Abstract
It is becoming increasingly clear that innate immune mediators play a role in regulating adaptive immune responses in asthma pathogenesis. Cockroach exposure is a major risk factor for the development of asthma. In this study we asked whether German cockroach (GC) feces (frass) could initiate an innate immune response. Naive BALB/c mice were challenged with a single intratracheal inhalation of GC frass. Proinflammatory cytokines were significantly increased in the bronchoalveolar lavage fluid at 3 h and were maintained at higher than baseline levels for at least 24 h. Neutrophil migration into the airways was evident as early as 3 h but was maximal between 6 and 24 h postinhalation. The early increase in cytokine expression was independent of TLR2 or TLR4. Newly infiltrated airway neutrophils were responsible for maintaining high levels of cytokines in the airways. Using neutrophils as an early marker of the innate immune response, we show that show that neutrophils isolated from the airways following GC frass inhalation express TLR2 and release cytokines. GC frass directly affected neutrophil cytokine production via TLR2, but not TLR4, as evidenced by the use of TLR-neutralizing Abs and neutrophils from TLR-deficient mice. Activation of cytokine expression occurred via GC frass-induced NF-κB translocation and DNA binding. These data show that GC frass contains a TLR2 agonist and, to our knowledge, this is the first report of an allergen directly activating cells of the innate immune system via TLR2 and suggests an important link between innate and adaptive immunity.
The incidence and severity of asthma has risen dramatically over the past two decades. There is growing evidence that the indoor environment may play an important role in the pathogenesis of childhood asthma. Cockroach, house dust mite, and cat exposure are the most commonly associated with asthma exacerbations (1). Cockroach allergens are important sensitizing agents, particularly in individuals who live in substandard, multi-family dwellings located in highly populated areas (2). A few studies have suggested that sensitization to cockroach might be more important than other allergens. Children who were both allergic to cockroach allergen and exposed to high levels of this allergen had greater hospitalization rates, unscheduled medical visits, and more days of wheezing than children allergic to and exposed to dust mites or cat dander (3). A prospective birth cohort study of children of asthmatic/allergic parents showed that the level of cockroach allergen, but not that of dust mite or cat allergen, was a significant predictor of recurrent wheeze (4). Fecal remnants (frass) are a likely source of allergen exposure, because desiccated frass is thought to integrate into dust particles and become deposited into the airways (5). These studies highlight the importance of cockroach in the asthma phenotype and substantiate the use of German cockroach (GC)3 frass in the current study.
Although the specific cytokines that induce Th1 vs Th2 responses are well described, the early innate response to allergen is not fully understood. An early and transient infiltration of neutrophils into the airways was shown following allergen challenge in humans and following OVA challenge in mice (6). In a model of OVA-sensitized mice, allergen challenge resulted in increased airway neutrophilia at 8 h with resolution at 48 h. The initial neutrophilia was followed later by an influx of eosinophils and lymphocytes and much later by airway hyperresponsiveness (7). The factor(s) regulating transient neutrophil influx into the airways are not well defined. Using the neutrophil as an early marker of innate immune responses, we asked whether GC frass may have a direct effect on innate immunity.
The innate immune response has evolved to recognize pathogen-associated molecular patterns (PAMPs), which are common to many classes of pathogens. PAMPs are recognized by pathogen recognition receptors, which include TLRs. TLRs recognize conserved microbial molecular signatures. For example, TLR2 recognizes peptidoglycan and lipoteichoic acid from Gram-positive bacteria, TLR4 recognizes Gram-negative bacterial LPS, TLR5 recognizes flagella, and TLR9 recognizes unmethylated DNA. TLRs are expressed on most cell types; however, their expression does not guarantee recognition of the pathogen because coreceptors and adaptor molecules may also be required. It has recently been shown that polymorphisms in TLR2 were a major determinant of allergy and asthma susceptibility in children of European farmers (8). A few studies have examined the role of TLR2 on experimentally induced murine models of asthma by sensitizing mice with a TLR2 agonist (9 –11). The results of these studies were contradictory. In this study we investigated whether GC frass could directly induce early innate immune responses via TLR-dependent activation in early responding immune cells. To our knowledge, an allergen has never been shown to activate TLR signaling.
Materials and Methods
Cockroach frass
The frass from one cage of GCs was transferred to a sterile container and stored at 4°C. Frass was resuspended in endotoxin-free, double-distilled water (2 h at 4°C while rocking) and frozen in aliquots for use throughout the entire study. Extracts were centrifuged to remove debris (13,000 × g for 5 min at 4°C), supernatants were harvested, and total protein was measured using the Bio-Rad protein assay dye (Bio-Rad). Endotoxin levels were determined by Charles River Laboratories using the Limulus amebocyte lysate assay.
Animals
Six-week-old female BALB/c, C57BL/6, C3H/HeOuJ (control), and C3H/HeJ (spontaneous mutation in TLR4) mice were obtained from The Jackson Laboratory and housed in a laminar hood in a virus-free animal facility. TLR2-deficient mice were obtained from Dr. S. Akira (12). In some experiments, mice were injected i.p. with the anti-granulocyte mAb RB5-8C5 (also referred to as Ly6g; BD Pharmingen) at a concentration of 100 μg/mouse (13) 24 h before inhalation. In all experiments, mice were anesthetized with ketamine (45 mg/kg)/xylazine (8 mg/kg) before PBS or GC frass (40 μg/40 μl) exposure by a single inhalation as previously described (14). Mice were given a lethal dose of sodium pentobarbital 3–24 h later (in most studies after 18 h). Animal care was provided in accordance with National Institutes of Health guidelines. These studies were approved by the Cincinnati Children’s Hospital Medical Center Institutional Animal Care and Use Committee (Cincinnati, OH).
Assessment of airway inflammation
Lungs were lavaged thoroughly with 1 ml of HBSS without calcium or magnesium. The lavage fluid was centrifuged (1,800 rpm for 10 min) and the supernatant was removed for cytokine analysis and immediately stored at −80°C. Total cell numbers were counted on a hemocytometer. Smears of bronchoalveolar lavage (BAL) cells prepared with a Cytospin II centrifuge (Shandon Thermo) were stained with Diff-Quick (Thermo Electron) solution for differential cell counting.
Myeloperoxidase (MPO) assay
Whole lungs were snap frozen in liquid nitrogen and homogenized in a solution containing 0.5% hexadecyltrimethylammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 20000 × g at 4°C. An aliquot of the supernatant was allowed to react with a solution of tetramethylbenzidine (1.6 mM) and 0.1 mM H2O2. The rate of change in absorbance was measured by spectrophotometry at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 μmol of hydrogen peroxide per minute at 37°C and expressed in units per 100-mg weight of tissue (15).
Cell culture
HL-60 promyelocytic leukemia cells (American Type Culture Collection) were cultured in RPMI 1640 medium supplemented with 10% FBS, 50 μg/ml streptomycin, 2 U/ml penicillin, and 2 mM L-glutamine. For differentiation, cells (1 × 106/ml were incubated in the presence of 1% DMSO for 4 days. Cells were centrifuged, washed, and deprived of serum for 6 h. In some cases cells were pretreated with isohelenin (30 μM for 1 h; EMD Biosciences) or neutralizing Abs against TLR2, TLR4, or isotype control (10 μg/ml; eBioscience) before the addition of GC frass (100 ng/ml) for 16 h. Selected cells were also treated with LPS from Escherichia coli (O111:B4; Sigma-Aldrich) that had been purified by ion exchange chromatography or with 1 μg/ml (S)-[2,3-bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH, 3HCl (Pam3CSK4; Calbiochem).
Primary human neutrophil isolation
Polymorphonuclear leukocytes were isolated from blood collected from healthy volunteers. Blood was drawn into heparin and then subjected to dextran T500 sedimentation, Ficoll-Histopaque density gradient centrifugation, and hypotonic erythrocyte lysis (16). All steps during the neutrophil isolation were maintained constant and all assays were performed immediately after isolation was complete. Polymorphonuclear cells were resuspended in serum-deprived medium before treatment with GC frass (10 –100 ng/ml) for 4 h (for RNA isolation and real-time PCR experiments) or 18 h (for cytokine ELISA experiments) with continual rocking.
Primary mouse bone marrow-derived neutrophils
Femurs and tibias were removed from C57BL/6 or TLR2-deficient mice. Bone marrow was isolated and rinsed and RBCs were lysed. Resuspended cells were layered onto a three-step Percoll gradient (52, 64, and 72%) and centrifuged (1,000 rpm for 30 min at room temperature). Neutrophils contained in the bottom layer (64 –72%) were collected, counted, and plated.
Isolation of neutrophils from mouse BAL fluid
Naive mice were administered a single intratracheal inhalation of GC frass, and BAL fluid was harvested 18 h later. Neutrophils were magnetically labeled with an anti-Ly-6G microbead kit (Miltenyi Biotech). Neutrophils were isolated by placing the magnetically labeled sample on a column placed in the magnetic field of a MACS separator. Magnetically labeled Ly-6G+ cells were retained on the column while non-labeled cells were run through. After removal of the column from the magnetic field, the neutrophils were counted and either stained for flow cytometry or placed in culture for determination of cytokine expression. Over 90% of the cells were neutrophils as determined by differential cell counting using Diff-Quick solution (Thermo Electron).
Flow cytometry
Neutrophils (1 × 106) isolated from the BAL fluid of mice were washed and incubated with PE-labeled isotype control or PE-labeled anti-mouse TLR2 Abs (eBioscience) for 1 h on ice. Cells were washed to remove unbound Ab and fixed with 1% paraformaldehyde (EM Sciences). Samples were analyzed on the FACSCalibur flow cytometer using the CellQuest Pro software for analysis.
ELISA
Cell supernatants were collected and clarified (13,000 rpm for 10 min at 4°C) before being analyzed for IL-8, IL-6, or TNF-α by ELISA according to the manufacturer’s specifications (Amersham Biosciences).
Immunoblot analysis
Differentiated HL-60 cells were cultured in 6-well plates and serum-starved for 24 h before treatment. Selected wells were treated with frass, and cell lysates were harvested and resolved electrophoresis on a 10% SDS- poly-acrylamide gel as previously described (17). After incubation with an anti-IκBα (Santa Cruz Biotechnology), signals were amplified and visualized using ECL.
EMSA
Differentiated HL-60 cells were treated with GC frass (100 ng/ml) for 1 h. Cells were harvested and nuclear proteins were isolated as previously described (18). All nuclear extraction procedures were performed on ice with ice-cold reagents. Protein concentrations were determined by Bradford assay (Bio-Rad) and stored at −70°C until use.
The probe was labeled with [γ-32P]ATP using T4 polynucleotide kinase (Invitrogen) and purified in MicroBiospin chromatography column. The gel was run using 10 μg of nuclear protein as previously described (18). In some instances, Abs against p65 (Rel A) or p50 (NF-κB1; Santa Cruz Biotechnology) were added (10 min at room temperature). An oligonucleotide probe encoding the consensus sequence of NF-κB was purchased from Santa Cruz Biotechnology. Cold-specific or nonspecific probes were added at 5× the concentration of the radiolabeled probe. Gels were transferred to Whatman 3M paper, dried under a vacuum at 80°C for 1 h, and exposed using a PhosphorImager (GE Healthcare).
Statistical analysis
When applicable, statistical significance was assessed by one-way ANOVA. Differences identified by ANOVA were pinpointed by the Student-Newman-Keuls multiple range test.
Results
A single intratracheal inhalation of GC frass increased cytokine expression and neutrophilia in BALB/c mice
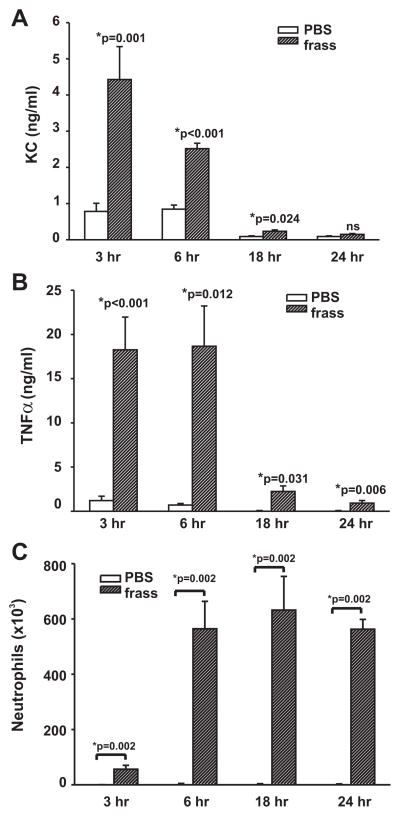
Naive BALB/c mice were given a single intratracheal inhalation of PBS or GC frass (40 μg) and killed 3, 6, 18, or 24 h later. Within 3 h there was a statistically significant increase in the level of KC in the BAL fluid of GC frass-treated mice compared with PBS-treated mice (Fig. 1A). The levels of KC began to decrease at 6 h but were still significantly higher at 18 h compared with levels in the PBS control. TNF-α levels were maximal between 3 and 6 h, after which time the levels began to decrease but were still significantly higher than in the PBS controls (Fig. 1B). IL-6 was also significantly increased at 3, 6, and 18 h following inhalation of GC frass compared with PBS (data not shown). The increase in cytokines preceded the increase in neutrophilia, which was small but statistically significant at 3 h postinhalation of GC frass, reached maximal levels around 6 h, and remained stable for 24 h (Fig. 1C). At the 18-h time point MPO levels in whole lung were significantly increased in the GC frass-treated mice compared with PBS-treated mice (12.9 ± 1.9 U per 100 mg of tissue for PBS-treated mice compared with 119.6 ± 4.6 U per 100 mg of tissue for frass-treated mice).
FIGURE 1.
A single inhalation of GC frass induced cytokine expression and airway neutrophilia. BALB/c mice were administered a single intra-tracheal inhalation of PBS or GC frass and harvested 3, 6, 18, 24 h later. BAL fluid was harvested and clarified and neutrophils were quantified following differential staining. BAL fluid was analyzed by ELISA. In all cases, means ± SEM (n = 5–7 mice per group) are reported. Statistical significance of the frass-treated mice compared with PBS-treated mice was determined by ANOVA. A, KC levels in the BAL fluid of mice. B, TNF-α levels in the BAL fluid. C, Neutrophil influx into BAL fluid expressed as mean cell number.
The initial increase in cytokine production is not mediated by TLR2 or TLR4
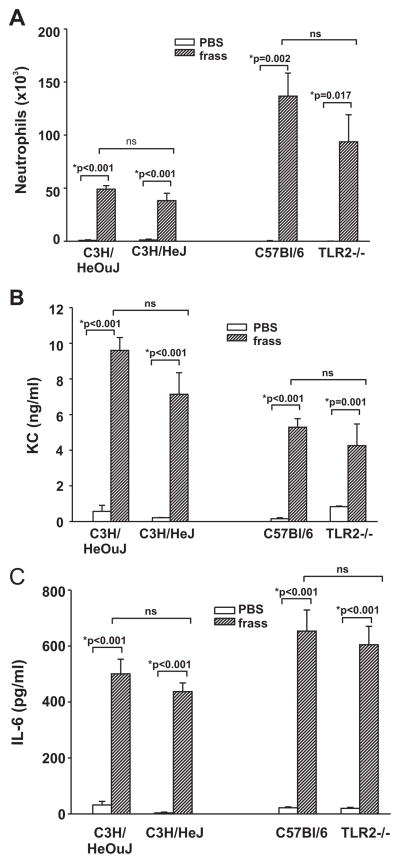
Because innate immune responses are thought to be regulated by TLRs, we asked whether TLR2 or TLR4 played a role in the initial cytokine release into the airways 3 h postinhalation. Although the trend toward a decrease was detected, there was no significant difference between neutrophil infiltration (Fig. 2A) or the levels of KC and IL-6 in the airways of wild-type or TLR2- or TLR4-deficient mice (Fig. 2, B and C). Because GC frass contains endotoxin (923 ng/mg frass; a 40-μg inhalation of GC frass contained ~37 ng of endotoxin), these data also suggest that the endotoxin alone in GC frass is not responsible for the activation of cytokines. In addition, these data suggest that GC frass-mediated early release of cytokines occurred independently of TLR2 or TLR4.
FIGURE 2.
The early response to GC frass is not mediated through TLR4 or TLR2. Wild-type (C3H/HeOuJ) and TLR4-deficient (C3H/HeJ) mice and wild-type (C57BL/6) and TLR2-deficient (TLR2−/−) mice were given a single intratracheal inhalation of PBS (40 μl) or GC frass (40 μg/40 μl). Three hours later, mice were given a lethal dose of sodium pentobarbital and BAL fluid was harvested and clarified and neutrophils were quantified following differential staining. In all cases, means ± SEM (n = 4 – 6 mice per group) were reported. Statistical significance of the frass-treated mice compared with PBS-treated mice was determined by ANOVA (ns, not significant). A, Neutrophil influx into BAL fluid. B, KC ELISA of BAL fluid. C, IL-6 ELISA of BAL fluid.
Maintenance of cytokine levels in the airways is dependent on neutrophil recruitment
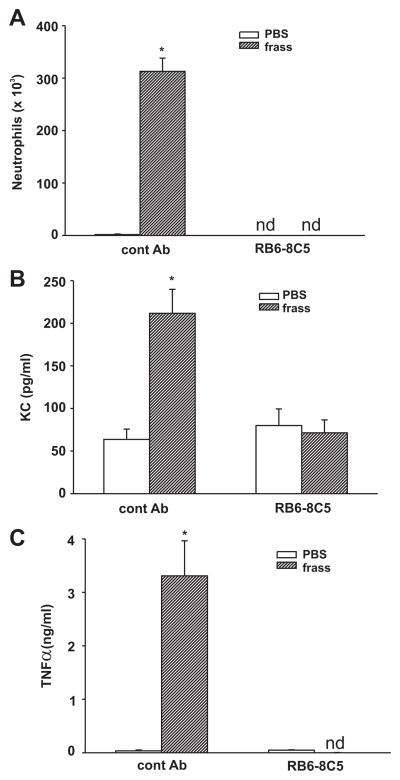
We hypothesized that neutrophils played a role in maintaining the cytokine levels in the BAL fluid of mice. To investigate this, we pretreated mice with R6B-8C5 (an Ab that depletes circulating neutrophils) 24 h before a single intratracheal challenge. We measured cytokine levels 18 h following PBS or GC frass treatment, because this time point correlated with high levels of BAL fluid neutrophilia. Pretreatment with the R6B-8C5 Ab totally abolished neutrophil recruitment into the BAL fluid as expected (Fig. 3A). Importantly, the increase in KC and TNF-α expression following GC frass inhalation was completely abolished in the RB6 – 8C5-pretreated mice compared with mice pretreated with isotype control Ab (Fig. 3, B and C). Of note, we also measured cytokine production 3 h following PBS or GC frass treatment and found that although R6B-8C5 abolished neutrophil infiltration, cytokine expression in the BAL fluid was only slightly reduced (data not shown). These data suggest that there is a population of cells that secrete cytokines for the immediate attraction of neutrophils and, once neutrophils infiltrate into the airways, they play a significant role in regulating cytokine expression.
FIGURE 3.
Neutrophil recruitment directly affected cytokine production. BALB/c mice were given a single injection of control Ab or RB5-8C5 (100 μg/mouse) 24 h before a single intratracheal inhalation of PBS (40 μl) or GC frass (40 μg/40 μl). Eighteen hours following the challenge with PBS or GC frass, mice were given a lethal dose of sodium pentobarbital and BAL fluid was harvested and clarified and neutrophils were quantified following differential staining. In all cases, means ± SEM (n = 5–7 mice per group) were reported. Statistical significance of the frass-treated mice compared with PBS-treated mice was determined by ANOVA (nd, none detected). A, Neutrophil influx into BAL fluid (*, p < 0.001). B, KC ELISA of BAL fluid (*, p < 0.001). C, TNF-α ELISA of BAL fluid (*, p < 0.001).
TLR2, but not TLR4, regulated GC frass-induced cytokine maintenance
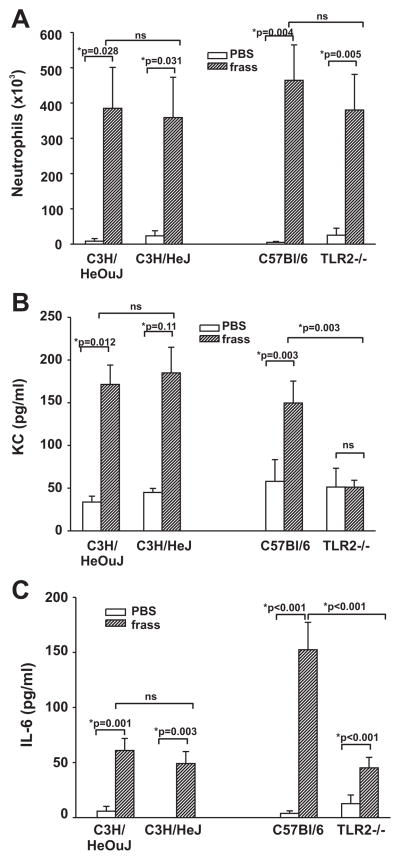
We next asked whether TLR2 or TLR4 played a role in GC frass-induced cytokine release into the airways 18 h postinhalation. To this end, we treated naive wild-type (C3H/HeOuJ or C57BL/6), TLR4-deficient (C3H/HeJ), or TLR2-deficient (TLR2−/−) mice with a single inhalation of PBS or GC frass and measured cytokine production and cellular infiltration into the airways 18 h later. Neutrophil infiltration into the airways was unaffected following GC frass treatment in TLR4- and TLR2-deficient mice compared with their wild-type controls (Fig. 4A). Interestingly, cytokine expression was significantly attenuated in the TLR2-deficient mice compared with the TLR4-deficient mice (Fig. 4, B and C). These data suggest that although TLR2 is not required for neutrophil recruitment into the airways, it is necessary for neutrophil-derived cytokine expression. This led us to hypothesize that GC frass directly affected neutrophil function via TLR2.
FIGURE 4.
Neutrophil accumulation and cytokine levels in the airways of TLR4 and TLR2 mutant mice. Wild-type (C3H/HeOuJ) and TLR4-deficient (C3H/HeJ) mice or wild-type (C57BL/6) and TLR2-deficient (TLR2−/−) mice were given a single intratracheal inhalation of PBS (40 μl) or GC frass (40 μg/40 μl) and 18 h later BAL fluid was harvested and clarified and neutrophils were quantified following differential staining. BAL fluid was analyzed by ELISA. In all cases, means ± SEM (n = 4 –7 mice per group) were reported. Statistical significance of the frass-treated mice compared with PBS-treated mice was determined by ANOVA (ns, not significant). A, Neutrophil influx into BAL fluid expressed as mean cell number. B, KC levels in the BAL fluid of mice. C, IL-6 levels in the BAL fluid of mice.
Mouse neutrophils express TLR2 and secrete cytokines
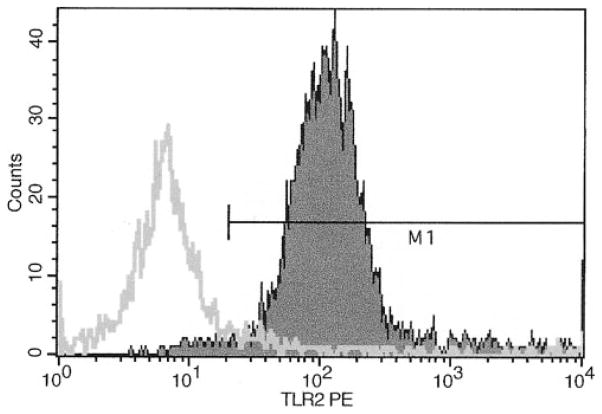
Naive BALB/c mice were administered a single intratracheal inhalation of GC frass and BAL fluid was recovered 18 h later. Neutrophils were magnetically labeled with anti-Ly-6G microbeads and isolated using a magnetic column. Ninety-five percent of the newly infiltrated neutrophils express TLR2 on their cell surface as determined by flow cytometry (Fig. 5) and secrete TNF-α (300.6 ± 36 pg/ml × 106 cells) and KC (43 ± 2 pg/ml × 106 cells). These data demonstrate that neutrophils recruited into the airways following GC frass inhalation express TLR2 and are secreting cytokines.
FIGURE 5.
TLR2 is expressed on the cell surface of neutrophils recruited into the airways. BALB/c mice were given a single intratracheal inhalation of GC frass (40 μg/40 μl) and BAL fluid was harvested 18 h later. Neutrophils were magnetically labeled and isolated before staining with either PE-conjugated isotype control (line) or PE-conjugated TLR2 (filled area) Abs. This experiment was repeated twice.
GC frass increased IL-8 and TNF-α expression in primary human neutrophils and differentiated HL-60 cells
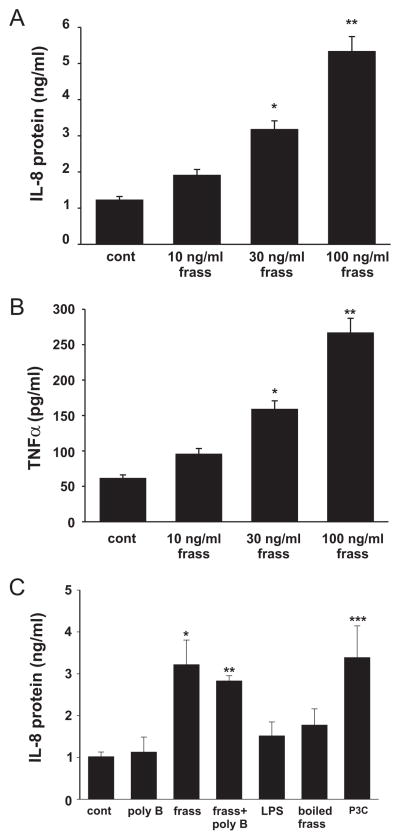
To address whether GC frass would directly affect neutrophil function, we used primary human neutrophils from normal healthy donors. We confirmed that >98% of the cells isolated were neutrophils. Incubation of isolated neutrophils with GC frass resulted in a dose-dependent increase in IL-8 and TNF-α protein expression (Fig. 6, A and B). GC frass treatment increased IL-8 and TNF-α mRNA levels in primary human neutrophils (4.3- and 11.6-fold, respectively when cells were treated with 100 ng/ml GC frass for 4 h), suggesting transcriptional up-regulation. Incubation of cells with 100 ng/ml frass resulted in the addition of 92 pg/ml endotoxin. However, treatment of the cells with 100 pg/ml column-purified endotoxin did not increase IL-8 expression, nor did polymyxin B have an effect on GC frass-induced IL-8 protein expression (Fig. 6C). We acknowledge that the addition of purified endotoxin from E. coli to cells should be interpreted with caution, as this may not represent the same source of endotoxin or the complexity of components in GC frass (i.e., TLR4 adaptor molecules or coreceptors). However, combined with the polymyxin B experiments and the in vivo data in TLR4 mutant mice, together these data suggest that GC frass can mediate cytokine expression and release from neutrophils independently of endotoxin. In addition, treatment of cells with boiled frass (boiled for 1 h before use) attenuated GC frass-induced IL-8 production from primary human neutrophils, suggesting that the TLR2 agonist activity is heat sensitive (Fig. 6C). Lastly, treatment of cells with the TLR2 agonist Pam3CSK4 increased IL-8 expression in neutrophils, confirming the activation of TLR2 on primary human neutrophils (Fig. 6C).
FIGURE 6.
GC frass increased IL-8 and TNF-α protein abundance in primary human neutrophils. Primary human neutrophils were isolated and treated with increasing concentrations of GC frass (10 –100 ng/ml) for 18 h. Supernatant was harvested and clarified and run on an ELISA for cytokine expression. A, IL-8 ELISA. Data are expressed as means ± SEM for four experiments (*, p = 0.003; **, p < 0.001; ANOVA). B, TNF-α ELISA. Data are expressed as means ± SEM for four experiments (*, p = 0.008; **, p < 0.001; ANOVA). C, Primary human neutrophils were pretreated with polymyxin B (50 mg/ml for 1 h) before treatment with GC frass (100 ng/ml) for 18 h. Some cells were treated with LPS (100 pg/ml), boiled frass (100 ng/ml, boiled for 1 h), or Pam-3-Cys (1 μg/ml). IL-8 ELISA was performed. Data are expressed as means ± SEM for four experiments (compared with control (cont); *, p = 0.009; **, p = 0.011; ***, p = 0.007; ANOVA).
We also tested the effects of GC frass on DMSO-differentiated HL-60 cells. We found a similar level of IL-8 activation (100 ng/ml GC frass increased IL-8 levels from a baseline of 6.0 ± 0.6 ng/ml to 35.2 ± 5.3 ng/ml, n ± 7; p < 0.001). Because we were obtaining a similar cytokine regulation using HL-60 cells compared with primary human neutrophils, we performed the remaining mechanistic studies using HL-60 cells.
GC frass regulates cytokine production via TLR
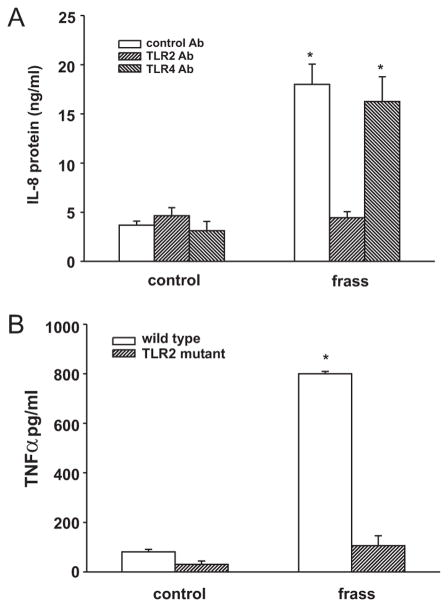
To assess the roles of TLR2 and TLR4 in GC frass-mediated cytokine expression, we pretreated differentiated HL-60 cells with a neutralizing Ab against TLR2 or TLR4 before treatment with GC frass. GC frass-mediated IL-8 expression was inhibited by the TLR2-neutralizing Ab, but not the TLR4-neutralizing Ab (Fig. 7A). To further test this, we isolated bone marrow derived-neutrophils from TLR2-knockout and wild-type mice and treated them ex vivo with GC frass. Neutrophils from wild-type mice were responsive to GC frass as depicted by the significant increase of TNF-α (Fig. 7B). In contrast, neutrophils from TLR2-knockout mice did not respond to GC frass, suggesting the importance of TLR2 for GC frass-mediated activation of neutrophils. Importantly, these data suggest that GC frass contains a TLR2 agonist.
FIGURE 7.
TLR2 is required for GC frass-induced regulation of cytokine production from neutrophils. A, Differentiated HL-60 cells were pretreated with neutralizing Abs against TLR2, TLR4, or isotype control Abs for 1 h before stimulation with GC frass (100 ng/ml). Cell medium was harvested at 18 h and analyzed by ELISA for IL-8 production. Data are expressed as means ± SEM for four experiments. GC frass increased IL-8 production in the presence of the isotype control and TLR4 Abs, but not the TLR2 Ab (*, p < 0.001; ANOVA). B, Primary bone marrow-derived mouse neutrophils were treated with GC frass. Cell medium was harvested at 18 h and analyzed by ELISA for TNF-α production. Data are expressed as means ± SEM for three experiments. GC frass increased TNF-α production compared with control treatment (*, p < 0.001; ANOVA).
GC frass increased NF-κB translocation and transactivation in human neutrophils
We initially investigated the role of NF-κB in GC frass-induced cytokine expression by using a chemical inhibitor of the NF-κB signaling pathway. We found that pretreatment of DMSO-differentiated HL-60 cells with isohelenin completely abolished GC frass-induced IL-8 release (Fig. 8A). To determine the role of NF-κB in GC frass-mediated cytokine expression in HL-60 cells, we asked whether GC frass regulated IκBα degradation. As would be expected with NF-κB activation, frass induced degradation of IκBα in a time-dependent fashion (Fig. 8, B and C). Next, we treated HL-60 cells with GC frass for 1 h and nuclear extracts were harvested for EMSA. GC frass increased the binding of nuclear proteins to an oligonucleotide encoding the consensus sequence NF-κB binding site. Coincubation of nuclear extracts with Abs against p65 RelA and p50 NF-κB1 each induced a supershift of the DNA binding complex, demonstrating the presence of these NF-κB family transcription factors (Fig. 8D). Taken together, these data suggest that GC frass increased neutrophil-derived cytokine expression in a NF-κB-dependent manner.
FIGURE 8.
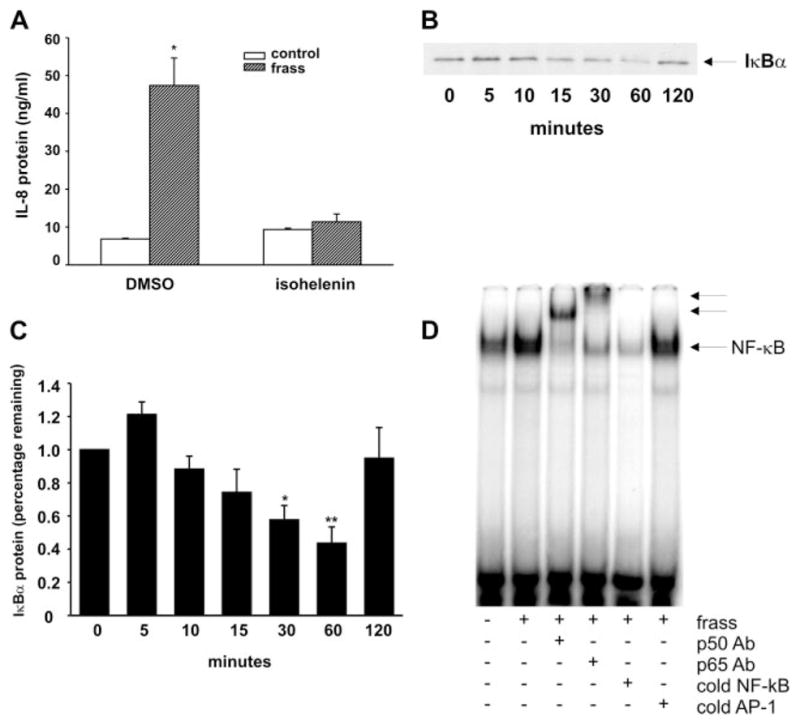
GC frass regulated NF-κB activation. A, HL-60 cells were pretreated with isohelenin (30 μM) for 1 h before treatment with GC frass for 18 h. Supernatants were harvested, clarified, and run on IL-8 ELISA. Data are expressed as means ± SEM for four experiments (*, p < 0.001; ANOVA). B, HL-60 cells were treated with frass (100 ng/ml) for the times as indicated (0 –120 min). Cell lysate was harvested, run on a Western blot, and probed for IκBα. These data are representative of four separate experiments. C, Quantification of the IκBα Western blots. Data are expressed as percentage of IκBα remaining compared with the untreated sample (means ± SEM, n = 4; *, p = 0.046; **, p = 0.023; ANOVA). D, HL-60 cells were treated with GC frass for 1 h and nuclear extracts were harvested for EMSA. Coincubation of nuclear extracts from frass-treated cells with Abs against p65 Rel A and p50 NF-κB1 were also performed. These data are representative of three separate experiments.
Discussion
In this study we found that a single intratracheal inhalation of GC frass in naive mice significantly increased neutrophil recruitment into the airways in a TLR2- and TLR4-independent manner. A novel finding in this study is that GC frass contains a TLR2 agonist and that an allergen directly regulated neutrophil function via TLR2. Direct proof of this came from the following findings: 1) neutralizing Abs against TLR2, but not TLR4, inhibited GC frass-mediated cytokine production in differentiated HL-60 cells; and 2) bone marrow-derived neutrophils from TLR2-deficient mice treated ex vivo with GC frass failed to increase cytokine production compared with neutrophils from wild-type mice. In addition, GC frass-induced cytokine expression in neutrophils was shown to be dependent on NF-κB activation. One limitation of this study is that the NF-κB studies were performed in the differentiated HL-60 cell line and not in primary human neutrophils because the isolation of neutrophils resulted in autoactivation, as evidenced by increased IκBα activation (K. Page, unpublished observations). Nevertheless, these data present strong evidence that GC frass contains a TLR2 agonist that directly activated neutrophils via TLR2 and NF-κB and suggests a link between innate and adaptive immunity to GC frass.
Neutrophils are important regulators of airway cytokine expression, as inhibition of neutrophil infiltration abrogated BAL cytokine expression following GC frass inhalation. The influx of neutrophils into the airways occurred independently of TLR2 agonists as evidenced by the increase in BAL neutrophils in GC frass-treated TLR2-knockout mice. Although neutrophil recruitment into the airways appeared to be unaffected by TLR2, maintenance of airway cytokine levels is dependent on TLR2. Increased levels of these cytokines may be important to maintain neutrophil recruitment, prolong the life of the neutrophil, or attract other inflammatory regulatory cells. A recent study showed that neutrophils treated with the TLR2-specific agonist Pam3CSK4 had increased IL-8 production and phagocytosis (19), suggesting an important role for TLR2 in regulating innate immune functions. In another study, stimulation of TLR2 with Pam3CSK4 increased neutrophil function as determined by increased respiratory burst, enhanced IL-8 generation, and prolonged life span in highly purified neutrophils (20). Our data confirm the role of TLR2 agonists in regulating neutrophil cytokine production. Although we did not study all of the parameters of neutrophil function (i.e., phagocytosis, chemotaxis, and respiratory burst), our data suggest an important role for GC frass in mediating neutrophil function in the airways.
The first cells to respond to GC frass inhalation and secrete cytokines to mediate immune and inflammatory reactions would most likely be airway epithelial cells or resident alveolar macrophages, both of which express TLR2 (21–23). Our preliminary data suggest that although mouse tracheal epithelial cells respond to GC frass by secreting cytokines, the cytokine levels in TLR2-deficient mice are not significantly altered (K. Page, unpublished observation). Although it has been shown that both alveolar epithelial cells and tracheobronchial epithelial cells express TLR2, the presence of TLR2 does not guarantee participation in responding to a pathogen. Consider that airway epithelial cells express TLR4 but are not responsive to endotoxin due to low levels of an essential adaptor molecule, MD-2 (24). It is not completely clear what additional adaptor proteins or coreceptors are involved in amplifying TLR2 signaling. It is known that TLR2 forms a heterodimer with either TLR1 or TLR6. In the current study, we did not investigate the effect of GC frass on either TLR1- or TLR6-deficient mice. It is possible that the presence of adaptor proteins or the profile of TLR2 heterodimers in airway epithelium compared with neutrophils may be important factors in GC frass-induced signaling.
The role of TLR2 in asthma is controversial. Previous studies have suggested that TLR2 agonists administered during sensitization enhanced allergic inflammation (10, 11), while other studies have suggested these agonists inhibit allergic airway inflammation (9, 25). It was shown that priming with the TLR2 agonist Pam3CSK4 increased OVA-induced experimental asthma in mice (10). Two studies have recently shown that Pam3CSK4 induced a Th2 bias in murine bone marrow-derived dendritic cells (10) and uncommitted, immature human monocyte-derived dendritic cells (26) as determined by increased Th2-associated effector molecules and up-regulation of costimulatory molecules. This would implicate an important role for the TLR2 agonist in GC frass, as activation of TLR2 could mediate allergic asthma. These data, along with our recent study, suggest that the TLR2 agonist in the allergen GC frass could be the signal that skews the immune response toward Th2 and the allergic phenotype.
Cockroach feces (frass) are likely sources of allergen exposure (27), as desiccated fecal remnants may easily incorporate into dust particles. It has been shown that after disturbance, dust particles containing cockroach proteins >10 μm in diameter may be deposited in the airway (5). GC frass is a complex mixture of a variety of biologically active molecules, including the allergens Bla g 1 and Bla g 2 (27) and active serine proteases and endotoxin (28). Interestingly, our data do not show a role for endotoxin or TLR4 in either neutrophil recruitment or activation. The fact that GC frass contains a TLR2 agonist suggests a possible mechanism by which cockroach may be a potent allergen, i.e., it contains not only the Ag but a TLR agonist (a PAMP) that may help induce the asthma phenotype. Interestingly it has been shown that Ag exposure in the absence of PAMPs failed to induce lung DC maturation (29). It is currently thought that lipopeptides and lipoproteins from microorganisms and also peptidoglycans derived from Gram-positive bacteria are TLR2 agonists (30). Although we have not yet identified the TLR2 agonist in GC frass, it is possible that frass contains peptidoglycans or lipoproteins from digestive processes or from microbial life in the cockroach gut.
During status asthmaticus, neutrophils are the dominant leukocyte subpopulation within the airways (31). In fact, the importance of neutrophilia in the asthma phenotype is suggested by the finding of a closer correlation of neutrophils to airway obstruction and severity of asthma than eosinophils in some studies (31, 32). We have found that once inhaled GC frass maintains its activity in the airways for at least 3 h (K. Page, unpublished observation), suggesting that GC frass would have an opportunity to interact with neutrophils in the airways. Through this investigation we have found that GC frass contains a TLR2 agonist, that has a direct effect on neutrophils to regulate cytokine expression in a NF-κB-dependent manner. This suggests not only a link between innate and adaptive immune responses but also suggests that in a severe asthma state in which neutrophils are present in the airways, inhalation of GC frass could directly activate infiltrated neutrophils and exacerbate airway inflammation.
Acknowledgments
We thank Dr. Shizuo Akira for providing the TLR2−/− mice and Dr. Lesley Doughty and Dan Marmer for outstanding assistance with flow cytometry.
Footnotes
This work was supported by the National Institutes of Health Grant HL075568 (to K.P.) and HL67736 (to M.W.-K.).
Abbreviations used in this paper: GC, German cockroach; BAL, bronchoalveolar lavage; MPO, myeloperoxidase; Pam3CSK4, (S)-[2,3-bis(palmitoyloxy)-(2-RS)-pro-pyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH, 3HCl; PAMP, pathogen-associated molecular pattern.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Gelber LE, Seltzer LH, Bouzoukis JK, Pollart SM, Chapman MD, Platts-Mills TA. Sensitization and exposure to indoor allergens as risk factors for asthma patients presenting to hospital. Am Rev Resp Dis. 1993;147:573–587. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 2.Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, Jankun T, Ren P, McSherry JE, Platts-Mills TAE, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the Northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen is causing morbidity among inner city children with asthma. N Eng J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 4.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills TA, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness and maternal smoking. Am J Resp Crit Care Med. 1999;160:227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 5.De Lucca SD, Taylor DJ, O’Meara TJ, Jones AS, Tovey ER. Measurement and characterization of cockroach allergens detected during normal domestic activity. J Allergy Clin Immunol. 1999;104:672–680. doi: 10.1016/s0091-6749(99)70341-6. [DOI] [PubMed] [Google Scholar]
- 6.Lommatzsch M, Julius P, Kuepper M, Garn H, Bratke K, Irmsher S, Luttmann W, Renz H, Braun A, Virchow JC. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J Allergy Clin Immunol. 2006;118:91–97. doi: 10.1016/j.jaci.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Taube C, Dakhama A, Rha YH, Takeda K, Joetham A, Park JW, Balhorn A, Takai T, Poch KR, Nick JA, Gelfand EW. Transient neutrophil infiltration after allergen challenge is dependent on specific antibodies and Fcγ III receptors. J Immunol. 2003;170:4301–4309. doi: 10.4049/jimmunol.170.8.4301. [DOI] [PubMed] [Google Scholar]
- 8.Eder W, Klimecki W, Yu L, Mutius Evon, Riedler J, Braun-Fahrländer C, Nowak D, Martinez FD, Team AS. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol. 2004;113:482–488. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- 9.Akdis C, Kussebi F, Pulendran B, Akdis M, Lauener RP, Schmidt-Weber CB, Klunker S, Isitmangil G, Hansjee N, Wynn TA, et al. Inhibition of T helper 2-type responses, IgE production, and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003;33:2717–2726. doi: 10.1002/eji.200323329. [DOI] [PubMed] [Google Scholar]
- 10.Redecke V, Häcker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 11.Chisholm D, Libet L, Hayashi T, Horner AA. Airway peptidoglycan and immunostimulatory DNA exposures have divergent effects on the development of airway allergen hypersensitivities. J Allergy Clin Immunol. 2004;113:448–454. doi: 10.1016/j.jaci.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443– 451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 13.Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H. Administration of anti-granulocyte mAb RB6 – 8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 14.Walters DM, Breysse PN, Wills-Karp M. Ambient urban Baltimore particulate-induced airway hyperresponsiveness and inflammation in mice. Am J Respir Crit Care Med. 2001;164:1438–1443. doi: 10.1164/ajrccm.164.8.2007121. [DOI] [PubMed] [Google Scholar]
- 15.Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med. 2004;10:55–62. doi: 10.2119/2004-00032.aneja. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tennenberg SD, Zemlan FP, Solomkin JS. Characterization of N-formyl-methionyl-leucyl-phenylalanine receptors on human neutrophils. J Immunol. 1988;141:3937–3944. [PubMed] [Google Scholar]
- 17.Page K, Strunk VS, Hershenson MB. Cockroach proteases increase IL-8 expression in human bronchial epithelial cells via activation of protease-activated receptor (PAR)-2 and ERK. J Allergy Clin Immunol. 2003;112:1112–1118. doi: 10.1016/j.jaci.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 18.Allen GL, Menendez IY, Ryan MA, Mazor RL, Wispe JR, Fielder MA, Wong HR. Hyperoxia synergistically increases TNF- α-induced interleukin-8 gene expression in A549 cells. Am J Physiol. 2000;278:L253–L260. doi: 10.1152/ajplung.2000.278.2.L253. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 20.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MKB. Selective roles for toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol. 2003;170:5268–5275. doi: 10.4049/jimmunol.170.10.5268. [DOI] [PubMed] [Google Scholar]
- 21.Soong G, Reddy B, Sokol S, Adamo R, Prince A. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J Clin Invest. 2004;113:1482–1489. doi: 10.1172/JCI20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong L, Medford AR, Uppington KM, Robertson J, Witherden IR, Tetley TD, Millar AB. Expression of functional toll-like receptor-2 and -4 on alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:241–245. doi: 10.1165/rcmb.2004-0078OC. [DOI] [PubMed] [Google Scholar]
- 23.Hertz CJ, Wu Q, Porter EM, Zhang YJ, Weismüller KH, Godowski PJ, Ganz T, Randell SH, Modlin RL. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human β defensin-2. J Immunol. 2003;171:6820–6826. doi: 10.4049/jimmunol.171.12.6820. [DOI] [PubMed] [Google Scholar]
- 24.Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PBJ. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol. 2004;287:L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 25.Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, Liew FY. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J Immunol. 2005;174:7558–7563. doi: 10.4049/jimmunol.174.12.7558. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984 – 4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 27.Yun YY, Ko SH, Park JW, Lee IY, Ree HI, Hong CS. Comparison of allergic components between German cockroach whole body and fecal extracts. Ann Allergy Asthma Immunol. 2001;86:551–556. doi: 10.1016/S1081-1206(10)62904-3. [DOI] [PubMed] [Google Scholar]
- 28.Hughes VS, Page K. German cockroach frass proteases cleave pro-matrix metalloproteinase-9. Exp Lung Res. 2007;33:135–150. doi: 10.1080/01902140701356561. [DOI] [PubMed] [Google Scholar]
- 29.Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered normally nonimmunogenic protein. J Exp Med. 2003;198:133–144. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaisho T, Akira S. Toll-like receptors and their signaling mechanism in innate immunity. Acta Odontol Scand. 2001;59:124 –130. doi: 10.1080/000163501750266701. [DOI] [PubMed] [Google Scholar]
- 31.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–1539. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 32.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biological significance. Am J Respir Crit Care Med. 2000;161:1185–1190. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]