Abstract
Narcolepsy-cataplexy is a neurological disorder associated with the inability to maintain wakefulness and abnormal intrusions of rapid eye movement sleep-related phenomena into wakefulness such as cataplexy. The vast majority of narcoleptic-cataplectic individuals have low or undetectable levels of orexin (hypocretin) neuropeptides in the cerebrospinal fluid, likely due to specific loss of the hypothalamic orexin-producing neurons. Currently available treatments for narcolepsy are only palliative, symptom-oriented pharmacotherapies. Here, we demonstrate rescue of the narcolepsy-cataplexy phenotype of orexin neuron-ablated mice by genetic and pharmacological means. Ectopic expression of a prepro-orexin transgene in the brain completely prevented cataplectic arrests and other abnormalities of rapid eye movement sleep in the absence of endogenous orexin neurons. Central administration of orexin-A acutely suppressed cataplectic behavioral arrests and increased wakefulness for 3 h. These results indicate that orexin neuron-ablated mice retain the ability to respond to orexin neuropeptides and that a temporally regulated and spatially targeted secretion of orexins is not necessary to prevent narcoleptic symptoms. Orexin receptor agonists would be of potential value for treating human narcolepsy.
Human narcolepsy-cataplexy is a debilitating neurological disease characterized by excessive daytime sleepiness, premature transitions to rapid eye movement (REM) sleep (so-called “sleep-onset REM periods”), and cataplexy (sudden bilateral skeletal muscle weakness without impairment of consciousness). Excessive sleepiness is treated by using amphetamines, modafinil, or γ-hydroxybutyrate (sodium oxybate), whereas cataplexy is treated with tricyclic antidepressants such as clomipramine (1, 2). This therapeutic regimen is problematic due to limited effectiveness, undesirable side effects such as insomnia or symptom rebounds, and the potential for abuse.
Recent studies (3-8) have concluded that narcolepsy-cataplexy in humans and animal models results from failure of signaling mediated by orexin (hypocretin) neuropeptides. Orexin-A and orexin-B are cleaved from a single precursor polypeptide prepro-orexin, which is expressed by a select population of neurons clustered around the perifornical lateral hypothalamus (LH) (9). Orexin neurons robustly innervate specific nuclei in basal forebrain, hypothalamus, and brainstem that are involved in sleep/wake regulation (6, 10). Intracerebroventricular (i.c.v.) injections of orexin peptides, administered acutely in mice and rats, have been shown to increase wakefulness and suppress both non-REM and REM sleep (11, 12).
Murine models of narcolepsy-cataplexy, generated by disrupting the prepro-orexin gene (orexin knockout mice) or inducing postnatal death of orexin-producing neurons (orexin/ataxin-3-transgenic mice), exhibit a phenotype remarkably similar to human narcolepsy-cataplexy: difficulty maintaining waking periods (sleep/wake state fragmentation), intrusions of REM sleep into wakefulness resembling cataplexy, and increased amounts of REM sleep during the active phase (6, 8). Whereas mutations in one of two known genes encoding orexin receptors, orexin receptor type 2 (OX2R), are responsible for an autosomal recessive form of inherited narcolepsy-cataplexy in canines (4), studies in mice suggest that loss of signaling through both orexin receptors is associated with a more severe form of the symptom complex analogous to that observed in humans (13, 14).
Indeed, orexin-A, a nonselective agonist for both orexin receptors (9), is low or undetectable in the cerebrospinal fluid of up to 95% of human cases of narcolepsy-cataplexy examined (15), and orexin mRNA and orexin-A immunoreactivity are drastically reduced in postmortem hypothalamic samples from narcoleptic-cataplectic humans (5, 7). Because of a strong association with certain HLA alleles, it has been speculated that narcolepsy-cataplexy may result from selective autoimmune degeneration of orexin neurons (16). These findings open up the possibility that replacement therapies based on administration of orexin receptor agonists might prove beneficial (1), a possibility not yet convincingly investigated in humans or animal models.
In this study, we examined whether symptoms of murine narcolepsy-cataplexy could be reversed either by ectopic production of orexin peptides from a prepro-orexin transgene or by pharmacological administrations of synthetic orexin-A. These interventions were evaluated by behavioral and electrophysiological criteria using orexin neuron-ablated orexin/ataxin-3-transgenic mice (8), an accepted biochemical and behavioral model of human narcolepsy-cataplexy.
Methods
Production of CAG/Orexin-Transgenic Mice. The CAG/orexin transgene was constructed so that the rat prepro-orexin gene is expressed under the control of the β-actin/cytomegalovirus (CAG) hybrid promoter, which drives widespread expression (17). Rat prepro-orexin cDNA was inserted between two EcoRI sites of pCAGGS (8). The transgene was excised by digestion with SalI and PstI, was gel-purified, and was then injected into pronuclei of fertilized eggs obtained from DBA1 mice as described (8). Full details of the characterization of these mice will be reported elsewhere.
Cataplexy Testing. Screening of eight orexin/ataxin-3-hemizygous-transgenic mice and 13 orexin/ataxin-3; CAG/orexin-double-hemizygous-transgenic mice (generally littermates, 12-20 weeks old, C57BL/6J N4 and N5 before filial crosses) was carried out as described (6, 13) during the dark phase by using infrared video recording, except that chambers contained additional novelties (plastic test tube racks and cotton nesting pads) to increase exploratory activity of the mice. Scoring criteria used by genotype- and treatment-blinded observers were described (6, 13).
After the cannulation of orexin/ataxin-3-transgenic animals for orexin administrations (see below), it was noted that cataplexy was not reliably elicited in some individuals, possibly due to stress associated with indwelling cannula, restraint, or injection procedures. Only animals consistently showing cataplectic attacks after vehicle administrations (5 of 16) were subjected to further analysis.
Cannulation (i.c.v.) and Orexin Administration. All procedures were approved by the appropriate institutional animal care and use committees, and were carried out in strict accordance with National Institutes of Health guidelines. Orexin/ataxin-3-hemizygous-transgenic mice (8) (16- to 19-week-old, male, C57BL/6J × 129SvEv F1 genetic background) were anesthetized with sodium pentobarbital (50-60 mg/kg i.p.) and were implanted with an indwelling stainless steel guide cannula into the left lateral ventricle (stereotaxic coordinates: 0.3 mm posterior to bregma, 0.9 mm lateral from midline, 2.4 mm depth from skull surface). Mice were treated postoperatively with penicillin-G benzathine/penicillin-G procaine suspension (100,000 units/kg s.c.). All animals were recovered for at least 10 days before experiments; mice used for further study regained presurgical body weights and exhibited no obvious signs of infection.
For cataplexy testing, doses of synthetic orexin-A (3 nmol per mouse i.c.v., generous gift of GlaxoSmithKline Pharmaceuticals) or vehicle [sterile artificial cerebrospinal fluid (12)] were administered by using a randomized crossover design at an interval of 4-7 days. All doses were delivered through cannula to gently restrained mice by using gas-tight syringes (Hamilton) with a blunt needle. An injection volume of 1 μl was delivered over a 30-sec period to each mouse, with the needle remaining in position for an additional 15 sec to ensure dispersal of the peptide. Mice were injected just before onset of the dark phase with cataplexy testing following immediately.
For i.c.v./electroencephalograph/electromyograph (EEG/EMG) experiments (see below), mice were injected either at the onset of the dark phase or, alternatively, at 3 h into the light phase. Mice were then returned to home cages for immediate recording of EEG/EMG.
EEG/EMG Recordings. For genetic studies, six orexin/ataxin-3-transgenic, five CAG/orexin;orexin/ataxin-3-double-transgenic littermates, and 10 wild-type control mice (all genotypes 14- to 15-week-old males, C57BL/6J N4 and N5 before filial crosses) were anesthetized and were surgically implanted for long-term EEG/EMG monitoring as described (6). For i.c.v./EEG/EMG experiments, four orexin/ataxin-3-transgenic and three wild-type mice (16- to 19-week-old males, C57BL/6J:129SvEv F1) were simultaneously implanted with a modified EEG/EMG implant (with only two EEG electrodes implanted over one hemisphere) in addition to a guide cannula placed as above. EEG/EMG signals were amplified, filtered, digitized, archived, and scored as described (6), and were further analyzed by using custom software.
Results
Genetic Rescue of Narcolepsy in Orexin Neuron-Ablated Mice. To examine whether the narcolepsy-cataplexy phenotype of orexin neuron-ablated mice could be rescued by ectopic production of orexin peptides, we produced transgenic mice that overexpress a prepro-orexin transgene under the control of a β-actin/cytomegalovirus hybrid promoter (CAG/orexin-transgenic mice). Several stable transgenic lines overexpressed the orexin as determined by Northern blots, radioimmunoassays, and anti-orexin-A immunohistochemistry (a more detailed description of the generation of CAG/orexin-transgenic lines will be published elsewhere; see Discussion). In this study, we used one line in which the whole-brain levels of orexin-A and -B peptides were increased by nearly 30- and 80-fold, respectively. Mice of this line were crossed to orexin/ataxin-3-transgenic mice to produce offspring that carry both transgenes (double hemizygous mice). Littermate pairs carrying either transgene (CAG/orexin- or orexin/ataxin-3-transgenic mice), both transgenes (orexin/ataxin-3;CAG/orexin-double-transgenic mice), or neither transgene (wild-type mice), as determined by genotyping with PCR, were selected for further experiments.
Anti-orexin-A immunohistochemistry of the brains of 15-week-old wild-type mice produced a pattern of staining in which orexin-producing cells were clustered in the perifornical LH, as described (refs. 9 and 10 and Fig. 1 a and b). In contrast, we found no orexin staining in any part of the brain of orexin/ataxin-3-transgenic littermates, which was consistent with destruction of endogenous orexinergic neurons by this age as reported (ref. 8 and Fig. 1 c and d). Littermates carrying the CAG/orexin transgene alone exhibited a widespread, diffuse orexin-immunoreactive staining throughout most of the brain, with highest levels observed in the hypothalamus, amygdala, hippocampus, and brainstem (Fig. 1 e and f). Notably, the native orexinergic neurons in CAG/orexin-transgenic mice were still visible above background in sections containing the LH, due to the extremely high levels of orexin immunoreactivity in endogenous neurons relative to those expressing the transgene ectopically. Orexin/ataxin-3;CAG/orexin-double-transgenic mice exhibited a similar pattern of widespread orexin signal throughout the hypothalamus and remaining brain. Notably absent, however, were endogenous orexinergic neurons (Fig. 1 g and h).
Fig. 1.
Immunohistochemical analysis of brains from transgenic mice. Anti-orexin-A immunostaining of coronal sections of brain tissue from wild-type, orexin/ataxin-3-hemizygous-transgenic-, CAG/orexin-hemizygous-transgenic-, and double-hemizygous-transgenic mice were performed as described (31). Note the punctate staining of native orexin-A-immunoreactive neurons clustered in the perifornical LH of wild-type mice (a and b) and the absence of orexin-A in the brains of orexin/ataxin-3-transgenic mice in which native orexinergic neurons have degenerated (c and d). CAG/orexin-transgenic mice have widespread, diffuse, ectopic production of orexin-A in addition to that observed in native neurons (e and f). In contrast, orexin/ataxin-3;CAG/orexin-double-transgenic mice exhibit only the ectopic pattern of orexin-A; native neurons are absent (g and h). (a Inset) The location and size of magnified images in b, d, f, and h. f, fornix; Arc, arcuate nucleus of the hypothalamus; 3V, third ventricle. (Bar, 3 mm for a, c, e, and g and 0.5 mm for b, d, f, and h).
We tested mice of all four genotypes for the presence of cataplectic arrests during the dark (active) phase by using infrared video photography. We adapted a behavioral paradigm in which narcoleptic mice are exposed to a novel environment that tends to increase the frequency of such arrests (6, 8), a method that has proven effective in screening for pharmacological suppression by clomipramine of cataplexy in narcoleptic mice (13). As in wild-type mice, mice carrying the CAG/orexin transgene alone exhibited no behavioral arrests of any kind (data not shown). The frequencies of arrests in orexin/ataxin-3 mice and orexin/ataxin-3;CAG/orexin-double-transgenic littermates were then compared (Fig. 2). All orexin/ataxin-3 mice exhibited cataplexy-like arrests within the 3-h observation period as described (8). In contrast, no arrests were observed in any of the orexin/ataxin-3;CAG/orexin-double-transgenic littermates under these same conditions. Thus, ectopic transgenic expression of orexin peptides prevents cataplectic arrests in mice in which endogenous orexin neurons have been ablated.
Fig. 2.
CAG/orexin transgene prevents cataplectic behavioral arrests in orexin neuron-ablated mice. Cataplectic arrests are absent in orexin/ataxin-3;CAG/orexin-double-hemizygous-transgenic mice, compared with orexin/ataxin-3-hemizygous-transgenic littermates. The number of cataplectic arrests observed (a) and total time spent in cataplexy (b) in each mouse are shown for 3-h sessions. Bars represent medians. ***, P < 0.0001 by Mann-Whitney test.
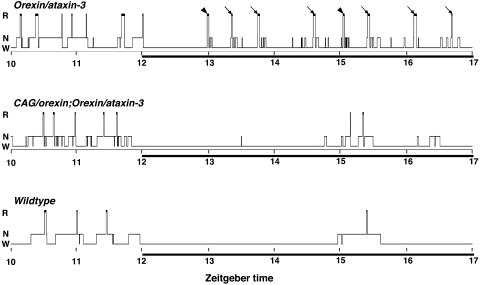
We then examined sleep/wake patterns over the entire 24-h day in each group of mice, by carrying out EEG/EMG recordings. As expected, the dark-phase sleep/wake patterns of orexin/ataxin-3 mice, summarized in hypnograms (Fig. 3), exhibited severe fragmentation of wakefulness and sleep with numerous brief non-REM sleep episodes and frequent transitions to REM sleep with little or no latency from preceding wakefulness. Such transitions were observed without exception in each mouse carrying the orexin/ataxin-3 transgene alone. In contrast, orexin/ataxin-3;CAG/orexin-double-transgenic littermates exhibited longer, more consolidated bouts of wakefulness and normalized amounts of REM sleep during the dark phase. Most importantly, no direct or premature wake-REM sleep transitions were ever detected in any double-transgenic mice. Hypnograms of wild-type mice from the same line were typical of the C57BL/6J strain, illustrating normal sleep/wake patterns during this period. Notably, compared with wild-type and narcoleptic mice, hypnograms of orexin/ataxin-3;CAG/orexin-double-transgenic mice exhibited increased levels of fragmentation of sleep specifically during the light (resting) phase, resulting possibly from chronic, abnormally high orexin tone due to the CAG/orexin transgene.
Fig. 3.
Sleep/wake cycles of typical transgenic and wild-type mice. Hypnograms represent concatenated 20-sec epochs of EEG/EMG activity, scored as awake (W), non-REM sleep (N), or REM sleep (R). Seven hours per mouse, including transitions from light phase to dark phase (solid bar), are shown. The orexin/ataxin-3-transgenic mouse exhibits fragmentation of wakefulness during the dark phase and frequent premature onsets of REM sleep that occur immediately after wakefulness (arrowheads) or after <1 min of preceding non-REM sleep (arrows). In contrast, the orexin/ataxin-3;CAG/orexin-double-transgenic mouse has more consolidated wakefulness during the dark phase. As in the wild-type mouse, no direct or premature transitions from wakefulness to REM sleep were ever observed in the double-transgenic mouse.
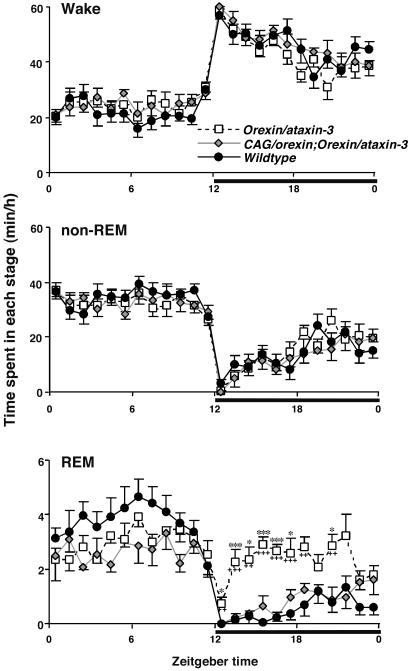
Sleep/wake states in these mice are also illustrated by hourly plots of amounts of time spent in wakefulness, non-REM sleep, and REM sleep over 24 h (Fig. 4). Consistent with a previous description (8), orexin/ataxin-3 mice showed selective increases in amounts of REM sleep during the dark phase, compared with wild-type controls. Importantly, mice carrying both transgenes exhibited a normalization of REM sleep amounts during the dark phase with an additional tendency toward reduced amounts of REM sleep during the light phase as well. Overall, ectopic expression of the CAG/orexin transgene prevented the development of symptoms of narcolepsy-cataplexy, despite the postnatal ablation of endogenous orexin neurons, supporting a specific role for orexin peptides in preventing narcolepsy-cataplexy.
Fig. 4.
Hourly plots of sleep/wake states in transgenic and wild-type mice. Narcoleptic orexin/ataxin-3-transgenic mice as well as orexin/ataxin-3;CAG/orexin-double-transgenic mice exhibit hourly amounts of wakefulness and non-REM sleep that are similar to those of wild-type mice. In contrast, orexin/ataxin-3-transgenic mice exhibit significantly increased amounts of REM sleep during the dark phase (solid horizontal bars). Double-transgenic mice exhibit a specific rescue of this abnormality compared with wild-type mice. *, P < 0.05; **, P < 0.005; ***, P < 0.0005 compared with wild-type mice; and +, P < 0.05; ++, P < 0.005; +++, P < 0.0005 compared with orexin/ataxin-3;CAG/orexin-double-transgenic mice by ANOVA and Tukey's post hoc tests. Values are means ± SE. (n = 10 for wild-type mice, n = 6 for orexin/ataxin-3 mice, and n = 5 for CAG/orexin;orexin/ataxin-3-double-transgenic mice.)
Pharmacological Rescue of Narcolepsy in Orexin Neuron-Ablated Mice. The rescue of the narcoleptic phenotype in orexin neuron-ablated mice by ectopic prepro-orexin expression led us to examine whether acute exogenous administrations of orexin might also rescue this phenotype. In pharmacological studies, we administered orexin-A, a nonselective agonist for orexin receptor type 1 (OX1R) and OX2R (9). The dose and route of administration chosen (3 nmol per mouse i.c.v.) is comparable to that used previously to examine the effects of orexin-A on behavior and metabolism in rodents (11, 18-20), and is effective in dose-response studies of sleep/wakefulness in wild-type mice, but produces no detectable effects in knockout mice lacking both OX1R and OX2R genes (M.M., unpublished observations).
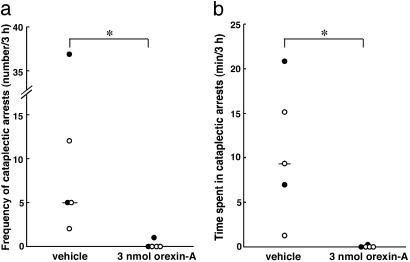
We examined whether i.c.v. orexin-A administration suppresses cataplectic arrests in orexin/ataxin-3-transgenic mice. By using a randomized crossover design, we administered vehicle and orexin-A to all mice during separate experimental sessions. When narcoleptic mice were treated with vehicle alone, they exhibited variable frequencies of arrests, as well as variable cumulative times spent in arrests during the first 3 h after injections (Fig. 5). In contrast, when the same mice were administered orexin-A, the frequency of arrests and overall time spent in cataplexy were significantly reduced in each mouse during the same 3-h time period, despite increases in observable wakeful activity. Thus, i.c.v. administration of orexin-A is sufficient to acutely suppress behavioral arrests in orexin/ataxin-3 mice.
Fig. 5.
Suppression of cataplectic arrests by i.c.v. orexin-A administration. Vehicle (artificial cerebrospinal fluid) and orexin-A (3 nmol per mouse) were administered by bolus injections into the lateral ventricles of five narcoleptic orexin/ataxin-3-transgenic mice before onset of the dark phase in a randomized crossover design, over two consecutive experimental sessions. The order in which animals were dosed is indicated by symbols: •, orexin-A first; ○, vehicle first. Number of cataplectic arrests observed (a) and total time spent in cataplexy (b) in each mouse are shown for 3-h sessions. Bars represent medians. *, P < 0.05 by Wilcoxon signed-ranks test.
To quantify the effects of orexin-A administration on sleep/wake status, we recorded EEG/EMG in orexin/ataxin-3 mice, as well as wild-type controls. Because narcoleptic humans and animals exhibit sleepiness and cataplexy during their respective active phases, it is likely that orexin-based therapies would be administered to humans during the active phase. Nevertheless, some pharmacological effects of exogenous orexins have been reported to vary with time of day (Zeitgeber-dependent effects, refs. 21 and 22). We therefore examined the effects of orexin-A administrations in mice both at the onset of the dark (active) phase as well as during the light (resting) phase, again by using a randomized crossover design.
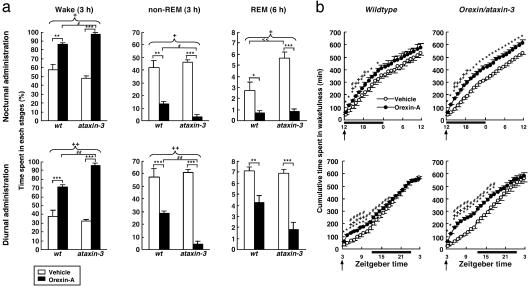
Patterns of wakefulness, non-REM sleep, and REM sleep revealed a robust arousal effect of orexin-A in transgenic narcoleptic as well as wild-type mice. Central administration of 3 nmol of orexin-A strikingly increased wakefulness and suppressed both non-REM and REM sleep, regardless of genotype and time of administration (Fig. 6a, and Fig. 7, which is published as supporting information on the PNAS web site). Interestingly, equivalent doses of orexin-A, administered during either the nocturnal or diurnal phases, produced arousal with greater effectiveness in narcoleptic mice compared with wild-type controls. Whereas both wild-type and narcoleptic mice exhibited similar amounts of wakefulness and non-REM sleep in a 3-h period after vehicle administrations, orexin-A induced significantly greater amounts of wakefulness in orexin/ataxin-3-transgenic mice than it did in wild-type mice. These increases in wakefulness were essentially mirrored by significantly greater suppressions of non-REM sleep in narcoleptic animals.
As expected, orexin/ataxin-3 mice exhibited significantly higher amounts of REM sleep than did wild-type controls during the dark phase under baseline (vehicle-administered) conditions. Critically, orexin-A effectively suppressed this elevation of REM sleep in narcoleptic mice. Again, orexin-A was more effective at suppressing REM sleep in narcoleptic mice than in wild-type controls, regardless of the time of administration (Figs. 6a and 7).
Fig. 6.
Orexin-A administrations increase wakefulness and suppress sleep in narcoleptic mice. (a) Amounts of time spent in each stage within 3 h (wake, non-REM) or 6 h (REM) after vehicle (open bar) and orexin-A (filled bar) administrations either at the beginning of the dark phase (nocturnal administration) or during the light phase started (diurnal administration) in wild-type (wt) or orexin/ataxin-3 (ataxin-3) mice. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vehicle vs. orexin-A in each genotype; #, P < 0.05; ##, P < 0.01 wild-type mice administered orexin-A vs. orexin/ataxin-3 mice administered orexin-A; <<, P < 0.01 wild-type mice administered vehicle vs. orexin/ataxin-3 mice administered vehicle; +, P < 0.05; ++, P < 0.01 interaction of dose and genotype, by two-way ANOVA. (b) Cumulative amounts of time spent in wakefulness after nocturnal and diurnal administrations of vehicle and orexin-A in wild-type or orexin/ataxin-3 mice. Times of administration are indicated by arrows. The dark phase is indicated by solid horizontal bars. *, P < 0.05 vehicle vs. orexin-A in each genotype; #, P < 0.05 wild-type mice administered orexin-A vs. orexin/ataxin-3 mice administered orexin-A; +, P < 0.05 interaction of dose and genotype, by two-way ANOVA. Values are means ± SE. (n = 3 for wild-type, n = 4 for orexin/ataxin-3 mice for both a and b.)
Unlike effects reported with amphetamine administrations in humans (23), rats (24), and mice (25), no immediate rebounds of sleep were observed after dark-phase or light-phase orexin-A administrations (Figs. 6b and 7). Only narcoleptic animals maintained a statistically significant increase of cumulative wakefulness (Fig. 6b) and a decrease of cumulative sleep (data not shown), even after 24 h after a nocturnal administration of orexin-A. This difference compared with wild-type controls likely resulted in part from the greater effectiveness of orexin-A in narcoleptic mice. In contrast, when orexin-A was administered diurnally, mice of both genotypes recovered sleep losses by the 24-h mark, but this recovery was gradual in nature. Overall, these results point to the feasibility of treating symptoms of narcolepsy-cataplexy by using pharmacological agonists of orexin receptors.
Discussion
Narcolepsy-cataplexy is a disorder of the organization of the sleep/wake cycle resulting, in a majority of patients, from absence of orexin peptides. Replacement therapy using orexin receptor agonists may provide treatment directed toward this fundamental pathophysiology. By using two distinct methods of replacing orexin peptides, we have demonstrated the reversal of symptoms of narcolepsy-cataplexy in a rodent model of the disorder in which native orexin neurons have degenerated.
Chronic overproduction of orexin peptides from an ectopically expressed transgene prevented the development of the narcolepsy-cataplexy syndrome after genetic ablation of endogenous orexin neurons. Similarly, bolus i.c.v. administrations of the nonselective orexin receptor agonist orexin-A acutely increased wakefulness, suppressed sleep, and inhibited cataplectic attacks in narcoleptic mice. Together, these findings provide strong evidence of the specific causal relationship between absence of orexin peptides in the brain and the development of the narcolepsy-cataplexy syndrome. From genetic studies alone, the formal possibility remains that the prepro-orexin transgene rescues the phenotype of orexin/ataxin-3 mice by precluding any period of orexin deficiency after the degenerative loss of native orexin neurons in young orexin/ataxin mice. However, our pharmacological rescue experiments indicate that even after substantial periods of complete orexin deficiency, the neural mechanisms required for orexin-mediated arousal and suppression of cataplexy; e.g., orexin receptors, intracellular signaling, postsynaptic neural networks, and other downstream neurotransmitter pathways, remain anatomically and functionally intact.
Our study suggested that acutely administered orexin-A has stronger arousal effects in orexin/ataxin-3-transgenic mice than in wild-type controls. The greater effectiveness may result from increased sensitivity of orexin-responsive pathways. Indeed, complex alterations in downstream neurotransmitter systems leading to cholinergic/monoaminergic imbalance are a well described feature of animal narcolepsy (26, 27). Whereas such changes in the brains of narcoleptics could theoretically result from compensatory increases in expression of excitatory orexin receptors, we have observed normal whole-brain levels of OX1R and OX2R mRNAs in prepro-orexin knockout mice (13), a phenotypic equivalent of the orexin/ataxin-3 mice used in this study.
We achieved definitive suppressions of behavioral cataplexy by using both genetic and pharmacological replacements of orexins. This study provides critical evidence that spatially or temporally specific activation of orexin receptors is not necessary to prevent cataplexy. These effects are unlikely to have resulted indirectly from increased arousal alone. The behavioral paradigm we used (exposure to novelty) induces high baseline levels of active, exploratory wakefulness among all genotypes (J.T.W., unpublished observations). Indeed, the elicitation of murine cataplectic attacks by novelty is itself probably related to this increased level of activity. Furthermore, other agents that promote wakefulness, such as amphetamines, modafinil, and caffeine, either have no effect on the frequency of human or mouse cataplexy or are mildly exacerbatory (2, 13, 26). Orexin peptides are therefore quite distinct from these agents in that they promote wakefulness while specifically suppressing cataplexy.
Another potential advantage of orexin agonist-based therapies follows from our results: arousal induced by a bolus administration of orexin-A resulted in no sharp rebounds of sleep afterward. When administered in coordination with the active phase, the orexin-induced gains in cumulative wakefulness lasted as long as 24 h after injection in narcoleptic mice. The absence of such rebounds, a potentially confounding factor in therapies for excessive daytime sleepiness based on classical psychostimulants (23), suggests that orexin-based therapies may more safely maintain wakefulness. Interestingly, recent studies suggest that orexins may promote attention and memory processes (28, 29); orexin-based therapies may therefore also improve cognitive function in coordination with increased arousal.
One other report, using OX2R-deficient narcoleptic Dobermans, has suggested that large peripheral doses of orexin-A may reverse sleep/wake fragmentation and cataplexy in some dogs (30). If true, such findings suggest that increased signaling mediated by OX1R may play a role in reducing cataplexy and normalizing sleep patterns. Unfortunately, other investigators have consistently failed to verify the findings presented in that study even at higher doses (S. Nishino and E. Mignot, personal communication). Considering that narcolepsy in Dobermans results from a defect in OX2R, lack of response to exogenous orexin would be a plausible finding in these dogs (4).
Our demonstration of a genetic rescue of narcolepsy-cataplexy may also provide implications for future human therapies, which might involve orexin gene therapy by using viral vectors, or transplantation of orexin neurons or stem cell precursors. Interestingly, we noted that fragmentation of sleep during the light (rest) phase was associated with chronic, unregulated overexpression of orexins, a phenotype to be described in greater detail elsewhere (J.T.W., T.S., and M.Y., unpublished data). As we have demonstrated pharmacologically, orexin-A has the potential to alter sleep/wake cycles when administered during either active or resting phases. Therefore, we predict that potential therapies based on orexin receptor agonists should rely on titration and timing of doses to reinforce rather than counteract normal circadian rhythms. Our observation that administrations of orexin-A promote wakefulness at the expense of non-REM and REM sleep in both wild-type and narcoleptic-cataplectic animals suggests that such agonists might also be useful in the treatment of other conditions of excessive daytime sleepiness in humans, including narcolepsy without cataplexy, idiopathic hypersomnia, and sleep deprivation of various causes. The effects of chronic orexin administrations in animals and the effects of orexin receptor agonists in humans clearly merit further investigation.
Supplementary Material
Acknowledgments
We thank S. A. Dixon and R. O. Floyd for technical support. M.Y. is an Investigator of the Howard Hughes Medical Institute. M.M. is a Long-term Fellow of the Human Frontier Science Program. J.T.W. is a Joint Fellow of the Department of Cellular Molecular Biology and Medical Scientist Training programs of University of Texas Southwestern Medical Center. This work was supported in part by research grants from the Perot Family Foundation and from the Exploratory Research for Advanced Technology/Japan Technology and Science Agency.
Abbreviations: CAG, β-actin/cytomegalovirus hybrid promoter; EEG/EMG, electroencephalograph/electromyograph; i.c.v., intracerebroventricular; LH, lateral hypothalamus; OX1R, orexin receptor type 1; OX2R, orexin receptor type 2; REM, rapid eye movement.
References
- 1.Mignot, E., Taheri, S. & Nishino, S. (2002) Nat. Neurosci. 5, Suppl., 1071-1075. [DOI] [PubMed] [Google Scholar]
- 2.Scammell, T. E. (2003) Ann. Neurol. 53, 154-166. [DOI] [PubMed] [Google Scholar]
- 3.Nishino, S., Ripley, B., Overeem, S., Lammers, G. J. & Mignot, E. (2000) Lancet 355, 39-40. [DOI] [PubMed] [Google Scholar]
- 4.Lin, L., Faraco, J., Li, R., Kadotani, H., Rogers, W., Lin, X., Qiu, X., de Jong, P. J., Nishino, S. & Mignot, E. (1999) Cell 98, 365-376. [DOI] [PubMed] [Google Scholar]
- 5.Peyron, C., Faraco, J., Rogers, W., Ripley, B., Overeem, S., Charnay, Y., Nevsimalova, S., Aldrich, M., Reynolds, D., Albin, R., et al. (2000) Nat. Med. 6, 991-997. [DOI] [PubMed] [Google Scholar]
- 6.Chemelli, R. M., Willie, J. T., Sinton, C. M., Elmquist, J. K., Scammell, T., Lee, C., Richardson, J. A., Williams, S. C., Xiong, Y., Kisanuki, Y., et al. (1999) Cell 98, 437-451. [DOI] [PubMed] [Google Scholar]
- 7.Thannickal, T. C., Moore, R. Y., Nienhuis, R., Ramanathan, L., Gulyani, S., Aldrich, M., Cornford, M. & Siegel, J. M. (2000) Neuron 27, 469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara, J., Beuckmann, C. T., Nambu, T., Willie, J. T., Chemelli, R. M., Sinton, C. M., Sugiyama, F., Yagami, K., Goto, K., Yanagisawa, M. & Sakurai, T. (2001) Neuron 30, 345-354. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., Williams, S. C., Richardson, J. A., Kozlowski, G. P., Wilson, S., et al. (1998) Cell 92, 573-585. [DOI] [PubMed] [Google Scholar]
- 10.Peyron, C., Tighe, D. K., van den Pol, A. N., de Lecea, L., Heller, H. C., Sutcliffe, J. G. & Kilduff, T. S. (1998) J. Neurosci. 18, 9996-10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piper, D. C., Upton, N., Smith, M. I. & Hunter, A. J. (2000) Eur. J. Neurosci. 12, 726-730. [DOI] [PubMed] [Google Scholar]
- 12.Huang, Z. L., Qu, W. M., Li, W. D., Mochizuki, T., Eguchi, N., Watanabe, T., Urade, Y. & Hayaishi, O. (2001) Proc. Natl. Acad. Sci. USA 98, 9965-9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willie, J. T., Chemelli, R. M., Sinton, C. M., Tokita, S., Williams, S. C., Kisanuki, Y., Marcus, J. N., Lee, C., Elmquist, J. K., Kohlmeier, K. A., et al. (2003) Neuron 38, 715-730. [DOI] [PubMed] [Google Scholar]
- 14.Willie, J. T., Chemelli, R. M., Sinton, C. M. & Yanagisawa, M. (2001) Annu. Rev. Neurosci. 24, 429-458. [DOI] [PubMed] [Google Scholar]
- 15.Mignot, E., Lammers, G. J., Ripley, B., Okun, M., Nevsimalova, S., Overeem, S., Vankova, J., Black, J., Harsh, J., Bassetti, C., et al. (2002) Arch. Neurol. (Chicago) 59, 1553-1562. [DOI] [PubMed] [Google Scholar]
- 16.van den Pol, A. N. (2000) Neuron 27, 415-418. [DOI] [PubMed] [Google Scholar]
- 17.Niwa, H., Yamamura, K. & Miyazaki, J. (1991) Gene 108, 193-199. [DOI] [PubMed] [Google Scholar]
- 18.Hagan, J. J., Leslie, R. A., Patel, S., Evans, M. L., Wattam, T. A., Holmes, S., Benham, C. D., Taylor, S. G., Routledge, C., Hemmati, P., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 10911-10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubkin, M. & Stricker-Krongrad, A. (1998) Biochem. Biophys. Res. Commun. 253, 241-245. [DOI] [PubMed] [Google Scholar]
- 20.Stricker-Krongrad, A., Richy, S. & Beck, B. (2002) Regul. Pept. 104, 11-20. [DOI] [PubMed] [Google Scholar]
- 21.Haynes, A. C., Jackson, B., Overend, P., Buckingham, R. E., Wilson, S., Tadayyon, M. & Arch, J. R. (1999) Peptides (Tarrytown, NY) 20, 1099-1105. [DOI] [PubMed] [Google Scholar]
- 22.Espana, R. A., Plahn, S. & Berridge, C. W. (2002) Brain Res. 943, 224-236. [DOI] [PubMed] [Google Scholar]
- 23.Valerde, C., Pastrana, L. S., Ruiz, J. A., Solis, H., Jurado, J. L., Sordo, C. M., Fernandez-Guardiola, A. & Maisterrena, J. A. (1976) Neuroendocrinology 22, 57-71. [DOI] [PubMed] [Google Scholar]
- 24.Edgar, D. M. & Seidel, W. F. (1997) J. Pharmacol. Exp. Ther. 283, 757-769. [PubMed] [Google Scholar]
- 25.Kitahama, K. & Valatx, J. L. (1979) Psychopharmacology 66, 291-295. [DOI] [PubMed] [Google Scholar]
- 26.Nishino, S. & Mignot, E. (1997) Prog. Neurobiol. 52, 27-78. [DOI] [PubMed] [Google Scholar]
- 27.Nishino, S., Fujiki, N., Ripley, B., Sakurai, E., Kato, M., Watanabe, T., Mignot, E. & Yanai, K. (2001) Neurosci. Lett. 313, 125-128. [DOI] [PubMed] [Google Scholar]
- 28.Wu, M., Zhang, Z., Leranth, C., Xu, C., van den Pol, A. N. & Alreja, M. (2002) J. Neurosci. 22, 7754-7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaeger, L. B., Farr, S. A., Banks, W. A. & Morley, J. E. (2002) Peptides (Tarrytown, NY) 23, 1683-1688. [DOI] [PubMed] [Google Scholar]
- 30.John, J., Wu, M. F. & Siegel, J. M. (2000) Sleep Res. Online 3, 23-28. [PMC free article] [PubMed] [Google Scholar]
- 31.Nambu, T., Sakurai, T., Mizukami, K., Hosoya, Y., Yanagisawa, M. & Goto, K. (1999) Brain Res. 827, 243-260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.