Abstract
The 4T1 mammary carcinoma cell line produces TSLP. We had hypothesized that TSLP promotes the development of a permissive environment for the growth and metastasis of primary tumor and that this is associated with a Th2 polarized anti-tumor immune response. We found that, in Tslpr−/− mice, the mean tumor diameters were smaller from days 27–40 and relatively fewer tumor cells were present in the lung, compared to wild type mice. Polarization of the Th2 cytokine profile was also diminished in Tslpr−/− mice. These findings confirmed those reported previously by others. Here, we further show that primary tumors are established less often in Tslpr−\− mice and that, unexpectedly, the relative number of tumor cells in the brain is greater in Tslpr−/− mice compared to wild type mice. Findings from our cytotoxicity assays show that 4T1-directed lysis is undetectable in both WT and Tslpr−/− mice, ruling out the possibility that altered cytotoxic responses in Tslpr−/− mice are responsible for the differences we observed. In a human tissue microarray, positive staining for TSLP was seen in tumor cells from breast cancer tissue but it was also seen in normal glandular epithelial cells from normal breast tissue, which has not been shown before. Thus, our findings provide new insight into the effects of TSLP in metastatic breast cancer.
Keywords: breast cancer, solid tumors, thymic stromal lymphopoietin, tumor immunity, immune regulation, mouse models, cytotoxicity
1.0 Introduction
The nature of the developing anti-tumor immune response is important in shaping the evolution of cancer. Both the innate and adaptive immune responses have the ability to either inhibit tumor development or generate a permissive environment in which tumor cells can thrive[1]. Chronic inflammation also plays an important role in the pathogenesis of cancer, including carcinoma of the breast[2–4]. Thymic stromal lymphopoietin (TSLP) is a cytokine that is made primarily by epithelial cells and has the ability to promote a Th2-mediated, inflammatory immune response[5–8]. The purpose of this study was to determine whether TSLP helps to create a permissive environment for the establishment, growth and metastasis of the primary tumor in the 4T1 mouse model of metastatic breast cancer.
TSLP is an IL-7-like cytokine derived primarily from epithelial cells. Although murine and human TSLP show only 43% amino acid identity, this cytokine performs similar functions in both species[9, 10]. Murine TSLP, originally identified in the supernatant from a murine thymic epithelial cell line, was found to support the growth of early T and B cell progenitors[11]. The functional TSLP receptor (Tslpr) consists of the IL-7Rα chain and a unique Tslpr chain. The latter can alone bind to TSLP with low affinity, [10, 12, 13] but when combined with IL-7Rα binds with higher affinity. Functional TSLP receptors are expressed on a range of cell lineages, including dendritic cells[14], CD4+ T cells[15–17], CD8+ T cells[18], and pro-B cells[19–21]. Several studies have shown that TSLP can promote the development of immune responses in which Th2 cytokines are dominant[15, 22–26]. Interestingly, human dendritic cells activated by TSLP can prime naïve CD4+ T helper cells to differentiate into Th2 cells that produce the pro-inflammatory cytokine, TNFα, in addition to classic Th2 cytokines[5]. Several studies have demonstrated that TSLP has a prominent role in inflammation. For example, chronic, systemic inflammation is seen in TSLP transgenic mice[6, 7] and atopic diseases in humans are associated with increased TSLP expression levels[5, 27, 28]. The latter has also been demonstrated in mouse models in which augmented TSLP expression in the skin is associated with an inflammatory response dominated by Th2 cytokines, as well as the histological features resembling atopic dermatitis in humans[29]. Several studies have shown that TSLP is an important mediator in the development of allergy-associated airway inflammation in mouse models. One study showed that Tslpr-deficient mice are resistant to the development of inflammation in the ovalbumin-plus-alum priming model and that reconstitution of Tslpr-deficient mice with T cells from Tslpr-expressing mice restores features of the inflammatory disease[16, 30]. These findings provide evidence that TSLP promotes an inflammatory response in which Th2-cytokines predominate.
We began this study when we discovered that TSLP is secreted into supernatants collected from cultures of 4T1 tumor cells. We hypothesized that this cytokine might therefore be a factor involved in the growth and progression of breast cancer. Since we started our study, two articles were published, also reporting a role for thymic stromal lymphopoietin (TSLP) in the development of breast cancer[31, 32]. In the study by Pedroza-Gonzalez and colleagues[31], supernatants from human breast tumor fragments showed a mixed Th1/Th2 cytokine profile but the levels of Th2 cytokines and TNFα were higher in these supernatants than in those from the tissue surrounding the tumor. The percentage of infiltrating IL-4 and IL-13-secreting CD4+CD3+ T cells was also higher in the tumor tissue. TSLP expression was seen in both human breast cancer cell lines and malignant breast tissue from patients. Using a xenogeneic mouse model, they further showed that neutralizing OX40L or TSLP with specific antibodies inhibited breast cancer growth and IL-13 production. In the second study, Olkhanud and colleagues[32] used the 4T1 mouse model of metastatic breast cancer to show that TSLP induced the differentiation of CD4 cells into Th2 cytokine-producing cells, which support cancer progression. The mean diameter of the primary tumor was smaller in Tslpr−/− mice than in WT mice by day 26 post-injection and TSLP levels in the sera correlated with the tumor growth in WT mice. Tumor growth and lung metastases were not seen in mice that had been injected with 4T1 cells that were unable to produce TSLP, suggesting that tumor-derived TSLP plays a pivotal role in tumor growth and metastasis in this model. Furthermore, metastatic foci in a lung biopsy from a patient with metastatic breast cancer stained positively for TSLP.
In our study, we used the 4T1 mouse mammary tumor model to compare the establishment and growth of the primary tumor, as well as its effect on the quantity of tumor cells in the lungs and brains of WT and Tslpr−/− mice. Furthermore, we wanted to determine whether TSLP responsiveness plays a role in shaping the de novo cytotoxic response and cytokine profile, as well as the cytotoxic and cytokine responses that develop over time in tumor-bearing mice. Although TSLP expression was previously shown in tumor tissue from patients with breast cancer[32, 31], we also wished to determine whether it is expressed in normal breast tissue. We therefore examined TSLP expression in a tissue microarray consisting of both normal breast tissue and tissue from patients with breast cancer.
2.0 Materials and Methods
2.1 Mice
Wild type Balb/c mice and TSLP receptor-deficient (Tslpr−/−) mice with a Balb/c genetic background were used. All mice were female and 8–10 weeks of age. Wild type mice were obtained from the local colony in the Genetic Models Center at the University of Manitoba. Tslpr−/− mice were generated as previously described[15]. Breeding pairs were provided by Dr. W. Leonard, National Heart, Lung and Blood Institute, Bethesda, MD and bred in the Genetic Models Center at the University of Manitoba. All of the experiments were performed in accordance with the standards of the Canadian Council on Animal Care.
2.2 Cell lines
Cell lines were maintained in complete RPMI 1640 culture medium (Life Technologies, Grand Island, NY) supplemented with 10% FBS (Gibco, Grand Island NY) and 1% penicillin-streptomycin (Gibco; 10000 units/ml Penicillin, 10000 μg/ml Streptomycin). The 4T1 mouse mammary carcinoma cells (H-2d) used in this study were obtained from Dr. Gary Sahagian at Tufts University, Boston, MA. This cell line, designated 4T1-12B, was derived by co-transfecting 4T1 cells with a firefly luciferase-containing vector and a puromycin resistance-vector [33]. 4T1-12B cells were derived from 4T1 cell obtained from Dr. Fred Miller at Karmanos Cancer Institute. The Moloney virus-induced lymphoma cell line, YAC-1 (H-2k/d) was obtained from the American Type Culture Collection (Rockville, MD). 4T1-12B cells were treated with 0.25% Trypsin-EDTA (Gibco) for two minutes and washed once in culture medium prior to being passaged.
2.3 Experimental Design
We used the 4T1 mouse mammary tumor model to determine how TSLP responsiveness affects the establishment, growth and metastasis of primary tumors, as well as certain aspects of the anti-tumor immune response. Two experimental groups were established, one in which 4T1-12B cells were injected into WT Balb/c mice and another in which 4T1-12B cells were injected into Tslpr−/− mice on a Balb/c genetic background. Wild type and Tslpr−/− mice were injected in the right mammary fat pad with 7 × 10−3 4T1-12B cells s.c., based on the protocol described by Pulaski and Ostrand-Rosenberg[34]. The effect of TSLP responsiveness on the establishment and growth of the primary tumor was studied by palpating the injection site and measuring the diameter of the primary tumor every 3–4 days using digital vernier calipers. In another series of experiments, we euthanized tumor-bearing mice from the two experimental groups at several time points and compared the cytokine profiles and 4T1-12B-directed lysis in both WT and Tslpr−/− mice bearing tumors. De novo cytokine and cytotoxic responses were also studied by co-culturing splenocytes from naive WT and Tslpr−/− mice with 4T1-12B cells at various ratios. Some mice in each group were allowed to reach their humane end point, as described in our animal use protocol. The relative number of tumor cells in the lung and brain were compared at these late time points. Controls for these experiments consisted of age and gender-matched WT and Tslpr−/− Balb/c mice that had not been injected with 4T1-12B tumor cells.
2.4 Determining the relative quantities of tumor cells in the lung and brain
In WT and Tslpr−/− mice that had reached their humane end point (mean=day 55 p.i.), we compared the relative quantities of tumor cells at two sites of metastasis, the lung and brain. Because the 4T1-12B cells contain a luciferase construct, we used the number of photons emitted per second in whole organ lysates that had been treated with luciferin as an indicator of the relative number of tumor cells present. Briefly, the tissue was snap frozen in liquid nitrogen and stored at −80°C until analysis. Frozen tissue was homogenized mechanically, mixed with cell culture lysis reagent (CCLR; Promega, Madison, WI, USA), vortexed for 15 minutes, subjected to three freeze-thaw cycles and then centrifuged at 13 000 rpm for 3 minutes at 4°C. The lysis procedure was then repeated for each cell pellet, without the freeze-thaw cycles. The supernatants from each sample were combined, adjusted to a volume of 1 ml and stored at −80°C until analysis. This method is described in greater detail elsewhere[35]. A Lowry protein quantification assay (BioRad Laboratories, Inc., Mississauga, ON, Canada) was used to determine the concentration of protein in the lysate. The Luciferase Assay System (Promega, Madison, WI, USA) was used to quantify free firefly luciferase in the tissue lysates. Lysates were adjusted to a concentration of 30 mg/ml of protein to ensure that readings were taken in the optimal range that had been determined in our validation assays, and two-fold serial dilutions were performed in CCLR. Twenty microliters of the resulting lysates were added to the wells of a white, opaque, 96-well, round-bottomed Costar microtiter plate (Corning Inc., Corning, NY). One hundred microliters of Luciferase Assay Reagent (Promega) were added to each well immediately before reading. Plates were read for 2 seconds using the BioTek Synergy 4 microplate reader (BioTek Instruments, Inc., Winooski, VT). Lysate from 4T1-12B cells was used as a positive control. CCLR alone was used as a negative control. To determine the relative luciferase signal for each whole organ, we multiplied the numbers of photons/second emitted by each sample by the dilution factor that was used earlier when adjusting the lysates to a protein concentration of 30 mg/ml.
2.5 Measuring cytokine levels in tumor-bearing mice
Spleens were harvested from WT and Tslpr−/− mice on days 7, 14, 21, 28 and 60 following tumor cell injection. The organs were minced with scissors, pressed through a stainless steel wire mesh to obtain a single-cell suspension and counted. Suspensions were then placed in a 24-well culture plate at a concentration of 1 × 107 cells/ml in avolume of 2 ml and incubated at 37° in 5% CO2. Supernatants were collected 48 hours later and stored at −20°C until the ELISAs were performed. Suspensions of splenocytes were prepared and cultured in complete RPMI-1640 supplemented with 5% FBS and 1% penicillin-streptomycin. OptEIA ELISA kits (BD Biosciences, San Diego, CA) were used to measure IFN-, IL-4, IL-12(p40). A DuoSet ELISA Development System (R&D Systems, Minneapolis, MN) was used to measure TSLP. All ELISAs were performed according to the manufacturer’s instructions.
2.6 Measuring cytotoxic activity in tumor-bearing mice
A 4-hour chromium-51 release assay was used to measure the levels of 4T1-12B-directed lysis in splenocytes from WT and Tslpr−/− tumor-bearing mice on days 7, 21, and 35. This method is described in detail elsewhere[36]. Briefly, spleens were harvested, minced with scissors and pressed through a stainless steel mesh to obtain a single cell suspension. Cells were counted, adjusted to a concentration of 107 cells/ml, and serially diluted 4 times to provide effector-to-target (E:T) cell ratios ranging from 100:1 to 12.5:1. 4T1-12B and YAC-1 target cells were labeled with chromium-51 radionuclide, sodium chromate in normal saline (Perkin Elmer, Woodbridge, Ontario, Canada) at a dose of 50 μCi/1 × 106 cells for 60 min. Cells were then washed three times in RPMI 1640 supplemented with 5% FBS, and adjusted to a final concentration of 105 cells/ml. Cytotoxicity was measured at each E:T cell ratio in triplicate cultures consisting of 100 μl of effector cell suspension and 100 μl of target cell suspension combined in wells of a plastic 96-well V-bottom microtiter plate. After centrifugation, 100 μl of supernatant was harvested from each well and counted for 2 min in a Wallac Wizard2 Automatic Gamma (Perkin Elmer, Waltam, MA). Spontaneous release of chromium-51 was measured by taking 100 μl of supernatant from cultures containing of 100 μl of target cells and 100 μl of medium without effector cells. Maximum chromium-51 release was measured re-suspending cultures containing 100 μl of target cells and 100 μl of medium and removing 100 μl of the cell suspension. The plates were incubated at 37°C for 4 h in 5% CO2. The percent lysis for each sample was calculated as follows:
The SE of the mean percent lysis for each triplicate was calculated, and dose-response curves were drawn. The number of lytic units per 107) effector cells (LU5/107) was calculated using exponential fit as described by Pross et al[37].
2.7 Measuring de novo cytokine and cytotoxicity responses
Spleens were harvested from normal WT and Tslpr−/− control mice without tumors. The organs were minced with scissors, pressed through a stainless steel wire mesh to obtain a single-cell suspension. The cells were counted, the suspension was adjusted to a concentration of 1×107 splenocytes/ml in complete RPMI 1640 medium supplemented with 5% FBS and 1% penicillin/streptomycin and two-fold serial dilutions were performed. The resulting suspensions were added to a 24-well cell culture plate at a volume of 1 ml/well. Confluent 4T1-12B cells were harvested by treatment with trypsin-EDTA, as described above, adjusted to a concentration of 1×105 cells/ml in RPMI/5% FBS and added to the splenocytes at a volume of 1 ml/well. Suspensions of splenocytes were prepared and cultured in complete RPMI-1640 supplemented with 5% FBS and 1% penicillin-streptomycin. Controls consisted of WT or Tslpr−/− splenocytes only at a concentration of 1 × 107 cells/ml or 4T1-12B cells only at a concentration of 1 × 105 and supernatants were collected 24 or cells/ml. Plates were incubated at 37° C in 5% CO2 48 hours later. Cytokines were measured in supernatants by ELISA, as described above.
A 4-hour chromium-51 release assay was used to measure splenic cytotoxic activity directed against tumor cells by splenocytes from naïve WT and naïve Tslpr−/− control mice. Spleens were harvested and a splenocyte suspension was prepared as described above. Cells were counted, adjusted to a concentration of 107 cells/ml, and serially diluted 4 times to obtain the following effector-to-target cell ratios: 100:1, 50:1, 25:1, 12.5:1. 4T1-12B and YAC-1 target cells were labeled with chromium-51 radionuclide, as described above and adjusted to a final concentration of 105 cells/ml. Cytotoxicity at each E:T cell ratio was measured in triplicate cultures consisting of 100 μl of effector cell suspension and 100 μl of target cell suspension combined in wells of a plastic 96-well V-bottom microtiter plate. Spontaneous release and maximum chromium-51 release were measured as described above. The plates were incubated and 100 μl of supernatant was harvested from each well and counted as described above. The percent lysis and SE for each triplicate sample were calculated and dose-response curves were drawn. The number of lytic units per 107 effector cells (LU5/107)were calculated as described above.
2.8 Detecting TSLP expression in normal human breast tissue and human breast cancer samples
Tumor microarrays were prepared using tissue samples from the Manitoba Breast Tumor Bank, which operates with the approval of the Faculty of Medicine, University of Manitoba, Research Ethics Board[38]. Core tissue samples (0.6 mm diameter; 50 from patients with breast cancer and 50 from normal breast tissue obtained from mammoplasty reduction surgery) were taken from selected areas using a tissue arrayer instrument (Beecher Instruments, Silver Spring, MD). Serial sections of TMAs were de-waxed, rehydrated and submitted to heat-induced antigen retrieval for 8 min, in the presence of citrate buffer using an automated tissue immmunostainer (Discovery Staining Module, Ventana Medical Systems, Tucson, Arizona). Immunohistochemical staining for TSLP was performed using anti-human TSLP antibody (ab79493; Abcam, Cambridge, MA) at a dilution of 1:100, as described elsewhere[39]. The entire glass slide containing the tissue microarray was scanned at room temperature using an Aperio ScanScope system (Leica Biosystems, Vista, California) and Aperio Spectrum acquisition software (Version 11.1.1.765). A composite figure (Figure 7) containing six images of representative tissue cores (40X magnification) was prepared using iPhoto ’09 software (Version 8.1.2, Apple Inc., Cupertino, CA).
Figure 7. TSLP is expressed in both breast cancer cells and in normal, glandular breast epithelial cells.
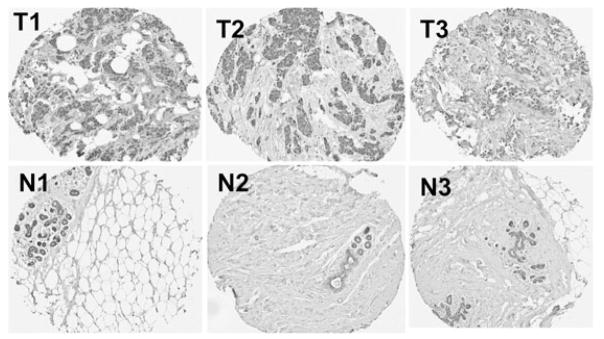
Tissue microarrays (TMA) were prepared from formalin-fixed, paraffin-embedded tissue from the Manitoba Breast Tumor Bank. TSLP expression (staining brown) is present in both breast tumors (T) and normal breast tissue (N) that had been procured from mammoplasty reduction surgery. Representative cores from three different patients (T1, T2 and T3) are shown, along with representative cores of normal breast tissue from three individuals (N1, N2 and N3). A total of 50 normal breast tissue cores and 50 malignant breast tissue cores were present in the TMA that was analyzed.
3.0 Results
3.1 TSLP production by 4T1-12B tumor cells
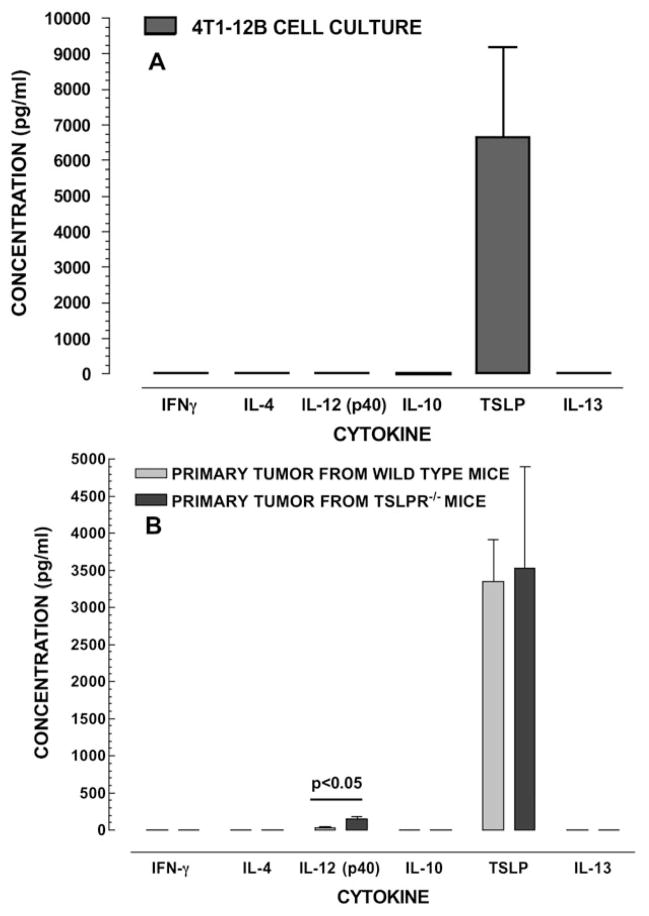
Cultures of 4T1-12B cells have the ability to produce TSLP in vitro. In our study, the TSLP concentrations were elevated in 4T1-12B cells, whereas other cytokines such as IFN-γ, IL-4, IL-12, IL-10 and IL-13 were not detected (Figure 1A). Our findings also show that the primary tumors that developed in WT and Tslpr−/− mice following the injection of 4T1-12B cells retain the ability to produce TSLP ex vivo. There was no significant difference in the TSLP concentrations in supernatants from WT and Tslpr−/− mice (p>0.05). It is noteworthy that the concentration of IL-12(p40) was significantly higher in cultures obtained from the Tslpr−/− group (p<0.05). In both groups, the concentrations of IFNγ, IL-4, IL-10 and IL-13 were below the level of detection.
Figure 1. 4T1-12B mouse mammary carcinoma cells secrete TSLP.
4T1-12B cells were grown in culture and cytokine concentrations were measured in the supernatant (A). Primary tumors from WT and Tslpr−/− mice were dissociated and the resulting cells were counted (B). Equal numbers of cells were plated for each group and cytokines levels were measured in the supernatants. Figure 1A shows that the mouse breast cancer cell line 4T1-12B secretes large amounts TSLP protein in culture. Figure 1B shows that TSLP is also produced by the primary tumor in both WT and Tslpr−/− mice bearing primary tumors (B). Concentrations of cytokines were compared using a Student’s t test (n=3).
3.2 Establishment and Growth of Primary Tumors
In these experiments, we determined whether the number of mice that established a primary tumor was different in WT and Tslpr−/− mice. Of the 19 WT and 18 Tslpr−/− mice that were injected with 4T1-12B tumor cells, the number in which primary tumors were successfully established was 18 and 11, respectively (Table 1; p<0.05). We then compared the diameters of the primary tumors in WT and Tslpr−/− mice at several time points and found that the mean primary tumor diameters were significantly higher in WT mice compared to Tslpr−/− mice from days 27 to 40 (p<0.05). These findings show that TSLP unresponsiveness is associated with slower tumor growth (Figure 2).
Table 1.
Primary tumors are established more often in WT mice than in TSLPR−/− mice over a 60-day period
| Number of mice with a primary tumor1 | No. of mice with no tumor2 | Total | p-value | |
|---|---|---|---|---|
| Wild-type | 18 | 1 | 19 | p<0.053 |
| TSLPR−/− | 11 | 7 | 18 | |
| Total | 29 | 8 | 37 |
The presence of a primary tumor was defined as a palpable mass of any size in the abdomen at any point post-injection (p.i.).
The absence of a primary tumor was defined as no palpable mass of any size in the abdominal area at any time point p.i.
The difference in the number of mice that either did or did not establish a primary tumor in the WT and TSLPR−/− groups were compared using a Fisher’s Exact test. Data shown is from three separate experiments, combined.
Figure 2. Primary tumors are smaller in Tslpr−/− mice than in WT mice 27–40 days after the tumor cells are injected.
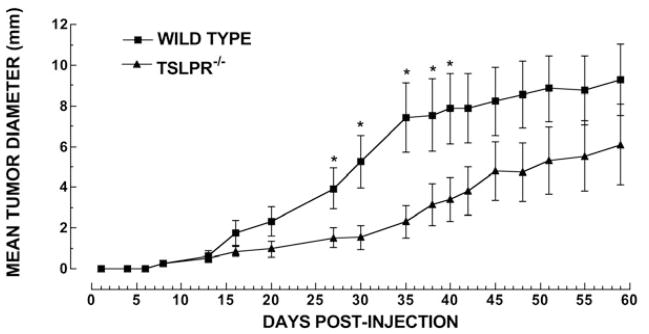
Female Balb/c mice were injected with 7 × 103 4T1 cells, s.c., into the mammary fat pad, at eight weeks of age. The mean tumor diameter (A) in WT (■) and Tslpr−/− (▲) mice was calculated as follows: diameter = •(l × w), where l=length and w=width. The mean tumor diameters were compared at each time point using a Student’s t test (n=8 mice/group). Asterisks indicate significant differences in the means. P values are as follows: day 27, p<0.05; day 30, p<0.03; day 35, p<0.02; day 38, p<0.05; and day 40 p<0.05. p.i: post-injection
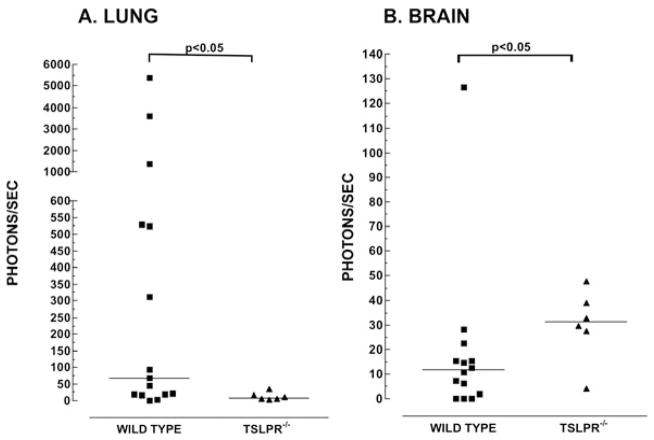
3.3 Relative quantities of tumor cells present in the lung and brain
It was previously shown that pulmonary metastasis of 4T1 tumor cells is diminished in Tslpr−/− mice[32]. In our study, we wished to determine the relative quantities of tumor cells in the lungs and brains of WT and Tslpr−/−. Figure 3 shows that the median number of photons/sec measured was higher in the lung in WT mice (55) compared to Tslpr−/− mice, (9 ; p<0.05). Conversely, the median number of photons/sec detected in the brain was lower in WT mice (12) than in Tslpr−/− mice (31; p<0.05). These findings show that TSLP responsiveness is associated with the presence of more tumor cells in the lung and, surprisingly, relatively fewer tumor cells in the brain.
Figure 3. The relative median number of tumor cells is lower in the lung and higher in the brain in Tslpr−/− mice.
Tumor-bearing mice that had reached their humane endpoint were euthanized and the lungs and brains were assayed by chemiluminescence for the presence of 4T1-12B cells. Symbols represent the number of photons/sec measured for a whole organ lysate obtained from an individual WT (■) or Tslpr−/− (▲) mouse. Medians, denoted by a horizontal line, were compared using a Mann-Whitney test. The number of mice in each group were as follows: Lung: n=15 for WT and n=6 for Tslpr−/−. Brain: n= 14 for WT and n=6 for Tslpr−/− mice. Data shown in Figures 6A and 6B are from three separate experiments, combined.
3.4 Cytokine Profiles
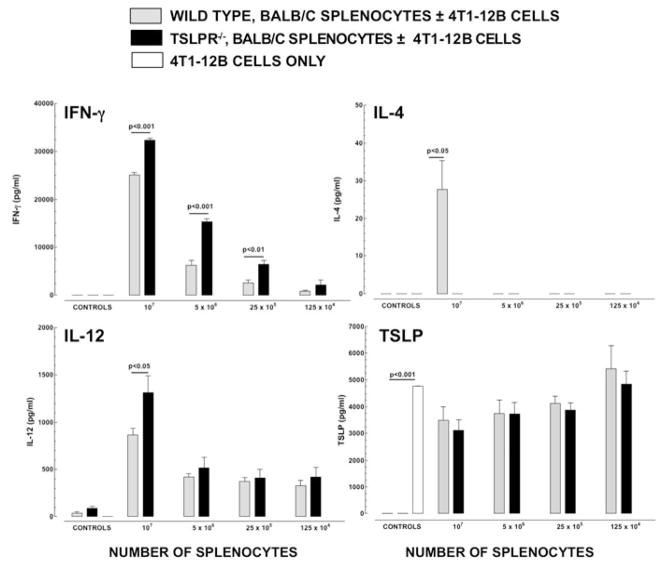
Because TSLP has been shown to promote the development of immune responses in which Th2 cytokines dominate, we compared the cytokine responses to 4T1-12B cells in splenocytes from WT and Tslpr−/− mice. Splenocytes were derived from both naïve and tumor-bearing mice. The de novo cytokine response to 4T1-12B tumor cells was shifted towards a greater level of Th1 cytokine production when splenocytes from Tslpr−/− mice were used (Figure 4). IFNγ was present in significantly higher concentrations in cultures containing Tslpr−/− splenocytes, compared to WT splenocytes, when the splenocytes concentration was 107 cells/ml (p<0.001), 5 × 106 cells/ml (p<0.001), and 25 × 104 cells /ml (p<0.01). IL-4 was only detectable in cultures containing splenocytes from WT mice at a splenocyte concentration of 107 cells/ml. It was not detectable in any of the other cultures tested. The concentration of IL-12 was significantly higher in cultures containing splenocytes from Tslpr−/− mice at the highest splenocyte concentration (p<0.05). The concentrations of TSLP in cultures containing splenocytes from either WT or Tslpr−/− mice were similar to those seen in cultures containing 4T1-12B alone (p>0.05 by ANOVA).
Figure 4. The de novo immune response to 4T1-12B tumor cells by splenocytes from naïve Tslpr−/− mice is shifted Away from Th1-dominated cytokine production.
One hundred thousand 4T1-12B cells were co-cultured with four concentrations of naïve, unstimulated splenocytes from WT or Tslpr−/− mice. Controls consisted of 1×107 WT or Tslpr−/− splenocytes alone and 1×105 4T1-12B cells alone. Cultures were incubated for 48 hours and supernatants were assayed by ELISA to determine the concentration of IFNγ, IL-4, IL-12(p40) and TSLP. Differences in the mean concentrations of cytokines in the WT and Tslpr−/− groups were compared for each concentration of splenocytes, using a Student’s t test (n=3/group/time-point). Data shown are from one representative experiment performed twice.
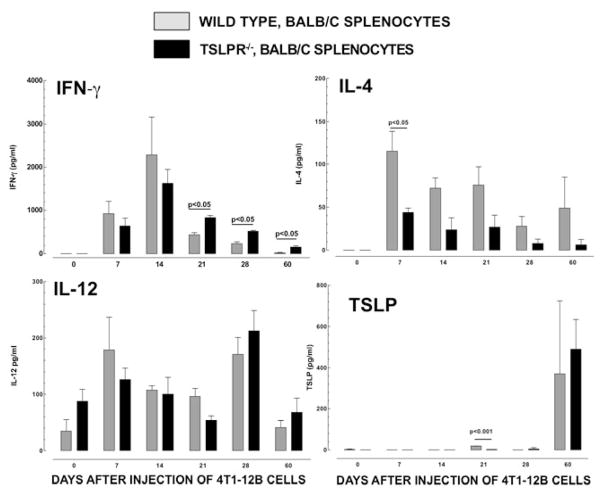
The cytokine profile in tumor-bearing Tslpr−/− mice was shifted away from Th2-dominated immunity over the course of the disease (Figure 5). The concentrations of IFN were significantly higher in splenocytes cultures from Tslpr−/− mice on days 21, 28 and 60, compared to those seen in cultures from WT mice (p<0.05). IL-4 reached higher levels in splenocyte cultures from WT mice compared to Tslpr−/− mice at each time point tested. This difference was significant on day 7 (p<0.05). There were no significant differences in the levels of IL-12(p40) observed in the two groups at any of the time points tested. The concentration of TSLP was significantly higher in cultures from WT mice on day 21 (p<0.001) but the levels were relatively low (<60 pg/ml). Higher levels of TSLP were seen on day 60 but the concentrations in cultures from WT and Tslpr−/− mice were not significantly different (p>0.05). Collectively, our findings show that TSLP responsiveness promotes the development of a Th2-polarized cytokine profile in immune responses to 4T1-12B tumor cells that develop in both naïve and tumor-bearing mice.
Figure 5. The cytokine profile in tumor-bearing Tslpr−/− mice is shifted away from Th2-dominated immunity and towards Th1-dominated immunity over a 60-day period.
Seven thousand 4T1-12B cells were injected into the mammary fat pad of 8-week female WT or Tslpr−/− mice. Spleens were harvested every 7 days p.i. until day 28, and then again on day 60 p.i. Splenocytes were cultured for 48 hours. ELISA was used to measure the following cytokines in the supernatants: IFNγ, IL-4, IL-12(p40) and TSLP. Differences in the mean concentrations of cytokines were compared in the WT and Tslpr−/− groups, at each time point, using a Student’s t test (n=3/group/time-point). Data shown are from one representative experiment performed twice.
3.5 Cytotoxicity Responses
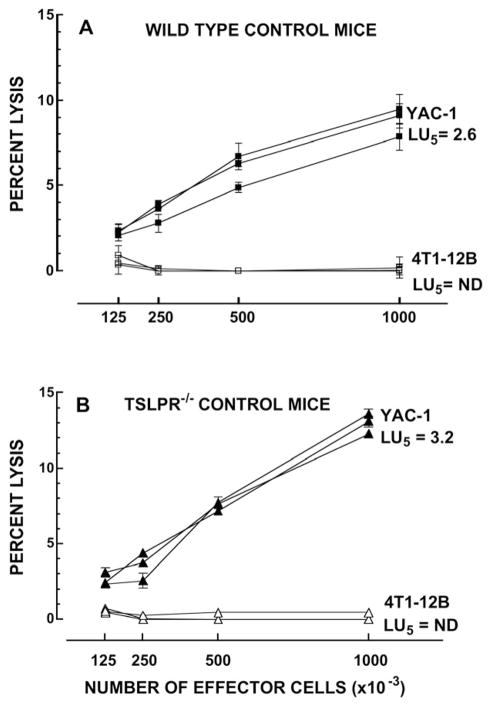
Whether TSLP can influence the development of cytotoxic responses to 4T1 tumor cells has not been studied previously. We therefore compared both the cytotoxicity responses to 4T1-12B cells in splenocytes from WT and Tslpr−/− mice that were either naïve or bearing tumors. Figure 6 shows the dose-response curves for splenic effector cells harvested from individual, naïve WT mice and Tslpr−/− mice. YAC-1-directed lysis was seen in both WT and Tslpr−/− mice indicating that the absence of TSLP-responsiveness does not inhibit the spontaneous lytic response directed against an NK-sensitive tumor target cell line. 4T1-12B-directed lysis was undetectable when naïve splenocytes from either WT or Tslpr−/− mice were used, which is consistent with previous observations that 4T1-12B cells are poorly immunogenic[40]. Table 2 shows that 4T1-12B-directed cytotoxicity was also undetectable in both the WT and Tslpr−/− mice bearing tumors. In addition, the levels of YAC-1 directed cytotoxicity decreased in both WT and Tslpr−/− over time and were consistently lower than those seen in control mice.
Figure 6. Splenocytes from naïve WT and Tslpr−/− mice do not exhibit 4 T1-12B-directed lytic activity.
Cell-mediated cytotoxicity was measured in a 4-hour chromium-51 release assay. Unstimulated splenocytes from individual naïve WT (A) or Tslpr−/− (B) mice were incubated for 4 hours with 51Cr-labeled 4T1-12B cells or YAC-1 cells at the following effector to target ratios: 100:1, 50:1, 25:1 and 12.5:1. Lytic units (LU5) were calculated using the exponential fit method, as described by Pross et al [46]. ND= not determined. n=3 mice/group/target. Symbols are representative of the following: ■: YAC-1-directed lysis by splenocytes from individual WT mice; □ 4T1-12B-directed lysis by splenocytes from individual WT mice; ▲: YAC-1-directed lysis by splenocytes from individual Tslpr−/− mice; △: 4T1-12B-directed lysis by splenocytes from individual Tslpr−/− mice. Data shown are from one representative experiment performed twice.
Table 2.
4T1-12B – directed cytotoxicity is undetectable in both WT and TSLPR−/− mice after the primary tumor has been established
| Wild-type mice (LU5)1 | TSLPR−/− mice (LU5) | |||
|---|---|---|---|---|
| 4T1-12B target cells | YAC-1 target cells4 | 4T1-12B target cells | YAC-1 target cells | |
| Control mice2 | ND3 | 2.6 ± 0.3 | ND | 3.1 ± 0.1 |
| Day 7 p.i. | ND | 0.4 ± 0.2 | ND | ND |
| Day 21 p.i. | ND | 0.9 ± 0.9 | ND | 1.1 ± 0.6 |
| Day 35 p.i. | ND | 0.8 ± 0.7 | ND | 0.3 ± 0.3 |
Lytic units were calculated according to the exponential fit as described in the Materials and Methods.
Age- and sex-matched WT or TSLPR−/− mice that had not been injected with tumor cells.
ND: LU5 not determined as percent lysis values were too low.
Positive control cell line.
3.6 TSLP expression in human breast tissue
To verify previously reported findings that TSLP is expressed in human breast tumors, we obtained tissue microarrays from the Manitoba Breast Tumor Bank to determine whether TSLP is expressed by epithelial cells from normal human breast tissue and/or by tumor cells in tissue biopsies from patients with breast cancer. Figure 7 shows representative images of positive staining for TSLP in breast tumors from three patients (T1, T2 and T3) and in normal breast tissue from three individuals (N1, N2 and N3). Tumor cells in the cores from breast cancer patients stained positively (T1-T3). Interestingly, normal glandular epithelial cells (N1-N3) from normal human breast tissue also stained positively. Some faint staining was observed in the stroma, which may be related to the fact that TSLP is a secreted protein.
4.0 Discussion
In this study, we confirmed findings from other investigators showing that the mouse mammary carcinoma cell line 4T1 produces TSLP in vitro and that the primary tumors that develop following the injection of these cells into Balb/c mice continue to produce this cytokine. Our findings further show that primary tumors from Tslpr−/− mice are smaller between days 27 and 40 compared to WT mice. Cytokine responses by splenocytes from either naïve or tumor-bearing WT mice are dominated by Th2 cytokines. As we had expected, the cytokine profile was shifted away from Th2 polarization when Tslpr−/− mice are used. Collectively, these findings indicate that TSLP facilitates the development of a permissive environment that promotes the growth of the primary tumor, which is consistent with the findings of others[32, 31].
In our study, primary tumors became established less frequently in mice lacking the TSLP receptor. Olkhanud et al previously reported that primary tumors did not become established in this model when TSLP production had been abrogated in the tumor cells by shRNA. These findings show that TSLP plays a pivotal role in the very early stages of the disease. We found that a cytotoxic response to the tumor cells failed to develop in both WT and Tslpr−/− mice bearing tumors. This novel finding indicates that the differences that we had observed during the course of the disease are not a result of differences in the levels of tumor-directed lysis. Although the relative quantity of tumor cells in the lung was diminished in Tslpr−/− mice, we unexpectedly found that it was greater in the brain. This novel but paradoxical observation suggests that TSLP mitigates metastasis to the brain. It was established several decades ago that the brain is an “immunologically privileged” site, a characteristic that helps to limit injury during inflammation in an organ with poor regenerative capacity[41]. This feature of the central nervous system (CNS) may become compromised if there is breakdown of the blood brain barrier (BBB)[41]. It is possible that the intrinsic properties of the BBB inhibit the entry of blood-borne 4T1-12B cells into the CNS in tumor-bearing WT mice. Since the anti-tumor response is shifted towards Th1 immunity in Tslpr−/− mice, it is possible that over production of Th1 cytokines may injure the BBB, thereby facilitating the movement of 4T1-12B cells across the BBB and into the brain more readily. This possibility is supported by studies showing that IFNγ and other Th1 cytokines increase BBB permeability and can enhance injury to the CNS, lending credence to this idea[42–45].
Our human tissue microarray study showed that, in addition to malignant epithelial cells, normal glandular epithelial cells also express TSLP protein. This novel finding suggests that TSLP is not specific to malignant epithelial cells in the breast but it is possible that TSLP or its receptor undergo either quantitative or qualitative changes in the microenvironment during the development of breast cancer.
In summary, our findings, as well as recent findings from other investigators support the idea that TSLP helps to create a permissive microenvironment for the development and pulmonary metastasis of mammary carcinoma in a mouse model using implanted 4T1 tumor cells. As such, they provide the rationale for new studies aimed at further evaluating the mechanisms of action for TSLP, as well as its prognostic value in human breast cancer.
Acknowledgments
This project was funded by an operating grant awarded to C. A. Ellison by the CancerCare Manitoba Foundation. We thank Ms. Sandra Troup and Michelle Parisien from the Manitoba Breast Tumor Bank for their expert assistance with the TMA experiments. We also thank Dr. John Wilkins for the use of his microplate reader for the chemiluminescence assays.
List of Abbreviations
- BBB
blood-brain barrier
- CCLR
Cell Culture Lysis Reagent
- CNS
central nervous system
- cpm
counts per minute
- E:T
cffector to target ratio
- ER
estrogen receptor
- G-CSF
granulocyte-colony stimulating factor
- LU
lytic Unit
- ND
not detected
- TMA
tissue microarray
- TSLP
thymic stromal lymphopoietin
- Tslpr
thymic stromal lymphopoietin receptor
- Tslpr−/−
TSLP receptor knockout
- WT
wild-type
- μci
microcurie
Footnotes
Disclosures: None
References
- 1.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9(4):212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008 Mar 1;371(9614):771–83. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002 Dec 19–26;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantovani A, Marchesi F, Porta C, Sica A, Allavena P. Inflammation and cancer: breast cancer as a prototype. Breast. 2007 Dec;16( Suppl 2):S27–33. doi: 10.1016/j.breast.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002 Jul;3(7):673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 6.Taneda S, Segerer S, Hudkins KL, et al. Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am J Pathol. 2001 Dec;159(6):2355–69. doi: 10.1016/S0002-9440(10)63085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osborn MJ, Ryan PL, Kirchhof N, Panoskaltsis-Mortari A, Mortari F, Tudor KS. Overexpression of murine TSLP impairs lymphopoiesis and myelopoiesis. Blood. 2004 Feb 1;103(3):843–51. doi: 10.1182/blood-2003-05-1557. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010 Apr;11(4):289–93. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YJ, Soumelis V, Watanabe N, Ito T, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 10.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001 Jul 1;167(1):336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 11.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994 Mar;22(3):321–8. [PubMed] [Google Scholar]
- 12.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000 Jul;1(1):59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 13.Park LS, Martin U, Garka K, Gliniak B, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000 Sep 4;192(5):659–70. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005 Nov 7;202(9):1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Shami A, Spolski R, Kelly J, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004 Jul 19;200(2):159–68. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005 Sep 19;202(6):829–39. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007 Jun 1;178(11):6720–4. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 18.Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 2008 Dec 1;181(11):7699–705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin SD, Koelling RM, Friend SL, et al. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999 Jan 15;162(2):677–83. [PubMed] [Google Scholar]
- 20.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat Immunol. 2003 Aug;4(8):773–9. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- 21.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):11070–5. doi: 10.1073/pnas.0402919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007 Feb 1;178(3):1396–404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 23.Shi L, Leu SW, Xu F, et al. Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin Immunol. 2008 Nov;129(2):202–10. doi: 10.1016/j.clim.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Jessup HK, Brewer AW, Omori M, Rickel EA, Budelsky AL, Yoon BR, et al. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J Immunol. 2008 Sep 15;181(6):4311–9. doi: 10.4049/jimmunol.181.6.4311. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura Y, Miyata M, Ohba T, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol. 2008 Dec;122(6):1208–14. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Zhou B, Headley MB, Aye T, Tocker J, Comeau MR, Ziegler SF. Reversal of thymic stromal lymphopoietin-induced airway inflammation through inhibition of Th2 responses. J Immunol. 2008 Nov 1;181(9):6557–62. doi: 10.4049/jimmunol.181.9.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005 Jun 15;174(12):8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 28.Miyata M, Hatsushika K, Ando T, et al. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur J Immunol. 2008 Jun;38(6):1487–92. doi: 10.1002/eji.200737809. [DOI] [PubMed] [Google Scholar]
- 29.Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005 Aug 15;202(4):541–9. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005 Oct;6(10):1047–53. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 31.Pedroza-Gonzalez A, Xu K, Wu TC, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011 Mar 14;208(3):479–90. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olkhanud PB, Rochman Y, Bodogai M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. 2011 May 15;186(10):5656–62. doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001 May;Chapter 20(Unit 20):2. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 35.Manthorpe M, Cornefert-Jensen F, Hartikka J, et al. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther. 1993 Aug;4(4):419–31. doi: 10.1089/hum.1993.4.4-419. [DOI] [PubMed] [Google Scholar]
- 36.Ellison CA, MacDonald GC, Rector ES, Gartner JG. Gamma delta T cells in the pathobiology of murine acute graft-versus-host disease. Evidence that gamma delta T cells mediate natural killer-like cytotoxicity in the host and that elimination of these cells from donors significantly reduces mortality. J Immunol. 1995 Nov 1;155(9):4189–98. [PubMed] [Google Scholar]
- 37.Pross HF, Baines MG, Rubin P, Shragge P, Patterson MS. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- 38.Watson PH, Snell L, Parisien M. The NCIC-Manitoba Breast Tumor Bank: a resource for applied cancer research. CMAJ. 1996 Aug 1;155(3):281–3. [PMC free article] [PubMed] [Google Scholar]
- 39.Skliris GP, Leygue E, Curtis-Snell L, Watson PH, Murphy LC. Expression of oestrogen receptor-beta in oestrogen receptor-alpha negative human breast tumours. Br J Cancer. 2006 Sep 4;95(5):616–26. doi: 10.1038/sj.bjc.6603295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998 Apr 1;58(7):1486–93. [PubMed] [Google Scholar]
- 41.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007 Jan;28(1):12–8. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Ramirez MA, Fischer R, Torres-Badillo CC, et al. Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J Immunol. 2012 Sep 15;189(6):3130–9. doi: 10.4049/jimmunol.1103460. [DOI] [PubMed] [Google Scholar]
- 43.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003 Dec;9(6):540–9. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt KE, Schumak B, Specht S, Dubben B, Limmer A, Hoerauf A. Induction of pro-inflammatory mediators in Plasmodium berghei infected BALB/c mice breaks blood-brain-barrier and leads to cerebral malaria in an IL-12 dependent manner. Microbes Infect. 2011 Sep;13(10):828–36. doi: 10.1016/j.micinf.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol. 2004 Dec;190(2):446–55. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Pross HF, Maroun JA. The standardization of NK cell assays for use in studies of biological response modifiers. J Immunol Methods. 1984 Mar 30;68(1–2):235–49. doi: 10.1016/0022-1759(84)90154-6. [DOI] [PubMed] [Google Scholar]