Abstract
Background
HIV and HCV infections may increase interleukin-6 (IL-6) and C-reactive protein (CRP). However, relationships between inflammatory biomarkers, chronic viral infections, clinical factors, and behavioral factors remain poorly understood.
Methods
Using linear regression, we modeled cross-sectional associations between loge IL-6 or loge CRP levels and HCV, HIV, injection drug use, and comorbidity among 1191 injection drug users.
Results
Mean age was 47 years, 46.0% reported currently injecting drugs, 59.0% were HCV-monoinfected, and 27% were HCV/HIV-coinfected. In multivariable models, higher loge IL-6 was associated with HCV monoinfection (β=0.191, 95%CI: 0.043 to 0.339) and HCV/HIV coinfection (β=0.394, 95% CI: 0.214 to 0.574). In contrast, HCV monoinfection (β=−0.523, 95%CI: −0.275 to −0.789) and HCV/HIV coinfection (β=−0.554 95%CI: −0.260 to −0.847) were associated with lower CRP. Lower CRP with HCV infection was independent of liver fibrosis severity, synthetic function, or liver injury markers; CRP decreased with higher HCV RNA. Increased injection intensity was associated with higher IL-6 (p=0.003) and CRP (p<0.001); increasing comorbidity (p<0.001) and older age (p=0.028) were associated with higher IL-6; older age was associated with higher CRP among HCV-uninfected participants (p=0.021).
Conclusion
HIV and HCV infections contribute to chronic inflammation; however, reduced CRP possibly occurs through HCV-virally-mediated mechanisms. Findings highlight potentially modifiable contributors to inflammation.
Keywords: inflammation, interleukin-6, C reactive protein, HCV, HIV, injection drug use, comorbidities
INTRODUCTION
Chronic inflammation, characterized by low-level elevation in circulating pro-inflammatory mediators,1 is hypothesized to underlie a wide range of age-associated conditions.2–6 Human immunodeficiency virus (HIV)-associated activation of inflammatory pathways is thought to contribute to increased susceptibility to non-AIDS-defining chronic disease and poorer health.7, 8 Higher levels of two pro-inflammatory mediators—interleukin-6 (IL-6) and C-reactive protein (CRP)—have been associated with mortality among healthy, non-disabled older persons9 and with HIV disease progression,10 AIDS events, 11 and death12, 13 among HIV-infected persons. Injection drug users (IDUs) also have chronically elevated levels of pro-inflammatory cytokines.14, 15 Hepatitis C virus (HCV) infection, which is highly prevalent among IDUs, also may contribute to chronic inflammation.16 Given that IDUs are at increased risk for HCV and HIV infections17 and that HIV-infected and injection drug using populations are aging,18, 19 the burden of disease related to chronic inflammation is likely to increase among IDUs.
Nevertheless, the interrelationships between chronic inflammation with injection drug use, age, comorbidity, and HCV and HIV infections are poorly understood. Given that inflammation lies on a common pathophysiological pathway to age-related disease and other poor health outcomes, characterizing factors associated with chronic inflammation in high-risk populations is important. We therefore assessed clinical and behavioral factors associated with elevation of IL-6 and CRP levels among a cohort of HIV-infected and at-risk IDUs with a high burden of non-AIDS-defining chronic conditions20 and a high prevalence of HCV infection.17
METHODS
Study Population
The AIDS Linked to the IntraVenous Experience (ALIVE) study, described previously,21 follows a cohort of current and former IDUs in Baltimore, Maryland. Study visits comprise an interview, a physical examination, and biospecimen collection. The study sample includes 1191 ALIVE participants after excluding participants with CRP>100 mg/l suggesting active infection22 (n=3), an infection (including bacterial or viral pneumonia, Pneumocystis jiroveci pneumonia, pulmonary tuberculosis, sepsis, and bacteremia) within +/−28 days of inflammatory marker testing and confirmed through medical record abstraction (n=8), and missing data on more than 2 of 6 measured comorbidities (Supplemental Table 1) (n=203). Those with HIV monoinfection (n=24) were excluded due to unique characteristics of this group and small sample size. Participants missing data on more than 2 comorbidities tended to be older (p=0.03), but did not differ in terms of sex (p=0.69), race (p=0.21), injection drug use frequency (p=0.44), or HCV/HIV infection status (p=0.90) compared to those with data on at least 4 of 6 comorbidities. All participants provided written informed consent; the Johns Hopkins University Institutional Review Board approved the study.
Study Measurements
Trained interviewers obtained socio-demographic information and medical history. Risk behaviors (cigarette, alcohol, and drug use) in the prior 6-month interval were ascertained through audio-computer assisted self-interview (ACASI). Participants provided biospecimens for testing, which included HIV serology using a commercially available enzyme-linked immunosorbent assay (ELISA) with Western blot confirmation (Dupont, Wilmington, DE). For HIV-infected participants, T-cell subset assays (CD4+ and CD8+) and RT-PCR testing for HIV RNA (COBAS Amplicor HIV-1 Monitor test, version 1.5, Roche Molecular Systems, Branchburg, New Jersey) were performed; the limit of detection for HIV RNA was considered to be ≤400 copies/ml to be consistent with prior data. Nadir CD4+ count was defined as minimum CD4+ count measured during time in study or the lowest self-reported CD4+ count prior to study entry. HCV infection was determined using an HCV 3.0 enzyme immunoassay (Ortho Diagnostic Systems, Raritan, NJ) according to manufacturer specifications. HCV RNA was measured on a subset of participants (n=999) using an RT-PCR assay (limit of detection ≤50 IU/ml) (Amplicor HCV Monitor Test kit; Roche Diagnostic Systems, Branchburg, NJ). We measured 6 non-AIDS-defining comorbidities (chronic kidney disease, anemia, diabetes, hypertension, liver fibrosis, and obstructive lung disease) described previously20 with the exceptions that significant fibrosis was defined as a fibrosis score (as measured through elastography; Fibroscan, EchoSens, Paris20) cut-point of ≥8.0 kPA17 and body mass index (BMI) was examined separately from number of comorbidities (Supplemental Table 1).
Covariates used in secondary analysis included albumin (g/dl), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) measured from non-fasting serum samples (Quest Laboratories). ALT and AST testing were performed using an Olympus 5200 Multichannel Chemistry Analyser with a coefficient of variance of <3%.23 These variables were treated categorically with ALT and AST assessed as a function of the upper limit of normal (ULN) of 30 U/l for males and 19 U/l for females.24
IL-6 and CRP Levels
IL-6 and CRP were measured once on serum samples collected and stored at −80°C using commercially-available ELISA kits (High-Sensitivity Quantikine Kit, R&D Systems, Minneapolis, MN) with detection ranges of 0.156–10.0 pg/ml and 31.25–2000 pg/ml and interassay coefficients of variance of 5.7% and 6.4%, respectively. Measurements were performed in duplicate and repeated if the measures differed by more than 15% or were out of the measurable range. The average of the two measures was used for analysis. The average time between serum collection and IL-6 and CRP testing was 6.4 years (SD: 1.2).
Statistical Analysis
In this cross-sectional analysis, univariate associations were explored using Fisher’s exact tests for categorical variables and analysis of variance tests (normal distribution) or Hodges-Lehmann’s tests for equal medians (non-normal distribution) for continuous variables. IL-6 and CRP were assessed continuously to maximize efficiency, log transformed (natural logarithm) to account for non-normally distributed residuals, and modeled as separate outcomes. Potential correlates of elevated inflammatory biomarkers were assessed in univariate and multivariable linear regression models. Bootstrapping was used to estimate 95% confidence intervals (CIs) to account for non-normally distributed residuals remaining after transformation in the final models.25 Effect modification of the association between elevated inflammatory biomarkers with age or with sex by HCV or HIV status was assessed. Akaike Information Criterion (AIC) and R2 were used to compare model fit; covariates that were statistically significant or improved model fit were kept in the final models. Multicollinearity was determined using variance inflation factors (VIFs), and covariates with VIF<2 were included. All analyses were performed using STATA software version 12.1 (College Station, TX).
RESULTS
Participant Characteristics
The average age of participants was 47 years, 87.6% were African American, 35.3% were female, 46.0% reported injecting drugs daily, and 41.1% had ≥2 comorbidities. Compared to HCV/HIV-uninfected participants, those with HCV monoinfection were more likely to be older, married, inject drugs daily, and have more comorbidities while participants with HCV/HIV co-infection were more likely to be older, unemployed, not use alcohol, have BMI <25, and have more comorbidities. Compared to participants with HCV-monoinfection, HCV-HIV coinfected participants were more likely to be African American (Table 1). Among HCV/HIV-coinfected participants, median current CD4+ was 304 cells/mm3 (IQR: 179–473), median nadir CD4+ was 176 cells/mm3 (IQR: 74–279), 45.0% had undetectable HIV RNA, and 52.7% had received HAART within the prior 6 months. Among HCV-infected participants, 3.9% reported ever receiving treatment for HCV infection.
Table 1.
Participant characteristics, stratified by HCV and HIV infection status
| HCV/HIV Infection Status | ||||
|---|---|---|---|---|
| Uninfected (n=166) |
HCV-monoinfected (n=703) |
HCV/HIV-coinfected (n=322) |
p-valuea | |
| Age, mean [SD] | 42.9 [7.9] | 47.6 [8.3] | 46.8 [6.4] | <0.001 |
| Female sex | 40.4 | 33.7 | 36.0 | 0.26 |
| African Americanb | 89.2 | 84.5 | 93.5 | <0.001 |
| Married, ever | 27.1 | 40.2 | 27.2 | <0.001 |
| Employedc | 31.3 | 27.3 | 20.2 | 0.012 |
| Homelessc | 13.9 | 16.8 | 14.3 | 0.49 |
| Cigarette usec | 83.7 | 85.8 | 81.9 | 0.27 |
| Alcohol use, ≥1 day/weekc | 61.5 | 55.2 | 45.5 | 0.001 |
| Injection drug use intensityc | ||||
| None | 61.5 | 50.5 | 57.8 | 0.001 |
| <Daily | 21.7 | 24.5 | 27.3 | |
| ≥Daily | 16.9 | 25.0 | 14.9 | |
| Depressive symptoms | 25.9 | 21.6 | 26.5 | 0.17 |
| No. of comorbiditiesd, median [IQR] | 1 [0, 1] | 1 [0, 2] | 2 [1, 3] | <0.001 |
| Chronic kidney disease | 9.7 | 20.8 | 49.8 | <0.001 |
| Anemia | 14.5 | 20.2 | 37.0 | <0.001 |
| Diabetes | 8.4 | 8.7 | 7.1 | 0.73 |
| Hypertension | 31.7 | 42.8 | 37.2 | 0.020 |
| Liver fibrosis | 12.3 | 29.9 | 40.8 | <0.001 |
| Obstructive lung disease | 16.6 | 15.6 | 16.5 | 0.92 |
| BMI | ||||
| <25 | 45.2 | 44.5 | 54.1 | 0.019 |
| 25–30 | 26.8 | 33.1 | 27.7 | |
| ≥30 | 28.0 | 22.3 | 18.2 | |
| IL−6 (pg/ml), median [IQR] | 1.37 [0.90, 2.26] | 1.56 [0.98, 2.78] | 1.82 [1.21, 3.15] | 0.002 |
| CRP (mg/l), median [IQR] | 2.13 [0.94, 5.57] | 1.56 [0.53, 4.82] | 1.36 [0.51, 3.88] | 0.003 |
Abbreviations: SD = standard deviation, IQR = interquartile range, HCV = hepatitis C virus, HIV = human immunodeficiency virus, BMI = body mass index
All values given as percentages unless otherwise indicated.
p-values comparing determined using Fisher’s exact tests for categorical variables and analysis of variance for continuous variables (normal distribution) or Hodges-Lehmann’s tests on equality of medians for continuous variables (non-normal distribution).
African American race is in comparison to white/other which is 81% white, 12% other, and 7% Asian, Alaskan/Native American, and white Hispanic.
In prior 6 months.
Comorbidities include a summary score of chronic kidney disease, anemia, diabetes, hypertension, liver fibrosis, and obstructive lung disease.
Correlates of Elevated IL-6
Median IL-6 level was 1.37 pg/ml (IQR: 0.90–2.26), 1.56 pg/ml (IQR: 0.98–2.78), and 1.82 pg/ml (IQR: 1.21–3.15) among HCV-uninfected, HCV-monoinfected, HCV/HIV-coinfected participants (p=0.002) (Table 1). In multivariable analysis (Table 2), older age, female sex, daily injection drug use, weekly alcohol use, BMI ≥30, having more comorbidities, HCV monoinfection, and HCV/HIV coinfection were independently associated with elevated IL-6 levels. Each 10-yr increase in age was associated with 0.083 (95% CI: 0.009 to 0.156) higher mean loge IL-6. Even after accounting for BMI, participants who injected drugs at least daily had 0.226 (95% CI: 0.076 to 0.376) higher mean loge IL-6 levels compared to those who did not inject in the prior 6 months. For each additional comorbidity, mean loge IL-6 increased by 0.091 (95% CI: 0.041 to 0.140).
Table 2.
Adjusted associations between inflammatory biomarkers and their correlates
| IL-6 (pg/ml in log scale) β coefficient (95% CI)a |
CRP(mg/l in log scale) βcoefficient (95% CI)a |
|
|---|---|---|
| HCV*Age | −0.317 (−0.589 – −0.044)* | |
| HCV/HIV status | ||
| HCV-uninfected | ref | ref |
| HCV-monoinfected | 0.191 (0.043 – 0.339)* | −0.523 (−0.257 – −0.789)* |
| HCV/HIV-coinfected | 0.394 (0.214 – 0.574)* | −0.554 (−0.260 – −0.847)* |
| CD4d | 0.056 (0.016 – 0.097)* | |
| Ageb, per 10 yr increase | 0.083 (0.009 – 0.156)* | 0.289 (0.044 – 0.535)* |
| Sex | ||
| Male | ref | ref |
| Female | 0.172 (0.059 – 0.285)* | 0.206 (0.027 – 0.385)* |
| Race | ||
| African American | ref | ref |
| White/other | 0.003 (−0.170 – 0.175) | 0.360 (0.092 – 0.628)* |
| BMI | ||
| <30 | ref | ref |
| ≥30 | 0.257 (0.119 – 0.394)* | 0.361 (0.150 – 0.571)* |
| Injection drug use intensityc | ||
| None | ref | ref |
| <Daily | 0.060 (−0.074 – 0.194) | 0.278 (0.057 – 0.500)* |
| ≥Daily | 0.226 (0.076 – 0.376)* | 0.621 (0.376 – 0.848)* |
| Alcohol usec | ||
| <1 day/week | ref | |
| ≥1 day/week | 0.137 (0.026 – 0.247)* | |
| No. of comorbidities | 0.091 (0.041 – 0.140)* |
Abbreviations: HCV = hepatitis C virus, HIV = human immunodeficiency virus, BMI = body mass index
Statistically significant at p<0.05
β coefficients estimated using linear regression, and 95%CIs estimated from bootstrapping using 1000 replications to account for non-Gaussian residuals.
Centered at the mean age of 47 years.
In prior 6 months.
Per 100 cell decrease (centered at 200 cells/mm3) for HIV-seropositive participants. Thus, a 100 cell decrease in current CD4 among HIV-infected participants is associated with a change of 0.056 in loge IL-6 in an adjusted model. CD4 was not included in the model for CRP because it was not statistically significantly associated with CRP and did not improve model fit.
Compared to having no infection, HCV monoinfection was associated with 0.191 (95% CI: 0.043 to 0.339) higher mean loge IL-6 levels, whereas HCV/HIV coinfection was associated with 0.394 (95% CI: 0.214 to 0.574) higher mean loge IL-6 levels after adjustment. Compared to HCV monoinfection, HCV/HIV coinfection was associated with 0.203 (95% CI: 0.062 to 0.344) higher mean loge IL-6. Furthermore, among participants with HIV infection, each 100 cell decrease in current CD4+ cell count was associated with an increase of 0.056 (95% CI: 0.016 to 0.097) in mean loge IL-6 (Table 2); neither nadir CD4+ count (p = 0.65), HIV RNA (p = 0.72) nor HAART use in the prior 6 months (p = 0.74) was associated with IL-6 levels in adjusted models.
No evidence of effect modification of the association between IL-6 and age by HCV (p for interaction = 0.73) or HIV infection (p for the interaction = 0.63) was found. Likewise, no evidence of effect modification of the association between IL-6 and sex by HCV (p for the interaction = 0.43) or HIV infection (p for the interaction = 0.87) was found.
Correlates of Elevated CRP
Median CRP level was 2.13 mg/l (IQR: 0.94–5.576), 1.56 mg/l (IQR: 0.53–4.82), and 1.36 mg/l (IQR: 0.51–3.88) among HCV-uninfected, HCV-monoinfected, HCV/HIV-coinfected participants (p=0.003) (Table 1). In multivariable analysis (Table 2), female sex, non-African American race, increased intensity of injection drug use, and BMI ≥30 were independently associated with elevated CRP levels. Injection drug use intensity showed a dose-response association with elevated CRP (p for trend = 0.004): those injecting less than daily had 0.278 (95% CI: 0.057 to 0.500) and those injecting daily or more had 0.621 (95% CI: 0.376 to 0.848) higher mean loge CRP compared to those who did not inject in the prior 6 months. In contrast to IL-6 models, an increasing number of comorbidities was not associated with elevated CRP in adjusted models (p = 0.30) and was not included in the final model.
HCV infection demonstrated a strong, inverse association with CRP levels. Further, this paradoxical effect of HCV was not substantially altered by HIV coinfection. Both HCV moninfection (β=−0.523, 95% CI: −0.257 to −0.789) and HCV/HIV coinfection (β=−0.554, 95% CI: −0.260 to −0.847) were negatively associated with CRP levels with a similar magnitude of association (Table 2). Neither current CD4+ count (p = 0.75), nadir CD4+ count (p = 0.23), HIV RNA (p = 0.55), nor HAART use in the prior 6 months (p=0.48) was associated with CRP among HCV/HIV-coinfected participants.
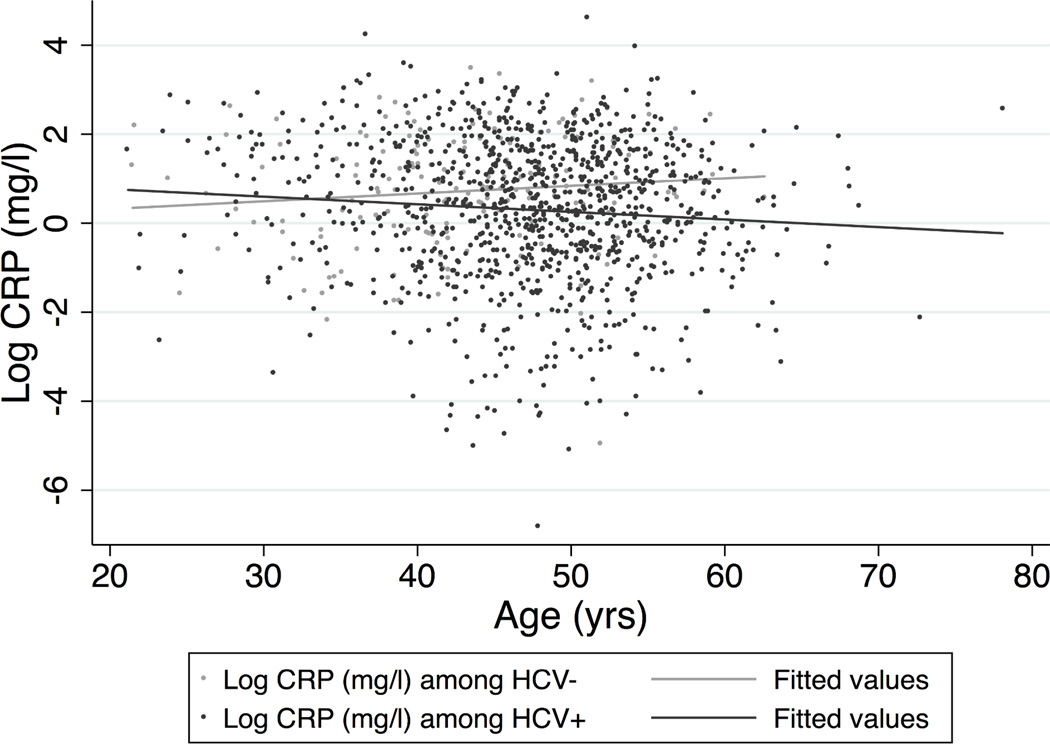
HCV infection modified the association between elevated CRP and age (Figure). While the expected increase in CRP with age was observed among HCV-uninfected participants, no significant association was observed in persons with HCV infection. For each 10-yr increase in age, mean loge CRP was 0.289 (95% CI: 0.044 to 0.528) higher among HCV-uninfected participants but did not change (β = −0.027; 95% CI: −0.158 to 0.103) among HCV-infected participants. No evidence of effect modification of the association between CRP and sex by HCV (p for the interaction = 0.30) was found.
Figure 1. Figure: Effect modification of the association between loge CRP and age by HCV status.
A scatterplot with lines depicting the fitted values from a linear regression model of the relationship between age and loge CRP (mg/l) adjusted for sex, race, BMI, and injection drug use intensity shows effect modification of this relationship by HCV infection (p-value for the interaction term between age and HCV infection in the adjusted model = 0.023). The fitted lines are estimated from the adjusted linear regression model and show that among HCV-infected participants, loge CRP increases with increasing age, whereas among HCV-uninfected participants, no statistically significant relationship between loge CRP and age is found.
Associations between HCV Status and CRP Levels Stratified by Liver-related Markers
Because CRP is produced in the liver, we further systematically evaluated whether the inverse association between HCV infection and CRP levels was explained by impaired liver function. We assessed markers of the severity of liver fibrosis, the degree of synthetic function (albumin levels),26 and liver injury (AST and ALT levels).23, 26 Moreover, we considered a potential HCV-virally-mediated pathway. The association between HCV and CRP after adjustment for confounders was examined within strata of each of these markers (liver fibrosis, albumin, AST, and ALT). We also estimated the association between HCV and CRP after adjustment for fibrosis score; the association between fibrosis score and CRP; and the association between HCV RNA and CRP.
HCV infection remained consistently associated with decreased CRP levels in all strata of liver fibrosis severity (none, mild, severe) (Table 3). Furthermore, CRP remained inversely associated with HCV infection (β=−0.463, 95%CI: −0.186 to −0.741) in an adjusted model including continuous fibrosis score. Although CRP levels also tended to be lower with higher fibrosis score (β=−0.386, 95%CI: −0.860 to 0.088), this trend was not significant. Moreover, among 721 participants with detectable HCV RNA, a one log10 increase in HCV RNA was associated with 0.189 (95% CI: −0.091 to −0.288) lower mean loge CRP.
Table 3.
Effect modification of the association between HCV and CRP by liver-related markersa
| HCV-uninfected | HCV-infectedb | |
|---|---|---|
| Liver fibrosis (kPa) | ||
| <8 (n=704) | ref | −0.340 (−0.646 – −0.033)* |
| 8–12.2 (n=191) | ref | −1.50 (−2.12 – −0.886)* |
| ≥12.3 (n=118) | n/ac | |
| Albumin (g/dl) | ||
| <3.4 (n=54) | n/ac | |
| ≥3.4 (n=1060) | ref | −0.544 (−0.802 – −0.286)* |
| ASTd (ULN) | ||
| <1.5 (n=662) | ref | −0.326 (−0.575 – −0.077)* |
| 1.5–2.5 (n=265) | n/ac | |
| ≥2.5 (n=187) | n/ac | |
| ALTd (ULN) | ||
| <1.5 (n=749) | ref | −0.349 (−0.589 – −0.109)* |
| 1.5–2.5 (n=205) | n/ac | |
| ≥2.5 (n=160) | n/ac |
Abbreviations: CRP = C reactive protein, AST = apartate aminotransferase, ALT = alanine aminotransferase
Statistically significant at p<0.05.
Difference in mean loge CRP was estimated from a linear regression of loge CRP adjusted for age, sex, race, injection drug use intensity, and body mass index. Linear regression was performed for each strata of liver fibrosis, albumin, AST, or ALT; the difference in mean loge CRP was compared to the reference group which included HCV-uninfected participants. 95% confidence intervals around the difference were estimated from bootstrapping using 1000 replications to account for non-Gaussian residuals.
HCV-infected category includes both HCV-monoinfected (67.4%) and HCV-coinfected (32.6%) participants.
Coefficients unable to be estimated due to no or small numbers in the HCV-uninfected reference group.
Categories for serum aspartate aminiotransferase (AST) or alanine aminotransferase (ALT) were created by multiplying 1.5 or 2.5 times the upper limit of normal (ULN). ULN was based on the sex-specific cut-points of 30 U/l for males and 19 U/l for females.
Further, in contrast to expectations that CRP levels would only be reduced in the presence of advanced liver disease, we actually had very few HCV-infected participants with substantially reduced synthetic function (albumin levels <3.4 g/dl) or with significant hepatic inflammation or liver injury (AST or ALT levels >1.5 ULN). Due to these small numbers, multivariable associations between CRP and HCV could not be assessed for participants in these strata; however, we did find that the median CRP levels were lower in HCV-infected compared to HCV-uninfected participants within strata of albumin, AST, or ALT levels (Supplemental Table 2).
Sensitivity Analyses
To account for potential misclassification of chronic HCV infection among HCV-infected participants, we performed identical analyses using HCV RNA (present/absent) instead of HCV serostatus among the subset of participants in which HCV RNA was measured. In multivariable models using HCV RNA instead of HCV serostatus we found the following: compared to HCV-uninfected participants, HCV/HIV-coinfected participants (β=0.335, 95%CI: 0.134 to 0.535) had significantly higher levels of IL-6 while the association between HCV monoinfection and elevated IL-6 was no longer significant (β= 0.118, 95%CI: −0.051 to 0.287). This attenuation is likely due to the instability of the complex model with a large number of covariates and reduced sample size. Compared to HCV-uninfected participants, HCV-monoinfected (β=−0.549, 95%CI: −0.295 to −0.802) and HCV/HIV-coinfected participants (β=−0.529, 95%CI: −0.225 to −0.883) still had significantly lower levels of CRP. The majority of the inferences and magnitudes for the reported associations with other covariates were essentially unchanged with the following exceptions: the association between alcohol use and elevated IL-6, although still positive, was attenuated and no longer significant; the associations between sex and age with CRP levels were no longer statistically significant. We also performed identical analyses excluding those who reported ever receiving HCV treatment; inferences for associations of all correlates with either IL-6 or CRP were the same (data not shown).
Because cocaine use has been previously associated with elevated CRP levels,27, 28 we repeated analyses exploring whether drug type might be driving the association of higher inflammatory biomarkers with increased injection intensity. In models incorporating variables for any cocaine use or any heroin use (including injected and/or smoked) in the prior 6 months, cocaine and heroin use were not significantly associated with either CRP or IL-6, and the estimates for injection drug use intensity were not appreciably changed (data not shown).
DISCUSSION
In this study of almost 1200 current and former IDUs at high-risk for HCV and HIV, both infections were independently associated with elevated IL-6 levels. In contrast, a strong negative association between HCV and CRP levels was found. With clinical and laboratory data available to systematically characterize several liver-related markers, HCV maintained an inverse association with CRP irrespective of the severity of liver fibrosis or range of measures of synthetic function or liver injury. Moreover, we observed a dose-response decline in CRP levels with increasing HCV RNA viremia, suggesting that HCV may function to lower CRP through virally-mediated mechanisms. In addition to characterizing associations of HIV and HCV with inflammatory markers, our data highlight potentially modifiable contributors (e.g., injection drug use, obesity, comorbidity) to inflammation in this aging population at high-risk for non-AIDS, chronic inflammatory diseases.
Despite substantial prevalence of chronic HCV and HIV infections, active injecting, and comorbidity, the observed levels of IL-6 and CRP in our study population were similar to the US general population and lower than reported from other HIV-infected populations.12, 13, 29–31 Nevertheless, over 21% of study participants had IL-6 levels >3.2 pg/ml which has been associated with mortality in elderly persons.9 Both HCV and HIV infections were independently associated with elevated IL-6, with higher levels seen in HCV/HIV coinfection compared to HCV monoinfection (Table 2). As in other studies,13, 29, 31–33 we found little association between HAART use or HIV RNA with inflammatory biomarkers. Despite the lack of association with HIV RNA, HIV-infected persons with lower CD4+ count exhibited higher IL-6 levels suggesting a link between more advanced immunosupression and markers of inflammation. One mechanism through which HIV might contribute to inflammation is microbial translocation in which on-going HIV replication even at low levels leads to microbial transmigration across mucosal barriers and consequent systemic inflammation,34 particularly in the presence of liver disease.35
Despite numerous prior studies of inflammation in HIV or HCV infected populations, to our knowledge the relationship between injection intensity and inflammatory biomarkers has not been previously characterized. We found that increased frequency of injection drug use correlated with increased IL-6 and CRP in a dose-response fashion. Although not previously described, this finding is biologically plausible. Injection intensity may lead to increased inflammation through direct mechanisms including mild cutaneous injection-related injury or infection or in response to filler agents added to injected substances36, 37 or through indirect mechanisms involving high-risk behaviors (e.g. alcohol, cigarette, non-injection drug use) or living conditions associated with injecting.38–40 Despite cocaine being linked to atherosclerosis and elevated CRP,27, 28 we found no association with recent cocaine use.
As expected, an increased number of comorbidities positively correlated with IL-6. Recently in this same study population, we documented that multimorbidity was significantly associated with HIV infection and related immunosuppression.20 Prior studies of CRP and IL-6 in HIV-infected populations generally have not accounted for multimorbidity. Thus, some of the pro-inflammatory effects of HIV infection may be mediated through HIV-associated comorbidities. Interestingly, the number of comorbidities was not correlated with CRP. However, the lack of relationship likely was influenced by residual confounding by the strong negative association between HCV and CRP. We also document an independent effect of obesity on IL-6 and CRP levels even after controlling for the significant associations of injection intensity, comorbidity and other confounders.
Although lower CRP levels with HCV infection has been previously reported,16, 41 this finding has not been widely-recognized or investigated. In our study, HCV infection and viremia were strongly associated with lower CRP levels, yet we could not detect a strong association of CRP with the degree of liver fibrosis or other markers of synthetic liver function or of liver injury. Importantly, the low levels of CRP with HCV infection were observed in our cohort which has a low prevalence of advanced liver disease. In aggregate, these data refute the hypothesis that reduced CRP levels in HCV infection is simply a consequence of diminished production of CRP in persons with advanced liver disease. Further mechanistic studies are needed to investigate how HCV contributes to reduced CRP levels. Our finding of a strong reduction in CRP with higher HCV RNA levels raises the possibility that active viral replication may inhibit CRP production. In addition to experimental studies, longitudinal clinical studies could evaluate CRP and cytokine changes with effective antiviral therapy.
While dysregulation of the immune system occurring with normal aging may cause persistent low-level pro-inflammatory cytokine circulation,42, 43 we found the effects of older age differed by HCV infection status. Older age was associated with higher IL-6 levels. Similarly, among HCV-uninfected participants, CRP levels increased with age (Figure). However, among HCV-infected participants, the expected age-related increase in CRP was not observed, perhaps explained by the increased likelihood of being HCV-infected at older ages in our cohort.
The main strength of this study is that it concurrently investigates a number of factors potentially associated with elevated inflammatory biomarkers in a population at high risk for chronic inflammation-associated disease. Our study has several limitations. The cross-sectional approach precludes determining causal relationships. Results from this urban, largely male and African-American cohort may not be generalizable to other HIV or drug-using populations. Data were collected on a limited subset of cytokines; information on other inflammatory and coagulation markers (e.g., TNF-α, D-dimer) would provide a more complete picture of inflammation. We also did not have data on use of medications that influence levels of inflammation (e.g., non-steroidal anti-inflammatory drug, statins, angiotensin-converting enzyme inhibitors). Inflammatory biomarkers were measured at one visit leaving some uncertainty about long-term elevation; however, IL-6 and CRP levels obtained at a single timepoint have been shown to be reproducible and representative of extended time periods.44, 45
In conclusion, we identified several clinical and behavioral factors which contribute to a pro-inflammatory state in persons with or at-risk for HCV and HIV infections. Chronic infection with HCV and with HIV are associated with elevated IL-6 levels, whereas HCV and CRP share a strong inverse relationship. In addition to chronic viral infections, increased injection intensity, obesity, and comorbidity also contribute to increased inflammation. As HCV and HIV populations continue to age, the burden of non-AIDS disease associated with chronic inflammation is expected to increase. In addition to studies investigating anti-inflammatory interventions in these high-risk populations, our data advocate for continued clinical focus on optimizing uptake and responses to antiviral therapy for HIV and HCV infections, substance abuse treatment and reduction in injection-related harm, weight loss, and appropriate management of comorbidities. In addition to representing good clinical care, these interventions may also translate into secondary benefits of reduced chronic inflammation and overall improvements in health.
Supplementary Material
ACKNOWLEDGMENTS
We thank the ALIVE study participants and staff for their contributions to this research.
Funding: This work was supported by the National Institutes of Health (grants RC1-AI-086053, R01-DA-04334, R01-DA-12568, R01-HL-90483, R01-DA-16078 and K01-AI071754) and the American Cancer Society (MRSG-07-284-01-CCE to G.D.K.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No authors have a conflict of interest to report.
Author Contributions: All authors contributed: 1) to the conception and design or acquisition of data or analysis and interpretation of the data; 2) drafting the article or revising it critically for important intellectual content; and 3) final approval of the version to be published.
This work has not been presented previously.
REFERENCES
- 1.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004 May;39(5):687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003 Sep 1;92(5):522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 3.Il'yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005 Oct;14(10):2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 4.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001 Jul 18;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 5.Means RT., Jr Pathogenesis of the anemia of chronic disease: a cytokine-mediated anemia. Stem Cells. 1995 Jan;13(1):32–37. doi: 10.1002/stem.5530130105. [DOI] [PubMed] [Google Scholar]
- 6.Kuo HK, Bean JF, Yen CJ, Leveille SG. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. J Gerontol A Biol Sci Med Sci. 2006 Apr;61(4):380–387. doi: 10.1093/gerona/61.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011 Feb 18;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010 Nov;5(6):498–503. doi: 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999 May;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 10.Lau B, Sharrett AR, Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006 Jan 9;166(1):64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 11.Rodger AJ, Fox Z, Lundgren JD, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009 Sep 15;200(6):973–983. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011 Jun 1;203(11):1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold M, Meise U, Gunther V, Rossler H, Zangerle R. Serum concentrations of circulating endogenous granulocyte-macrophage colony-stimulating factor in HIV-1-seropositive injecting drug users. Presse Med. 1994 Dec 17;23(40):1854–1858. [PubMed] [Google Scholar]
- 15.Ownby RL, Kumar AM, Benny Fernandez J, et al. Tumor necrosis factor-alpha levels in HIV-1 seropositive injecting drug users. J Neuroimmune Pharmacol. 2009 Sep;4(3):350–358. doi: 10.1007/s11481-009-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castello G, Scala S, Palmieri G, Curley SA, Izzo F. HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol. 2010 Mar;134(3):237–250. doi: 10.1016/j.clim.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Mehta SH, Kirk GD, Astemborski J, Sulkowski MS, Afdhal NH, Thomas DL. Stability of liver fibrosis among HCV-infected injection drug users. Antivir Ther. 2012 Mar 15;17(5):813–821. doi: 10.3851/IMP2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong GL. Injection drug users in the United States, 1979–2002: an aging population. Arch Intern Med. 2007 Jan 22;167(2):166–173. doi: 10.1001/archinte.167.2.166. [DOI] [PubMed] [Google Scholar]
- 19.Smith G. Senate Special Committee on Aging, 2005. Washington, DC: 2005. Statement of Senator Gordon H. Smith. Aging Hearing: HIV over fifty, exploring the new threat. [Google Scholar]
- 20.Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis. 2011;53(12):1256–1264. doi: 10.1093/cid/cir673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 22.Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406–418. doi: 10.1111/j.1749-6632.1982.tb22153.x. [DOI] [PubMed] [Google Scholar]
- 23.Mehta SH, Netski D, Sulkowski MS, Strathdee SA, Vlahov D, Thomas DL. Liver enzyme values in injection drug users with chronic hepatitis C. Dig Liver Dis. 2005 Sep;37(9):674–680. doi: 10.1016/j.dld.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002 Jul 2;137(1):1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 25.Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1998. [Google Scholar]
- 26.Johnston DE. Special considerations in interpreting liver function tests. American Family Physician. 1999 Apr 15;59(8):2223–2230. [PubMed] [Google Scholar]
- 27.Tai H, Lai H, Jani J, Lai S, Kickler TS. HIV infection and cocaine use induce endothelial damage and dysfunction in African Americans. Int J Cardiol. 2012 Nov 15;161(2):83–87. doi: 10.1016/j.ijcard.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel AJ, Mendelson JH, Sholar MB, et al. Effect of cocaine usage on C-reactive protein, von Willebrand factor, and fibrinogen. Am J Cardiol. 2002 May 1;89(9):1133–1135. doi: 10.1016/s0002-9149(02)02289-0. [DOI] [PubMed] [Google Scholar]
- 29.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010 Jun 15;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005 May 1;39(1):44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 31.Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011 Jan 1;56(1):36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regidor DL, Detels R, Breen EC, et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011 Jan 28;25(3):303–314. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reingold J, Wanke C, Kotler D, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008 Jun 1;48(2):142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 35.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008 Jul;135(1):226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips KT, Stein MD. Risk practices associated with bacterial infections among injection drug users in Denver, Colorado. Am J Drug Alcohol Abuse. 2010 Mar;36(2):92–97. doi: 10.3109/00952991003592311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd-Smith E, Wood E, Zhang R, Tyndall MW, Montaner JS, Kerr T. Risk factors for developing a cutaneous injection-related infection among injection drug users: a cohort study. BMC Public Health. 2008;8:405. doi: 10.1186/1471-2458-8-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orosz Z, Csiszar A, Labinskyy N, et al. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol Heart Circ Physiol. 2007 Jan;292(1):H130–H139. doi: 10.1152/ajpheart.00599.2006. [DOI] [PubMed] [Google Scholar]
- 39.Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosom Med. 2008 Jul;70(6):646–652. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- 40.Eklund CM. Proinflammatory cytokines in CRP baseline regulation. Adv Clin Chem. 2009;48:111–136. doi: 10.1016/s0065-2423(09)48005-3. [DOI] [PubMed] [Google Scholar]
- 41.Nascimento MM, Bruchfeld A, Suliman ME, et al. Effect of hepatitis C serology on C-reactive protein in a cohort of Brazilian hemodialysis patients. Braz J Med Biol Res. 2005 May;38(5):783–788. doi: 10.1590/s0100-879x2005000500017. [DOI] [PubMed] [Google Scholar]
- 42.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 43.Hager K, Machein U, Krieger S, Platt D, Seefried G, Bauer J. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging. 1994 Nov-Dec;15(6):771–772. doi: 10.1016/0197-4580(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 44.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997 Jan;43(1):52–58. [PubMed] [Google Scholar]
- 45.Rao KM, Pieper CS, Currie MS, Cohen HJ. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994 Dec;102(6):802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.