Abstract
Mammalian skeletal muscle fibers can be classified into functional types by the heavy chain (MyHC) and light chain (MyLC) isoforms of myosin (the primary motor protein) that they contain. Most human skeletal muscle contains fiber types and myosin isoforms I, IIA and IIX. Some highly specialized muscle fibers in human extraocular and jaw-closing muscles express either novel myosins or unusual combinations of isoforms of unknown functional significance. Extrinsic laryngeal muscles may express the extraocular MyHC isoform for rapid contraction and a tonic MyHC isoform for slow tonic contractions. In jaw-closing muscles, fiber phenotypes and myosin expression have been characterized as highly unusual. The jaw-closing muscles of most carnivores and primates have tissue-specific expression of the type IIM or `type II masticatory' MyHC. Human jaw-closing muscles, however, do not contain IIM myosin. Rather, they express myosins typical of developing or cardiac muscle in addition to type I, IIA and IIX myosins, and many of their fibers are hybrids, expressing two or more isoforms. Fiber morphology is also unusual in that the type II fibers are mostly of smaller diameter than type I. By combining physiological and biochemical techniques it is possible to determine the maximum velocity of unloaded shortening (Vo) of an individual skeletal muscle fiber and subsequently determine the type and amount of myosin isoform. When analyzed, some laryngeal fibers shorten at much faster rates than type II fibers from limb and abdominal muscle. Yet some type I fibers in masseter show an opposite trend towards speeds 10-fold slower than type I fibers of limb muscle. These unusual shortening velocities are most probably regulated by MyHC isoforms in laryngeal fibers and by MyLC isoforms in masseter. For the jaw-closing muscles, this finding represents the first case in human muscle of physiological regulation of kinetics by light chains. To gether, these results demonstrate that, compared to other skeletal muscles, cranial muscles have a wider repertoire of contractile protein expression and function. Molecular techniques for reverse transcription of mRNA and amplification by polymerase chain reaction have been applied to typing of single fibers isolated from limb muscles, successfully identifying pure type I, IIA and IIX and hybrid type I/IIA and IIA/IIX fibers. This demonstrates the potential for future studies of the regulation of gene expression in jaw-closing and laryngeal muscles, which have such a variety of complex fiber types fitting them for their roles in vivo.
Keywords: Muscle, Myosin, Fiber types, Reverse transcription-polymerase chain reaction
Introduction
Mammalian skeletal muscles may be characterized by their motor neuron, motor unit and muscle fiber properties. Physiological characteristics of these structures include motor neuron axon conduction velocity, recruitment order of skeletal muscle motor units, muscle tension, muscle activity pattern (either `tonic' activity or `phasic' activity) and fatigue resistance. A common way to describe muscle tissue diversity is through classification of skeletal muscle fiber types which link protein composition and metabolism to contractile performance. Mammalian muscle is classified into slow (type I) and fast (type II) groups, with subclassification of fast fibers including types IIA, IIB and IIX. Motor axon diameter, motor unit size, muscle fiber diameter, fusion frequency, contraction speed and tetanic force increase by fiber type in the order of I < IIA < IIX ≤ IIB. Recruitment of motor units however is ordered from the slowest (in which slow-conducting motor axons innervate slow-contracting, type I, muscle fibers) to the fastest (in which fast-conducting motor axons innervate the largest and fastest, type IIB, muscle fibers) [Farina et al., 2002]. A large range of functional diversity is possible in skeletal muscle tissue by variations in fiber type distribution between muscle type, species differences and individual variation. Current evidence suggests that the IIB fiber type is rare or entirely absent in larger mammals including man [Snow et al., 1982; Horton et al., 2001], due probably to differences in isometric tension cost. Isometric tension cost, i.e. the ratio between energy expended and tension generated (PO), is lowest for type I fibers in rat muscle (0.66) and highest for type IIB fibers (2.90). Likewise in humans, tension cost increases from type I fibers (0.56) to type IIX fibers (1.76) [Stienen et al., 1996]. These ratios support the hypothesis that large mammals have a lower energy cost for activation of skeletal muscles than smaller animals. The absence of type IIB fibers in larger mammals is probably a necessary adaptation for size.
Variability beyond these general parameters is evidenced in `specialized' cranial muscles as exemplified by the jaw-closing, intrinsic laryngeal and extraocular muscles. Unlike their counterparts in the limb and abdominal areas these groups have evolved to serve functions that are distinct from postural tonicity and locomotion. Two of these groups have evolved characteristic fiber types containing unique isoforms of myosin, the main motor protein of muscle. Type II masticatory (`type IIM') myosin heavy chain (MyHC) isoform is found in jaw-closing muscles of some species [Rowlerson et al., 1983], and the extraocular MyHC isoform in extraocular muscles [Wieczorek et al., 1985]. Data has suggested the possibility of novel myosins in intrinsic laryngeal muscles [DelGaudio et al., 1995], but definitive molecular evidence does not currently exist.
Further, at least in rabbit muscle, it has been demonstrated with molecular techniques that the extraocular MyHC is expressed in laryngeal muscle [Briggs and Schachat, 2000].
The pattern of expression of these characteristics suggests that they are important functional specializations, of which extraocular and jaw-closing muscle groups can be regarded as extreme examples. The extraocular muscles have a similar function between species and therefore limited variability in anatomy and fiber type compositions. In contrast, the jaw-closing muscles have functions, anatomy and fiber types that vary tremendously between species. Laryngeal muscles have more modest variability in anatomy, perhaps more significant variability in function and their fiber type properties are currently under investigation [Brandon et al., 2003].
Adult type I and II fibers typically express only their specific MyHC isoform, yet in cranial muscles myosin coexpressions are an important and common feature. Myosin coexpression in these fibers has been shown to be relatively consistent and probably does not represent an orderly transition from one stable fiber type to another. Extraocular muscles are anatomically subdivided into global and orbital layers. The global layer contains fibers that may coexpress extraocular, slow, tonic and other fast MyHC isoforms. Orbital region fibers may coexpress tonic, extraocular, embryonic, neonatal or other fast MyHC isoforms [Sartore et al., 1987]. Hence, although many fast fibers in extraocular muscles are characterized by the presence of some `extraocular' MyHC, most are likely to be hybrids rather that the homogenous types expressing a single myosin (as commonly found in limb muscles). Jaw-closing muscles of carnivores, primates [Rowlerson et al., 1983] and some marsupial mammals [Sciote et al., 1995; Sciote and Rowlerson, 1998] contain the IIM fiber type since many of their fibers express only type IIM MyHC and myosin light chains (MyLC) [Rowlerson et al., 1981] (summarized in fig. 1). Laryngeal muscles have some normal type I and II fibers, and some fibers with apparent stable coexpressions of myosin including extraocular MyHC [DelGaudio et al., 1995] and tonic MyHC [Han et al., 1999].
Fig. 1.
Immunohistochemical staining of two serial sections cut in the transverse plane from an area associated with the posterior mandible, close to the temporomandibular joint in an adult Monodelphis domestica (South American opossum). Heads were hemidissected then rapidly fixed in methacarnoy to preserve antigenicity prior to staining [Sciote and Rowlerson, 1998]. a Reactivity of anti-type IIM MyHC antibody for fibers in masseter, a jaw-closing muscle. c Reactivity of anti-type II (IIA, IIX and IIB) antibody for fibers in digastric, a jaw-opening muscle. b Craniofacial skeleton of Monodelphis, with line indicating the relative area stained in a and c.
Of special clinical interest are adaptations found in human jaw-closing muscles. Although a member of the primate order, human jaw-closing muscles do not contain type IIM fibers. Rather there is expression of a variety of myosins including type I, IIA, IIX, neonatal and atrial (α-cardiac) [Sciote et al., 1994] and some embryonic MyLC [Butler-Browne et al., 1988]. Although some fibers express only one isoform, many have hybrid myosin content in which almost all possible combinations of two or more isomyosins may be found [Sciote et al., 1994; Monemi et al., 1996, 1998]. Hybrid fibers may also be identified in limb muscles, but in most cases represent cells in transition from one stable phenotype to another. In limb muscles, transitions between the stable fiber types I, IIA and IIX are found on an orderly continuum: I ↔ (I + IIA) ↔ IIA ↔ (IIA + IIX) ↔ IIX. Another unusual feature of human jaw closing muscles is the relatively small diameter of fibers containing fast (type II) myosins. These fast fibers are of such diminished size that their appearance is suggestive of disuse atrophy found in limb muscles (fig. 2).
Fig. 2.

Myofibrillar ATPase histochemical reactivity of serial sections of human sartorius muscle after incubation in buffer at pH 10.2 (a), 4.3 (b) and 4.6 (c). Type II fibers are reactive in alkali pH, type I in acid pH, and type IIX fibers moderately reactive in mild acid pH. ◯ = Type I fiber; ∎ = type IIX fiber; * = type IIA fiber. Magnification ×200.
In considering the role specialized cranial muscles may play in musculoskeletal disorders it is important to consider their intrinsic differences from other skeletal muscle tissues. This report seeks to describe some of these differences by presenting new data and, in addition, reviewing recent findings principally from jaw-closing and laryngeal muscles.
Scientific Methods and Techniques
Histochemistry and Immunohistochemistry of Tissue Sections
Tissue level investigations of skeletal muscle have sought to characterize the structure as an estimate of overall organ performance and contractility. Features such as fascicle organization, capillarity, fiber organization and fiber type classification are useful indicators of overall muscle tension, contraction speed and fatigability. Although opportunity is limited, where possible investigators have sampled muscle from living humans, usually as a needle biopsy in training and performance experiments or as a larger sample when muscle is resected as part of a standard surgical procedure. In the present study masseter muscle samples were taken from patients undergoing orthognathic surgical procedures for jaw repositioning. Although these patients demonstrated modest abnormalities in craniofacial growth, there is no indication of muscle abnormalities. Samples taken from similar subjects show characteristics of fiber type distribution and size [Daniel et al., 2001] resembling those described for subject populations including individuals with normal carniofacial growth [Thornell et al., 1984; Sciote et al., 1994; Monemi et al., 1998]. Laryngeal muscle samples were obtained from patients undergoing laryngectomy usually for treatment of neoplasm of epithelial origin, and in whom laryngeal muscle function was normal. Samples which represent normal limb muscle were collected subsequent to resections for treatment of orthopedic oncology. All surgical biopsies were obtained according to University Institutional Review Board guidelines for ethical use of human subjects in research. A standard preparation of a muscle biopsy is immediate snap-freezing of the tissue block at −80°C (or colder), followed by serial sectioning in a cryostat. Avoiding fixation assures appropriate tissue reactivity for histochemical and immunohistochemical staining. For determination of fiber type by myofibrillar ATPase histochemistry in human muscles a histochemical stain similar to that of Brooke and Kaiser [1970] is often used.
Our technique is a modification of this original method [as described previously in Snow et al., 1982], but with reaction incubation times doubled to increase the intensity of staining for human muscle. Immunohistochemical staining is conducted using an indirect immunoperoxidase method after incubation of tissue sections in MyHC-specific antibodies. The following MyHC-specific antibodies were used: anti-type I monoclonal (slow skeletal MyHC – Sigma Aldrich clone NOQ7), antifast monoclonal (fast skeletal IIA, IIB and IIX MyHC – Sigma Aldrich clone MY-32), anti-IIA monoclonal (fast IIA – American Type Culture Collection clone SC-71), anti-IIB (American Type Culture Collection clone BF-F3), antineonatal polyclonal [Scapolo et al., 1991], antitonic polyclonal [Mascarello and Rowlerson, 1992], anticardiac monoclonal (cardiac αMHC – Sera Lab clone MAS-366 and BAG5; American Type Culture Collection Clone BA-G5), and anti-IIM polyclonal [Sciote et al., 1995]. Subsequent to staining serial sections with a panel of antibodies and a series of histochemical stains after various acid and alkali preincubations, biopsies are characterized for the proportions, size and types of fibers present. These fiber-type characteristics are used to infer physiological activity of the whole muscle.
Single Fiber Physiology
There are many physiological tests which may be utilized to determine muscle activity. Perhaps the most common is electromyographic activity as determined by surface electrodes placed on the skin overlying a muscle belly [Svensson et al., 2001], but this technique only describes general gross activity in whole muscle. Of interest, especially in cranial muscles, are the fine movements and motor control in performance of highly specialized tasks. In jaw-closing muscles, bite force transducers are used to determine muscle activity as it is translated into force produced at the occlusal surfaces of teeth [Dechow and Carlson, 1983]. Working under the constraint of biopsy samples, it is still possible to directly assess muscle cell performance using fiber bundle [Zuurbier et al., 1995] and single fiber [Edman, 1979] preparations. Single fiber preparations allow direct assessment of cell physiology to be correlated with contractile protein expression and fiber type. This is particularly important in specialized cranial muscles where novel myosins or unusual protein coexpressions have been identified.
In data presented here we have used the slack-test protocol [Edman, 1979] to assess unloaded shortening velocity (Vo) of chemically skinned single fibers. Single fibers as short as 0.5–1.5 mm may be dissected from a skinned muscle fiber bundle and mounted via small stainless steel clamps between a force transducer and linear motor of the physiological testing equipment (Scientific Instruments, Heidelberg) in relaxing solution. Initial sarcomere length is adjusted to approximately 2.3–2.5 μm, as calculated by a first order laser diffraction pattern. The fiber is then activated to resting maximal tension using activating solution with an activating solution of ~pCa2+ (4.5). The slack test protocol measures repeated ramp shortenings of the activated fiber and the amount of time necessary to remove introduced slack by sarcomeric shortening as described previously [Sciote and Kentish, 1996; Morris et al., 2001; Sciote et al., 2002]. The test determines Vo, in muscle lengths/second (ML/s). Subsequent to physiological testing, fiber cells are prepared for contractile protein isolation and identification. Identification of MyHC isoforms requires an adapted electrophoresis protocol with low acrylamide concentrations and addition of glycerol [Blough et al., 1996]. A single fiber provides sufficient protein load for glycerol gel electrophoresis and standard discontinuous SDS-PAGE. Standard SDS-PAGE allows separation and identification of actin and other contractile proteins including MyLC isoforms, but does not separate MyHC isoforms (which require glycerol gels). All gels are silver stained and optically scanned with Quantity-1 software (BioRad) to quantify band intensities for calculation of protein content. Where necessary, Western blots were used for isoform identification according to the method of LaFramboise et al. [1990].
Identification of MyHC Gene Message in Single Fibers
As described above, transitions along a continuum from slow to fast MyHC isoforms occur in response to diverse physiological states that accompany changes in muscle fiber phenotypes. The changes between myosin isoforms are reversible, often resulting in mismatches between message and protein content within transitional fibers [Pette and Staron, 2001], as well as unusual patterns of coexpression for slow and fast myosin mRNAs [Horton et al., 2001] and the presence of some negative fibers that lack detectable myosin message [Smerdu et al., 1994; Ennion et al.,1995]. We are using reverse transcription (RT) and amplification by polymerase chain reaction (PCR) together with protein data from histochemistry and immunohistochemistry to investigate the plasticity of MyHC gene expression in tissue extracts and isolated single fibers of limb, laryngeal, extraocular and jaw-closing skeletal muscle complexes. For single fiber studies, muscle biopsies are quickly separated into thin longitudinal strips, snap frozen in isopentane-dry ice and lyophilized in sterile microfuge tubes. Individual fibers that range in length from 2 to 8.5 mm are dissected from the freeze-dried strips. RNA is isolated from the single fibers with guanidinium thiocyanate using RNAqueous™-4PCR kits (Ambion) and digested for 30 min at 37°C with 1 μg DNAse. Approximately 1.5% of the total RNA from each fiber is reverse-transcribed at 50°C for 1 h in the presence of 0.4 μM oligo dT(18) and 20 pg/μl random hexamer primers and amplified using Titanium™ One-Step RT-PCR kits (Clontech Laboratories) in an MJ Research PTC-200 thermal cycler. PCR reactions include an initial denaturation at 94°C for 5 min followed by 30 cycles of denaturation (30 s at 94°C), annealing (30 s at 60°C) and elongation (1 min at 72°C). Final elongation is done for 5 min at 72°C. Oligonucleotide primers for PCR (table 1) were selected from GenBank mRNA sequences for MyHC-I/β (accession XM-033374), MyHC-IIA (accession XM-012618), MyHC-IIX (accession XM-052590) and MyHC-IIB (accession XM-017815) using Jellyfish software (LabVelocity) and synthesized by a commercial source (Invitrogen). QuantumRNA™ 18S internal standards (Ambion) were amplified using a 7:3 primer to competimer ratio. Electrophoretic analyses of PCR products were done on 5-μl aliquots out of 15-μl reaction volumes in 3% NuSieve® 3:1 agarose gels containing 0.5 μg/ml ethidium bromide. Gels were photographed on a UV light box using an AlphaImager 2000 system (Alpha Innotech) and a densitometric analysis of bands was performed with Quantity One 4.1 (Bio-Rad Laboratories) quantitation software.
Table 1.
Primers for RT-PCR of MyHC genes
| MyHC | Sense primer | Antisense primer |
|---|---|---|
| β | 5′-AGTAGCTTTGCCACATCTTGATCT-3′ | 5′-TTCCTCCCAAGGAGCTGTTACACA-3′ |
| IIA | 5′-GAGCAGAGAAGGAGGAAAAGTGAC-3′ | 5′-CTGCATAACGTTCTTTGAGGTTGT-3′ |
| IIX | 5′-CTGCAAGCAAAGGTGAAATCCT-3′ | 5′-TGGTCACCTTTCAGCAGTTTAGATAA-3′ |
Results and Discussion
Fiber Types and Their Variability in Human Jaw-Closing Muscles
The jaw-closing muscles of carnivores and primates contain an additional fast fiber type, with a notably rapid ATPase activity and fast twitch speed, termed IIM or `type II masticatory' [Rowlerson et al., 1981]. This fiber type, which contains a distinct isoform of myosin, is thought to be characteristic of carnivorous behavior or aggressive biting [Rowlerson et al., 1983]. We have recently extended these observations to marsupial mammals including the North American [Sciote et al., 1995] and South American [Sciote and Rowlerson, 1998] opossum (fig. 1) and marsupial mammals of Australia including the microbat and kangaroo [Hoh et al., 1996]. These marsupial species demonstrate dramatic differences between fiber type composition among type II fibers when jaw-opening and jaw-closing muscles are compared. Jaw-closing muscles as exemplified by masseter are composed predominately of type IIM fibers, while the jaw-opening muscles (such as anterior digastric) contain predominately limb type II fibers.
The jaw-closing muscles of man are notably different, containing fibers of an exceptionally wide range of fiber diameters and MyHC composition, but no IIM fiber types as found in other primates. Before describing human jaw-closing muscles it is necessary to provide insight into human skeletal muscle in general since there are some species differences. In the limb muscles of small mammal types IIB and IIX may be found in abundance, but as animal size increases the relative proportion of IIB decreases substantially. In human muscle the IIB fiber type classified by ATPase histochemistry muscle actually contains the IIX homologue and no IIB myosin or transcripts [Smerdu et al., 1994; Ennion et al., 1995]. Hence, human limb and abdominal muscles of normal health and function are composed of type I, IIA and IIX fibers (fig. 2). Human jaw-closing muscles contain many fiber types, several of which coexpress MyHC isoforms [Sciote et al., 1994; Monemi et al., 1996, 1998]. The fiber type composition of human masseter is also distinctive compared to typical limb muscle in that its type II fibers are typically small in diameter compared to the normally sized type I fibers (fig. 3a–c). This unusual size of the type II fibers is reminiscent of a limb muscle that is subject to disuse, producing preferential atrophy and diameter decrease in type II fibers (fig. 3f). Another extreme phenotype occurs in masseter hypertrophy where type II fibers approach the average fiber diameter of their counterparts in limb muscle (fig. 3d). The subject we sampled with the condition of masseter hypertrophy possessed a long-term neurologic condition causing spasm of the masseter resulting in extreme muscle hyperfunction. Unlike our subject, masseter hypertrophy usually occurs as a gradual progressive increase in size of the muscle in the area located over the ramus of the mandible not accompanied by spasm or parotid gland involvement [Buchner et al., 1979]. Furthermore masseter hypertrophy may be associated with symptoms commonly described in temporomandibular disorders (TMD) such as temporal headache, jaw tension, trismus, indefinable masseter pain and joint pain [Beckers, 1977], and is probably the best example of direct muscle involvement in TMD.
Fig 3.
Staining of biopsy sections taken from the masseter muscle of a young healthy adult (serial sections a-c), a middle aged adult with masseter hypertrophy secondary to muscle spasm (serial sections d, e) and the vastus lateralis of a middle aged man with preferential type II fiber atrophy subsequent to inactivity (section f). a Reactivity of anti-type I MyHC antibody. b Reactivity of anti-type II MyHC antibody. c Reactivity for anti-type IIA MyHC antibody. Myofibrillar ATPase reactivity is shown after incubation in buffer at pH 10.2 (d, f) and after pH 4.6 (e). ◯, ● = Type I fiber; ◻, ∎ = type IIA fiber; * = fibers coexpressing both type I and II MyHC isoforms. Magnification ×600.
Jaw-closing muscles do contain type I, IIA and IIX fibers with homogeneous isoform content, but type I, IIA and IIX MyHC isoforms can all be coexpressed in various combinations with neonatal and α-cardiac MyHCs. We have distinguished two fiber types in jaw-closing muscles as `neonatal' and `atrial' on the basis of their MyHC expression; these types are found quite often in human jaw-closing muscles but rarely, if ever, in other skeletal muscles. The `neonatal' fiber type expresses the neonatal MyHC in various combinations of type I, IIA and IIX, but is a mature fiber in other respects. Likewise, the `atrial' fiber type expresses the cardiac α-MyHC in combination with type I, IIA and/or IIX even though it is a normal skeletal muscle fiber. In jaw-closing muscles of human subjects, these fiber types have characteristic sizes (fig. 4). These unusual fiber types characteristic of human jaw-closing muscles are currently the subject of physiological investigation since there are no known animal models that closely mimic this phenotype. An added complication of extrapolating physiological results obtained at single fiber level to the whole jaw-closing muscles is the level of heterogeneity that exists in these muscles across all organizational levels from individual fibers to the whole muscle in man. Although type I fibers predominate overall in human jaw-closing muscles, there are both systematic differences in the percentage of type I fibers in different parts of the muscles, and highly variable compositions of remaining fiber types (fig. 5) [Ringqvist et al., 1982; Eriksson and Thornell, 1983]. Thus, we are currently investigating the question of whether fiber type diameter and percent composition differ significantly in subject populations with varying craniofacial morphologies. A preliminary answer (published in abstract form) can be found in a report on a group of orthodontic patients from whom a masseter muscle biopsy was taken during an orthognathic surgical procedure to reposition one or both jaws as part of overall orthodontic treatment [Daniel et al., 2001]. There appear to be significant differences in fiber types between extremes of facial types, but the variability in fiber type in these orthodontic groups probably requires sampling from a larger patient population to develop firm conclusions.
Fig. 4.
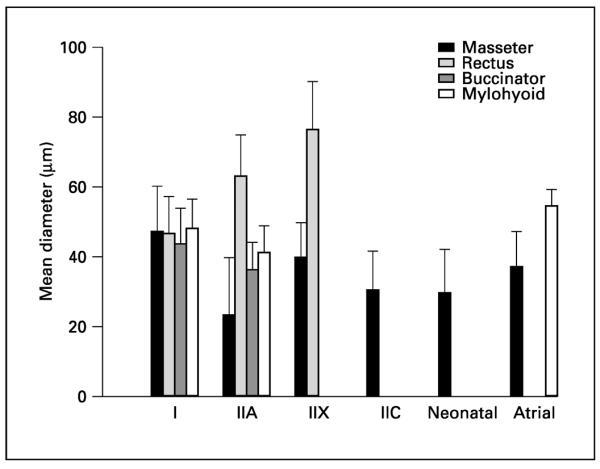
Morphometric analysis of average fiber type diameter after biopsy sampling from a young adult population (jaw closing, jaw opening, abdominal and facial). Number of subjects: masseter n = 28, rectus (abdominal muscle) n = 4, buccinator n = 3 and mylohyoid n = 3. A portion of this data is taken from Sciote et al. [1994].
Fig. 5.
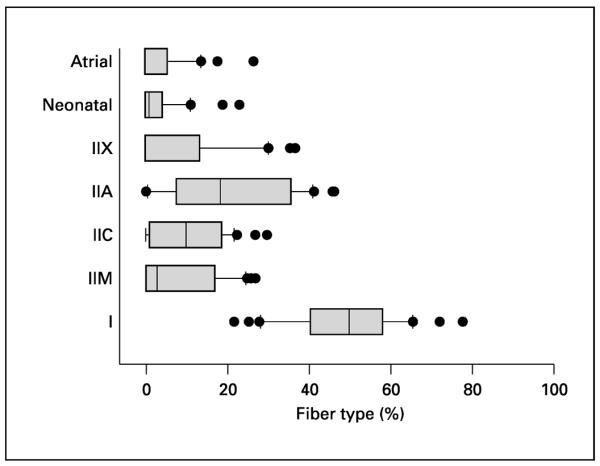
Percent composition for each fiber type in masseter muscle biopsies sampled in figure 4 presented as boxplots. The boxes contain the median and the fourths of data and resist gross distortion of outlying values, here demonstrating the masseter muscle's striking variability in % fiber type composition.
Single Fiber Physiology
In mammalian skeletal muscle, type I fibers containing type I MyHC are associated with a slower unloaded shortening velocity (Vo), and type II fibers containing type II MyHCs with a faster Vo, as measured by the slack test [Reiser et al., 1988; Bottinelli et al., 1991]. Fibers heterogeneous for type I and type II isoforms have intermediate shortening speeds, with Vo proportional to the amount of type II isoform present [Reiser et al., 1985]. The correlation of shortening velocity to MyHC isoform content within fast-contracting type IIA, IIB and IIX fibers is less certain when compared across species. In the rabbit [Sweeney et al., 1988] and human [Larsson and Moss, 1993], IIX fibers have a faster Vo than IIA fibers, which may be correlated to MyHC isoform content, but in the rat this correlation has been questioned [Bottinelli et al., 1994a]. The relative content of essential light chains (the actual amount of MyLC1f vs. MyLC3f in a muscle fiber) in type II fibers of rabbit may modify MyHC-dependent shortening speed, producing a greater range of Vo for each fiber type [Sweeney et al., 1988]. But in the rat, the relative essential light chain content may modify Vo to the extent that some IIB fibers may shorten more slowly than IIA fibers [Bottinelli et al., 1994b]. This strong correlation of MyLC content to Vo was not demonstrated in human type II limb muscle fibers [Larsson and Moss, 1993].
Similar experiments were first conducted by us in rabbit masseter muscle, since this species contains fibers that homogeneously express α-cardiac MyHC like that present in some human masseter fibers. When tested for Vo, α-fibers have a mean Vo of 0.78 ML/s, which was intermediate in speed between masseter and soleus type I and type II fibers [Sciote and Kentish, 1996]. The only slightly faster Vo of α-cardiac fibers relative to type I fibers is surprising since in cardiac muscle the α-cardiac MyHC is thought of as fast-contracting, in the range of 4–5 ML/s [Sweitzer and Moss, 1993]. However, shortening velocities in cardiac myocytes are regulated by other factors in addition to myosin proteins [Ford, 1991], so direct comparison to skeletal fibers is probably not appropriate. A further complication exists when comparing the results between cardiac and skeletal muscle cells. In cardiac myocytes α-cardiac MyHC is typically combined with the atrial MyLCs, MyLC1A and MyLC2A, while in α-cardiac fibers of masseter, α-cardiac MyHC is combined with the slow skeletal MyLCs, MyLC1s and MyLC2s [d'Albis et al., 1993].
We have now tested shortening velocity in chemically skinned fibers from human limb, masseter and two intrinsic laryngeal muscles, the posterior cricoarytenoid and thyroarytenoid, in relation to the type and amount of myosin isoforms present [Morris et al., 2001]. Sampling from a variety of limb or abdominal muscles, we were able to determine unloaded shortening velocities for fibers that were comparable to previous studies of human fibers and with average velocity increasing in the order of type I < IIA < IIX. This data, presented in figure 6, shows a three-dimensional bar chart with single fiber data separated into bins according to shortening velocity, fiber type and MyHC isoform content. The observed data for type I fibers of masseter and type II fibers of intrinsic laryngeal muscles exemplify the differences between cranial and limb muscle. Masseter fibers homogeneous for type I MyHC have shortening velocities that span almost the entire range of shortening values found in type I and type II limb fibers, so factors other than MyHC isoform content must help regulate contraction. Within this group of type I fibers there is a subpopulation of fibers that contracted at a much lower range of shortening speeds than that of limb muscle. This spread of data for six fibers with an unusually low Vo (<0.1 ML/s) is illustrated in masseter lane, bin 1 of figure 6. Subsequently we isolated and identified MyLC content in addition to MyHC content in these fibers. Through standard SDS-PAGE and Western blotting with MyLC-specific antibodies we were able to identify an additional MyLC isoform present in masseter fibers with unusually slow Vo, namely MyLC embryonic, but absent in the majority of fibers studied (fig. 7a). There is only one other report of changes in MyLC isoform content modifying shortening velocity in human muscle [D'Antona et al., 2002]. In skinned human thyroarytenoid muscle fibers they found relatively large variability in Vo of type IIA fibers. Faster IIA fibers contained decreased amounts of MyLC2f (the regulatory MyLC usually associated with fast MyHC isoforms) and IIA fibers with slower Vo contained increased amounts of MyLC2s (the regulatory MyLC usually associated with the type I or slow MyHC isoform). Further physiological techniques, such as the in vitro motility assay could help confirm these results.
Fig. 6.
Summary of Vo (unloaded shortening velocity) values for chemically skinned single fibers from selected limb, masseter and laryngeal muscles. Fibers are positioned on lanes based upon muscle of origin and MyHC isoform composition, and into bins based upon shortening velocity. Please note that the Vo scale changes at a rate of 0.2 ML/s in the range of 0.0–2.2, then in 1.0 ML/s changes from 3.0 to 5.0.
Fig. 7.
a Electrophoretic analysis of MyLC isoenzymes in standard 14% acrylamide discontinuous SDS-PAGE with single human muscle fiber cells sampled from poster cricoarytenoid (PCA), masseter (Mass.), triceps, gastrocnemius (Gastroc) and thyroarytenoid (TA) muscles. Two slow and three fast MyLC isoforms are resolved with the addition of one embryonic MyLC species found in some masseter fibers. The masseter fiber in lane 3 has a Vo value found in bin 1 of the masseter type I fiber lane (fig. 6). b Electrophoretic analysis of MyHC isoenzymes in modified 9% polyacrylamide gels containing 0.1% SDS and 6% glycerol and visualized by silver staining. The control marker was taken from a whole biopsy sample homogenate of human tibialis muscle (lane 1). Other muscle samples are labeled by lane: atria, fetal, extraocular (EO), thyroarytenoid (TA) and posterior cricoarytenoid (PCA) muscles. Relative mobility of known protein species was neonatal > I > IIA > α > IIX. Two additional bands (labeled MHCa and MHCb) may be identified in EO, TA and PCA muscles only.
Some type II fibers from intrinsic laryngeal muscles have an exceptionally fast shortening velocity. In figure 6, bins 5–7 contain fibers with velocities ranging from approximately 3– 5 ML/s that are very fast in comparison to the fastest IIX fiber bin in limb muscle with approximately 2.2 ML/s. Electrophoretic isolation of MyHC isoforms from these fibers has been inconclusive since myosin content is heterogeneous, including variable expression of two additional species (fig. 7b). This information suggests that further work is necessary to determine MyHC isoform content in human laryngeal muscle before final physiological observations are made.
MyHC Gene Message in Single Fibers
Single-fiber methods for the analysis of MyHC-RNA by RT-PCR were used to detect individual representatives of homogeneous and hybrid gene expressions that typically comprise the continuum of fiber-type transitions in skeletal muscle. The RT-PCR products of four such representative fibers are shown in figure 8, which is a composite from different agarose gels that were run under identical conditions of electrophoresis. Each panel in the figure displays a data set from different single fibers whose RNA was consecutively reverse-transcribed and amplified under the same conditions, but in separate reaction tubes containing 18S, MyHC-I/β, MyHC-IIA or MyHC-IIX-specific PCR primers. Type I, I/IIA, IIA and IIA/IIX fibers were obtained from lyophilized strips of human sartorius whereas the type IIX fiber came from human semitendinous muscle. Equivalent volumes between samples were maintained throughout for RNA isolation, RT-PCR reactions and agarose gel electrophoresis. However, concentrations of RNA in the single fiber samples were not adjusted for equivalency, so that the intensities of the ethidium bromide-stained 18S control bands, as determined by densitometric analysis using Quantity One™ software, differ between the data sets. Although the dissected fibers were approximately the same length, variations in their diameter and weight, as well as some losses encountered during isolation steps could all contribute to differences in RNA recoveries. Nevertheless, the intensities of bands for RT-PCR of 18S rRNA were approximately equal in the type I, I/IIA and IIA/IIX fibers whereas intensities of the bands in the IIA and IIX sets were, respectively, 75 and 23% of maximum values. Because of the possible inequalities between amounts of RNA and efficiencies of RT-PCR for the MyHC species examined here, comparisons between data sets are primarily qualitative, with the identification of MyHC-β/I, IIA and IIX expressing pure fibers and I/IIA and IIA/IIX expressing hybrid fibers being the foremost observation. Secondarily, comparisons of the intensities between 18S internal controls and PCR product bands were done to determine a relative abundance of MyHC RNA within the data set for each fiber. Analysis shows that amounts of I/β, IIA and IIX message, relative to 18S rRNA, were 74, 58 and 211%, respectively, in the pure fibers. In the I/IIA hybrid fiber, the relative abundance of MyHC-I/β was 62% and IIA was 9% of 18S. In the IIA/IIX hybrid fiber, IIA was 64% and IIX was 101% of 18S. Both hybrid fibers may be in transition to the faster phenotype. Also, according to the `nearest neighbor rule' proposed for combinations of MyHC isoforms in fibers [Pette and Staron, 2001], the relative quantities of RNA detected here indicate that the first hybrid fiber contained ~6.7 × more MyHC-I/β than IIA and it is properly classified I/IIA or IC. The relative abundance of MyHC-IIX RNA was ~1.6-fold greater than IIA in the second hybrid fiber, which is more correctly termed type IIX/IIA. Of course this classification only applies if there is directly proportionate translation of message into protein. In future studies it will be interesting to directly compare message to protein amounts, especially in cases of dysfunction or at least altered function. The power of single fiber techniques and quantitative RT-PCR [Wright et al., 1997] for analysis of gene expression in muscle has previously been demonstrated. The further application of single fiber methods presented here with modifications for quantitative analysis of mRNA in relation to protein isoform composition will be extremely important for characterization of the diverse fiber types and transitional states in muscles of the temporomandibular complex.
Fig. 8.

Representative human muscle fiber types analyzed by agarose gel electrophoresis of RT-PCR products from single fibers. RNA from isolated single fibers of sartorius (I, I/IIA, IIA and IIA/IIX types) and semitendinous (IIX type) muscles was reverse-transcribed and amplified using primers for 18S rRNA internal control (a), MyHC-I/β (b), MyHC-IIA (c) and MyHC-IIX (d). Amplified cDNA products were 489 bp (18S), 89 bp (IIB), 176 bp (IIA) and 224 bp (IIX).
Applications for Skeletal Muscle Biology in Temporomandibular Joint Research
TMD may encompass a wide variety of more specific diseases or disorders which often result in pain, tenderness and other symptoms in the joint, surrounding connective structures and/or skeletal muscle. In the extreme, pathological changes such as jaw-closing muscle inflammation and hypertrophy may be imaged and described, most commonly with magnetic resonance imaging [Yang et al., 2001]. Masticatory muscles are directly involved in positioning of the joint and disc, especially the lateral pterygoid muscle which inserts directly into the anteromedial portion of the articular disc. The articular disc also attaches to the fascia of the masseter and temporalis muscles. Beyond these direct connections afferent information from muscle spindles from all of the masticatory muscles is used to provide overall jaw posture including positioning of the temporomandibular joint [Schmolke, 1994]. Although it is likely that muscle tissue does not `cause' TMD this does not exclude the possibility that it plays a role in exacerbating or prolonging joint problems.
Although recent investigations involving cell biology and physiology of cranial muscles have provided interesting information, they have not so far provided evidence for muscle tissue involvement in the genesis of TMD disorders. At present we are still in the initial stages of understanding the range of metabolic and physiological diversity found in human cranial muscle tissue. The greatest challenge for the immediate future is a precise description of this diversity, given a number of practical and ethical constraints. Any investigation of cranial muscle as an organ must be conducted on living subjects undergoing clinical treatments. Cadaver specimens which allow greater volumes of tissue sampling are of limited value, as they generally do not allow direct investigation of physiological functions. Hence findings in ongoing work often depend upon relatively small sample sizes in comparison to the overall size of muscle. An additional complication with regard to jaw-closing muscles is the tremendous amount of variability observed between different subjects, even when samples are collected from the same local area of muscle by one surgeon and processed and analyzed using the same techniques by the researcher. One major problem for the future will be to determine the extent to which individual variations in normal jaw function are related to these differences in fiber type composition. Another will be identifying criteria which allow distinction between normal variation and pathological changes. This will require larger, multifaceted clinical studies of form and function in human subjects in whom adequate clinical diagnosis is known together with the results of skeletal muscle biology research. Ultimately the question remains: Are there clinical markers for developing TMD in muscle tissue, which may be incorporated into diagnosis and treatment of these complicated problems?
Abbreviations used in this paper
- ML/s
muscle lengths/second
- MyHC
myosin heavy chain
- MyLC
myosin light chain
- PCR
polymerase chain reaction
- RT
reverse transcription
- TMD
temporomandibular disorders
References
- Beckers HI. Masseter hypertrophy and its intraoral surgical correction. J Maxillofac Surg. 1977;5:28–35. doi: 10.1016/s0301-0503(77)80072-6. [DOI] [PubMed] [Google Scholar]
- Blough ER, Rennie ER, Zhang F, Reiser PJ. Enhanced electrophoretic separation and resolution of myosin heavy chains in mammalian and avian skeletal muscles. Anal Biochem. 1996;233:31–35. doi: 10.1006/abio.1996.0003. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Maximum shortening velocity and coexistence of myosin heavy chain isoforms in single skinned fast fibers of rat skeletal muscle. J Muscle Res Cell Motil. 1994a;15:413–419. doi: 10.1007/BF00122115. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibers. J Physiol. 1994b;478:341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon CA, Rosen C, Georgelis G, Horton MJ, Sciote J. Muscle fiber type composition and effects of vocal fold immobilization on the two compartments of the human posterior cricoarytenoid: A case study of four patients. J Voice. 2003 doi: 10.1016/s0892-1997(03)00027-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MM, Schachat F. Early specialization of the superfast myosin in extraocular and laryngeal muscles. J Exp Biol. 2000;203:2485–2494. doi: 10.1242/jeb.203.16.2485. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types: How many and what kind. Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Buchner A, David R, Temkin D. Unilateral enlargement of the masseter muscle. Int J Oral Surg. 1979;8:140–148. doi: 10.1016/s0300-9785(79)80009-5. [DOI] [PubMed] [Google Scholar]
- Butler-Browne GS, Eriksson PO, Laurent C, Thornell LE. Adult human masseter muscle fibers express myosin isozymes characteristic of development. Muscle Nerve. 1988;11:10–20. doi: 10.1002/mus.880110614. [DOI] [PubMed] [Google Scholar]
- d'Albis A, Anger M, Lompre AM. Rabbit masseter muscle expresses the cardiac α myosin heavy chain gene. Evidence from mRNA sequence analysis. FEBS Lett. 1993;324:178–180. doi: 10.1016/0014-5793(93)81388-g. [DOI] [PubMed] [Google Scholar]
- Daniel Y, Ferri J, Krivosic-Horber T, McDonald F, Raoul G, Reyford H, Rowlerson A. Masseter muscle fibre types in relation to craniofacial form. J Physiol. 2001;531:154–155P. [Google Scholar]
- D'Antona G, Megighian A, Bortolotto S, Antonietta Pellegrino M, Marchese Ragona R, Staffieri A, Bottinelli R, Reggiani C. Contractile properties and myosin heavy chain iso-form composition in single fibre of human laryngeal muscles. J Muscle Res Cell Motil. 2002;23:187–195. doi: 10.1023/a:1020963021105. [DOI] [PubMed] [Google Scholar]
- Dechow PC, Carlson DS. A method of bite force measurement in primates. J Biomech. 1983;16:797–802. doi: 10.1016/0021-9290(83)90003-9. [DOI] [PubMed] [Google Scholar]
- DelGaudio JM, Sciote JJ, Carroll WR, Escalmado RM. Atypical myosin heavy chain in rat laryngeal muscle. Ann Otol Rhinol Laryngol. 1995;104:237–245. doi: 10.1177/000348949510400310. [DOI] [PubMed] [Google Scholar]
- Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennion E, Sant'Ana PJ, Sargeant AJ, Young A, Goldspink G. Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. J Muscle Res Cell Motil. 1995;16:35–43. doi: 10.1007/BF00125308. [DOI] [PubMed] [Google Scholar]
- Eriksson PO, Thornell LE. Histochemical and morphological muscle-fibre characteristics of the human masseter, the medial pterygoid and the temporalis muscles. Arch Oral Biol. 1983;28:781–795. doi: 10.1016/0003-9969(83)90034-1. [DOI] [PubMed] [Google Scholar]
- Farina D, Fosci M, Merletti R. Motor unit recruitment strategies investigated by surface EMG variables. J Appl Physiol. 2002;92:235–247. doi: 10.1152/jappl.2002.92.1.235. [DOI] [PubMed] [Google Scholar]
- Ford LE. Mechanical manifestations of activation in cardiac muscle. Circ Res. 1991;68:621–637. doi: 10.1161/01.res.68.3.621. [DOI] [PubMed] [Google Scholar]
- Han Y, Wang J, Fischman DA, Biller HF, Sanders I. Slow tonic muscle fibers in the thyroarytenoid muscles of human vocal folds; a possible specialization for speech. Anat Rec. 1999;256:146–157. doi: 10.1002/(SICI)1097-0185(19991001)256:2<146::AID-AR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Hoh JF, Hugues P, Thomas Q. Masticatory myosin expression in marsupial mammals of Australia. Basic Appl Myol. 1996;18:32–45. [Google Scholar]
- Horton MJ, Brandon CA, Morris TJ, Braun TW, Yaw KM, Sciote JJ. Abundant expression of myosin heavy-chain IIB RNA in a subset of human masseter muscle fibres. Arch Oral Biol. 2001;46:1039–1050. doi: 10.1016/s0003-9969(01)00066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFramboise WA, Daood MJ, Guthrie RD, Moretti P, Schiaffino S, Ontell M. Electrophoretic separation and immunological identification of type 2X myosin heavy chain in rat skeletal muscle. Biochim Biophys Acta. 1990;1035:109–112. doi: 10.1016/0304-4165(90)90181-u. [DOI] [PubMed] [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarello F, Rowlerson AM. Myosin isoform transitions during development of extraocular and masticatory muscles in the fetal rat. Anat Embryol. 1992;185:143–153. doi: 10.1007/BF00185915. [DOI] [PubMed] [Google Scholar]
- Monemi J, Eriksson PO, Dubail I, Butler-Browne GS, Thornell LE. Fetal myosin heavy chain increases in human masseter muscle during aging. FEBS Lett. 1996;386:87–90. doi: 10.1016/0014-5793(96)00402-4. [DOI] [PubMed] [Google Scholar]
- Monemi J, Eriksson PO, Eriksson A, Thornell LE. Adverse changes in fibre type composition of the human masseter versus biceps brachii muscle during aging. J Neurol Sci. 1998;154:35–48. doi: 10.1016/s0022-510x(97)00208-6. [DOI] [PubMed] [Google Scholar]
- Morris TJ, Brandon CA, Horton MJ, Sciote JJ. Maximum shortening velocity and myosin heavy-chain isoform expression in human masseter fibers. J Dent Res. 2001;80:1845–1848. doi: 10.1177/00220345010800091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Kasper CE, Greaser ML, Moss RL. Functional significance of myosin transitions in single fibers of developing soleus muscle. Am J Physiol. 1988;254:C605–C613. doi: 10.1152/ajpcell.1988.254.5.C605. [DOI] [PubMed] [Google Scholar]
- Reiser PJ, Moss RL, Giulian GG, Greaser ML. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985;260:9077–9080. [PubMed] [Google Scholar]
- Ringqvist M, Ringqvist I, Eriksson PO, Thornell LE. Histochemical fibre-type profile in the human masseter muscle. J Neurol Sci. 1982;53:273–282. doi: 10.1016/0022-510x(82)90012-0. [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Mascarello F, Veggetti A, Carpene E. The fibre-type composition of the first branchial arch muscles in carnivora and primates. J Muscle Res Cell Motil. 1983;4:443–472. doi: 10.1007/BF00711949. [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Pope B, Murray J, Whalen RB, Weeds AG. A novel myosin present in cat jaw-closing muscles. J Muscle Res Cell Motil. 1981;2:415–438. [Google Scholar]
- Sartore S, Mascarello F, Rowlerson A, Gorza l., Ausoni S, Vianello M, Schiaffino S. Fibre types in extraocular muscles: A new myosin isoform in the fast fibers. J Muscle Res Cell Motil. 1987;8:161–172. doi: 10.1007/BF01753992. [DOI] [PubMed] [Google Scholar]
- Scapolo PA, Rowlerson A, Mascarello F, Veggetti A. Neonatal myosin in bovine and pig tensor tympani muscle fibers. J Anat. 1991;178:255–263. [PMC free article] [PubMed] [Google Scholar]
- Schmolke C. The relationship between the temporomandibular joint capsule, articular disc and jaw muscles. J Anat. 1994;184:335–345. [PMC free article] [PubMed] [Google Scholar]
- Sciote JJ, Kentish JC. Unloaded shortening velocities of rabbit masseter muscle fibres expressing skeletal or alpha-cardiac myosin heavy chains. J Physiol. 1996;492:659–667. doi: 10.1113/jphysiol.1996.sp021335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciote J, Morris T, Brandon C, Horton M, Rosen C. Unloaded shortening velocity and myosin heavy chain variations in human laryngeal fibers. Ann Otol Rhinol Laryngol. 2002;111:120–127. doi: 10.1177/000348940211100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciote JJ, Rowlerson A. Skeletal fiber types and spindle distribution in limb and jaw muscles of the adult and neonatal opossum, Monodelphis domestica. Anat Rec. 1998;251:548–562. doi: 10.1002/(SICI)1097-0185(199808)251:4<548::AID-AR10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Rowlerson AM, Carlson DS. Myosin expression in the jaw-closing muscles of the domestic cat and American opossum. Arch Oral Biol. 1995;40:405–413. doi: 10.1016/0003-9969(94)00181-a. [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Rowlerson AM, Hooper C, Hunt NP. Fibre type classification and myosin isoforms in the human masseter muscle. J Neurol Sci. 1994;126:15–24. doi: 10.1016/0022-510x(94)90089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdu V, Karsch-Mizrachi I, Campione M, Lienwand L, Schiaffino S. Type-IIx myosin heavy chain transcripts are expressed in type-IIb fibers of human skeletal muscle. Am J Physiol. 1994;267:C1723–C1728. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- Snow DH, Billeter R, Mascarello F, Carpene E, Rowlerson A, Jenny E. No classical type-IIB fibres in dog skeletal muscle. Histochemistry. 1982;75:53–65. doi: 10.1007/BF00492533. [DOI] [PubMed] [Google Scholar]
- Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myfibrillar ATPase activity in skinned human skeletal muscle fibres: Fibre type and temperature dependence. J Physiol. 1996;493:299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson P, Burgaard A, Schlosser S. Fatigue and pain in human jaw muscles during a sustained, low-intensity clenching task. Arch Oral Biol. 2001;46:773–777. doi: 10.1016/s0003-9969(01)00028-0. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Kushmerick MJ, Mabuchi K, Sreter FA, Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated muscle fibers. J Biol Chem. 1988;263:9034–9039. [PubMed] [Google Scholar]
- Sweitzer NK, Moss RL. Determinants of loaded shortening velocity in single cardiac myocytes permeabilized with α-hemolysin. Circ Res. 1993;73:1150–1162. doi: 10.1161/01.res.73.6.1150. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Billeter R, Eriksson PO, Ringqvist M. Heterogeneous distribution of myosin in human masticatory muscle fibres as shown by immunohistochemistry. Arch Oral Biol. 1984;29:1–5. doi: 10.1016/0003-9969(84)90034-7. [DOI] [PubMed] [Google Scholar]
- Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Coexpression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Haddad F, Qin AX, Baldwin KM. Analysis of myosin heavy chain mRNA expression by RT-PCR. J Appl Physiol. 1997;83:1389–1396. doi: 10.1152/jappl.1997.83.4.1389. [DOI] [PubMed] [Google Scholar]
- Yang XY, Pernu H, Pyhtinen J, Tiilikainen PA, Oikarinen KS, Raistoa AM. MRI findings concerning the lateral pterygoid muscle in patients with symptomatic TMJ hyper-mobility. J Craniomand Pract. 2001;19:260–268. doi: 10.1080/08869634.2001.11746177. [DOI] [PubMed] [Google Scholar]
- Zuurbier CJ, Heslinga JW, Lee-de Groot MB, Van der Laarse WJ. Mean sarcomere length-force relationship of rat muscle fibre bundles. J Biomech. 1995;28:83–87. doi: 10.1016/0021-9290(95)80009-3. [DOI] [PubMed] [Google Scholar]






