by 90 days, the last time point measured. Throughout the course of the study, no behavioral differences were observed between the nonhuman primates pre- and posttreatment as determined by blinded assessment of videotaped behavior. Vector-derived TPP-I activity above background was seen in an average of 32% of the brain volume at 90 days. Because the aim is to target as much of the brain volume as possible, this is a huge improvement on AAV2, which led to TPP-I activity over background in less than 10% of the volume of the brain (Sondhi et al., 2007). These observations of safety and transgene expression after AAVrh.10hCLN2 administration directly to the CNS establish the dose and safety profile for human clinical studies with AAVrh.10hCLN2 for the treatment of LINCL, which is primarily a disease that impacts the CNS. It is certainly possible that a CNS cure may be followed by longer term pathology in other organs, but the accessibility of these sites (without a blood–brain barrier) significantly reduces the hurdles to extending therapeutic coverage.
Efficacy of transgene expression
Our animal studies have demonstrated the long persistence of AAVrh.10 expression, and we hope that it will be adequate for a single administration therapy; there is, however, no guarantee of this. It is also possible that the disease is one of the developing brain and that the TPP-I therapeutic level will drop with age (not unlike the lack of pathology in other tissues that are CLN2 negative). Given the invasive procedure required for delivery, a second administration may be too burdensome and would require a new approach.
The primary conclusion of the study is that successful therapeutic long-term gene expression was achieved over a wide domain of the brain. The difference in TPP-I activity observed between the 7- and 90-day treated NHPs is consistent with known patterns of AAVrh.10 transgene expression (De et al., 2006). On the basis of comparisons of treated versus untreated CLN2 knockout mice, TPP-I levels of 5 to 10% of normal are sufficient to improve quality of life measures and enhance survival (Sleat et al., 1999; Sondhi et al., 2005). In the current study, we observed levels of TPP-I activity in NHPs that were 160±18% above endogenous levels at 90 days postadministration. These data support the idea that intraparenchymal administration of our vector would be therapeutic at the doses used and support transition into a human clinical study.
Immune responses to AAV capsid and transgene
The recombinant AAVrh.10hCLN2 vector has no viral genes that express viral proteins; therefore any vector-related host responses are attributable to the viral capsid or expressed transgene. Systemic administration of AAV vectors elicits anti-AAV neutralizing antibodies in monkeys and humans (Manno et al., 2006; Nathwani et al., 2007). In contrast, administration of AAV2 vectors to the brain of nonhuman primates did not result in an increase in humoral immunity (Hackett et al., 2005). In human studies of AAV2 administration to brain, less than half of the subjects in both Canavan disease and LINCL groups had transient increases in neutralizing titer (McPhee et al., 2006; Worgall et al., 2008).
In contrast, administration of AAVrh.10 to the nonhuman primate brains in the present study did result in an increase in serum neutralizing antibodies. On the basis of spread of vector to other organs after CNS administration to LINCL mice (Sondhi et al., 2007), this immune response may be related to spread of vector to antigen-presenting cells beyond the CNS. However, this humoral immune response to AAVrh10 is likely associated with the fact that nonhuman primates are the natural host of AAV serotype rh.10 and that the African green monkeys used in this study have been primed by prior exposure to immunologically similar AAV serotypes. In contrast, in human populations, preexisting immunity is most prevalent against serotypes AAV2 and AAV5, and is minimal against nonhuman primate serotypes such as AAVrh.10 (Calcedo et al., 2009). In this regard, humans, and especially children, have likely not been previously exposed to AAVrh.10. In support of this, humans show as much as 20% neutralizing activity to two rhesus-derived serotypes, AAV7 and AAV8 vectors (Calcedo et al., 2009, 2011; Boutin et al., 2010), where AAV8 is a clade E virus closely related to AAVrh.10 (Vandenberghe et al., 2009). The capacity of this preexisting immunity to cross-react to and impact gene transfer of AAVrh.10 will be an important parameter for the clinical study, but the significantly lower seroprevalence of these rhesus-derived serotypes than the AAV2 vector supports the use of the AAVrh.10 vector in human clinical trials.
Although the surgical procedure for the delivery of the AAVrh.10hCLN2 vector to the CNS was optimized to prevent trauma, the surgical administration of the vector (rather than the vector itself ) did pose some minor risks, as evidenced by our previous AAV2hCLN2 preclinical study and in a preclinical study in nonhuman primates with AAV2 expressing neurturin (Hackett et al., 2005; Herzog et al., 2008). In our prior AAV2-based study in nonhuman primates, slight to mild lesions in the brain were seen across all treatment groups, including the sham group (with needle insertion only and no fluid infusion) and PBS, AAV2Null, and AAV2hCLN2 groups (Hackett et al., 2005). This abnormal histopathology was attributed mainly to the mechanical trauma of the procedure.
In addition to these minor surgical-related lesions, there were localized, vector-related histopathological changes in the CNS, including lymphoplastic perivascular cuffing, gliosis, and swollen microvesiculated neurons near to the administration site. These changes likely reflect a localized inflammatory response. Because nonhuman primates have immunity against the AAVrh.10 capsid, it is unclear whether this observation is restricted to nonhuman primates and may or may not be applicable to humans. Also, these observed effects were localized to the area immediately surrounding the vector administration site and not found in areas removed from these sites. Most importantly, there were no clinical correlates to this histopathological finding as evidenced by the general safety, hematological, and behavioral analyses. Nevertheless, this study suggests a dose-limiting toxicity that must be factored into the design of the human study.
Supplementary Material
Acknowledgments
The authors thank Andrew Edelstein, Katrina del Fierro, Michael Baad, and Benjamin Van de Graaf for technical assistance; and N. Mohamed and Dudley Noel McCarthy for help in preparing this manuscript. These studies were supported, in part, by U01 NS047458.
Author Disclosure Statement
The authors declare there is no conflict of interest.
References
- Boustany R.M. Batten disease or neuronal ceroid lipofuscinosis. In: Moser H.W., editor. Handbook of Clinical Neurology. Elsevier Science; New York: 1996. pp. 671–700. [Google Scholar]
- Boutin S. Monteilhet V. Veron P., et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Calcedo R. Vandenberghe L.H. Gao G., et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R. Morizono H. Wang L., et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley C.N. Wolfe J.H. Transduction characteristics of adeno-associated virus vectors expressing Cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Crystal R.G. Sondhi D. Hackett N.R., et al. Clinical protocol: Administration of a replication-deficient adeno-associated virus gene transfer vector expressing the human CLN2 cDNA to the brain of children with late infantile neuronal ceroid lipofuscinosis. Hum. Gene Ther. 2004;15:1131–1154. doi: 10.1089/hum.2004.15.1131. [DOI] [PubMed] [Google Scholar]
- Daly T.M. Vogler C. Levy B., et al. Neonatal gene transfer leads to widespread correction of pathology in a murine model of lysosomal storage disease. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2296–2300. doi: 10.1073/pnas.96.5.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B.P. Heguy A. Hackett N.R., et al. High levels of persistent expression of α1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol. Ther. 2006;13:67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Ezaki J. Tanida I. Kanehagi N., et al. A lysosomal proteinase, the late infantile neuronal ceroid lipofuscinosis gene (CLN2) product, is essential for degradation of a hydrophobic protein, the subunit c of ATP synthase. J. Neurochem. 1999;72:2573–2582. doi: 10.1046/j.1471-4159.1999.0722573.x. [DOI] [PubMed] [Google Scholar]
- Gao G. Alvira M.R. Somanathan S., et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. U.S.A. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett N.R. Redmond D.E. Sondhi D., et al. Safety of direct administration of AAV2(CU)hCLN2, a candidate treatment for the central nervous system manifestations of late infantile neuronal ceroid lipofuscinosis, to the brain of rats and nonhuman primates. Hum. Gene Ther. 2005;16:1484–1503. doi: 10.1089/hum.2005.16.1484. [DOI] [PubMed] [Google Scholar]
- Haltia M. The neuronal ceroid-lipofuscinoses. J. Neuropathol. Exp. Neurol. 2003;62:1–13. doi: 10.1093/jnen/62.1.1. [DOI] [PubMed] [Google Scholar]
- Herzog C.D. Dass B. Gasmi M., et al. Transgene expression, bioactivity, and safety of CERE-120 (AAV2-neurturin) following delivery to the monkey striatum. Mol. Ther. 2008;16:1737–1744. doi: 10.1038/mt.2008.170. [DOI] [PubMed] [Google Scholar]
- Hu C. Busuttil R.W. Lipshutz G.S. RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J. Gene Med. 2010;12:766–778. doi: 10.1002/jgm.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. Lobel P. Production and characterization of recombinant human CLN2 protein for enzyme-replacement therapy in late infantile neuronal ceroid lipofuscinosis. Biochem. J. 2001;357:49–55. doi: 10.1042/0264-6021:3570049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- McPhee S.W. Janson C.G. Li C., et al. Immune responses to AAV in a phase I study for Canavan disease. J. Gene Med. 2006;8:577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- Nathwani A.C. Gray J.T. McIntosh J., et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings N.D. Barrett A.J. Tripeptidyl-peptidase I is apparently the CLN2 protein absent in classical late-infantile neuronal ceroid lipofuscinosis. Biochim. Biophys. Acta. 1999;1429:496–500. doi: 10.1016/s0167-4838(98)00238-6. [DOI] [PubMed] [Google Scholar]
- Sleat D.E. Donnelly R.J. Lackland H., et al. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science. 1997;277:1802–1805. doi: 10.1126/science.277.5333.1802. [DOI] [PubMed] [Google Scholar]
- Sleat D.E. Gin R.M. Sohar I., et al. Mutational analysis of the defective protease in classic late-infantile neuronal ceroid lipofuscinosis, a neurodegenerative lysosomal storage disorder. Am. J. Hum. Genet. 1999;64:1511–1523. doi: 10.1086/302427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat D.E. Wiseman J.A. El-Banna M., et al. A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the CLN2 gene results in a loss of tripeptidyl-peptidase I activity and progressive neurodegeneration. J. Neurosci. 2004;24:9117–9126. doi: 10.1523/JNEUROSCI.2729-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondhi D. Hackett N.R. Apblett R.L., et al. Feasibility of gene therapy for late neuronal ceroid lipofuscinosis. Arch. Neurol. 2001;58:1793–1798. doi: 10.1001/archneur.58.11.1793. [DOI] [PubMed] [Google Scholar]
- Sondhi D. Peterson D.A. Giannaris E.L., et al. AAV2-mediated CLN2 gene transfer to rodent and non-human primate brain results in long-term TPP-I expression compatible with therapy for LINCL. Gene Ther. 2005;12:1618–1632. doi: 10.1038/sj.gt.3302549. [DOI] [PubMed] [Google Scholar]
- Sondhi D. Hackett N.R. Peterson D.A., et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol. Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- Sondhi D. Peterson D.A. Edelstein A.M., et al. Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp. Neurol. 2008;213:18–27. doi: 10.1016/j.expneurol.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld R. Heim P. von Gregory H., et al. Late infantile neuronal ceroid lipofuscinosis: Quantitative description of the clinical course in patients with CLN2 mutations. Am. J. Med. Genet. 2002;112:347–354. doi: 10.1002/ajmg.10660. [DOI] [PubMed] [Google Scholar]
- Vandenberghe L.H. Breous E. Nam H.J., et al. Naturally occurring singleton residues in AAV capsid impact vector performance and illustrate structural constraints. Gene Ther. 2009;16:1416–1428. doi: 10.1038/gt.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines D.J. Warburton M.J. Classical late infantile neuronal ceroid lipofuscinosis fibroblasts are deficient in lysosomal tripeptidyl peptidase I. FEBS Lett. 1999;443:131–135. doi: 10.1016/s0014-5793(98)01683-4. [DOI] [PubMed] [Google Scholar]
- Williams R.E. Gottlob I. Lake B.D., et al. Classic late infantile NCL. In: Goebel H.H., editor. The Neuronal Ceroid Lipofuscinosis (Batten Disease) IOS Press; Fairfax, VA: 1999. pp. 37–54. [Google Scholar]
- Wisniewski K. Kida E. Golabek A.A., et al. Neuronal Ceroid Lipofuscinosis: Classification and Diagnosis. Academic Press; New York: 2001. pp. 1–34. [DOI] [PubMed] [Google Scholar]
- Worgall S. Kekatpure M.V. Heier L., et al. Neurological deterioration in late infantile neuronal ceroid lipofuscinosis. Neurology. 2007;69:521–535. doi: 10.1212/01.wnl.0000267885.47092.40. [DOI] [PubMed] [Google Scholar]
- Worgall S. Sondhi D. Hackett N.R., et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum. Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.