Abstract
Recent accounts of memory suggest that retrieval of a learning experience transforms that memory into a labile state that requires a period of protein synthesis to be reconsolidated into a fixed state. In this article, we show that the impairments in behavior caused by the protein synthesis inhibitor anisomycin given after retrieval are temporary and are thus not likely to reflect disruptions in a protein synthesis-dependent reconsolidation process. Mice received injections of anisomycin after either initial acquisition or retrieval of contextual fear conditioning. When anisomycin injections followed acquisition, freezing was impaired during memory tests the next day and 21 days later. When anisomycin injections followed normal retrieval of contextual fear conditioning, freezing was impaired the next day but recovered to levels of control mice when testing occurred 21 days later. This recovery effect occurred after short or long durations of exposure during the retrieval period and was specific to the conditioning context. These results suggest that anisomycin injections after retrieval do not retroactively affect the memory from conditioning.
Molecular accounts of memory suggest that memories are moved from a labile to a more fixed state in a protein synthesis-dependent consolidation process (1-3). Many experiments have demonstrated a role for protein synthesis during the consolidation of the learning that occurs after acquisition of a behavioral task, such as Pavlovian conditioning, in which a conditioned stimulus (such as a tone or a conditioning context) is paired with an unconditioned stimulus (such as a footshock; refs. 4 and 5). It also has been suggested that retrieval of the original association during reexposure to the conditioned stimulus induces another period of consolidation in which protein synthesis is needed for the original memory to be reconsolidated into a fixed state (6, 7). From this reconsolidation perspective, protein synthesis inhibition after retrieval, just like protein synthesis inhibition after acquisition, results in deficits in the consolidation of the original memory. The challenge for a reconsolidation account of memory is to demonstrate that impairments in behavior correspond to deficits in memory storage.
A number of studies have found that manipulations after retrieval impair performance. Some of the first studies of postretrieval impairments demonstrated that manipulations such as electroconvulsive shock (8) or hypothermia (9) after stimulus reexposure resulted in deficits in performance during subsequent tests. Although there were different accounts of these effects, much of the early theorizing described the behavioral deficits as reflecting impairments in memory retrieval (10-15). This focus on retrieval mechanisms resulted in part from the demonstrations that postretrieval performance impairments could be reversed through reminder treatments (14) or through the simple passage of time (9, 15). These findings suggest that postretrieval manipulations alter performance without eliminating the original memory.
This issue has been brought to a modern light by recent demonstrations that injections of the protein synthesis inhibitor anisomycin into the amygdala or hippocampus after reexposure to a discrete conditioned stimulus or a conditioning context disrupt freezing during a subsequent test (6, 7). These findings have led to the suggestion that retrieval causes reactivated memories to be moved into a labile state, as is thought to occur during initial memory formation (3, 7). During this labile state, it is argued, the synaptic connections that underlie the memory representation are destabilized and require protein synthesis to be returned to a stabile, permanent state. Reconsolidation theories therefore describe the effects of these postretrieval manipulations in terms of their deleterious effects on the original memory. Little is known about the long-term effects of protein synthesis inhibition after retrieval of contextual fear conditioning. As such, it is not clear whether the postretrieval deficit results from impaired reconsolidation or from some other process that does not affect the original memory. Some studies of avoidance learning have demonstrated that anisomycin-induced deficits after retrieval reverse with time (15, 16), which is consistent with the idea that the original memory is not affected by postretrieval anisomycin injections. However, some experiments that have examined the long-term retention of contextual fear conditioning after postretrieval manipulations, including anisomycin injections, have failed to find recovery (5, 6). Because much of the modern theorizing about reconsolidation is based on data collected with fear conditioning, a demonstration of the reversibility of deficits in freezing caused by anisomycin after contextual retrieval would be particularly challenging for those theories. In the following experiments, we show that anisomycin injections after conditioning cause long-term deficits in contextual freezing but that the same injections after retrieval have short-term effects that reverse with time.
Materials and Methods
Subjects. Two hundred seventy-six male C57BL/6 mice bred in our animal facility from mice originally obtained from The Jackson Laboratory were used in the experiments. They were 8-12 weeks old and had free access to food and water in their home cages. All experiments were conducted according to National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Injections. Anisomycin (Sigma) was diluted in saline and dissolved in 1 M HCl. The pH was adjusted to ≈7 with 1 M NaOH. Mice received subcutaneous injections of 50 or 75 mg of anisomycin/kg of body weight or an equivalent volume of saline in the first experiment (Fig. 1). Injections in all other experiments were 50 mg/kg of anisomycin or an equivalent volume of saline. This amount of anisomycin has been shown to yield >90% protein synthesis inhibition in the brain during the first 2 h (17). Mice received a single injection immediately or multiple injections immediately, 2, 4, and 6 h after acquisition (Figs. 1 and 2) or retrieval (Figs. 3, 4, 5). These multiple injections of anisomycin should sustain protein synthesis inhibition at levels >90% until 2 h after the final injection, resulting in strong protein synthesis inhibition for 8 h in mice that received multiple injections.
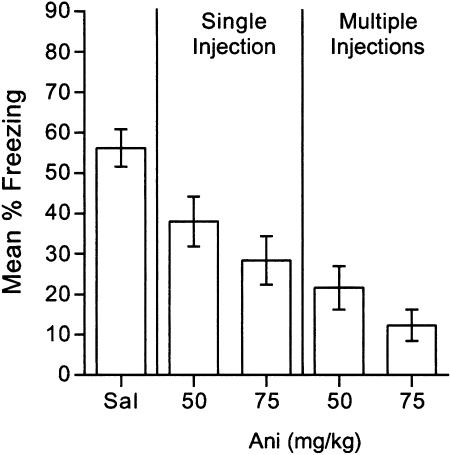
Fig. 1.
Single or multiple anisomycin injections after acquisition impair contextual fear conditioning. Freezing is shown in the conditioning context the day after conditioning, which was followed immediately (single injection) or immediately, 2, 4, and 6 h later (multiple injections) by injections of saline (Sal), 50 mg/kg anisomycin (Ani), or 75 mg/kg anisomycin. Error bars indicate SEM.
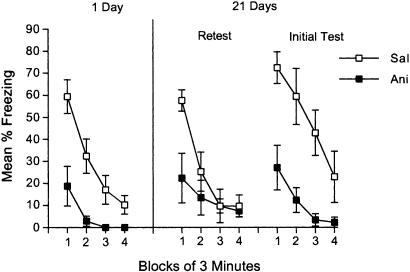
Fig. 2.
The impairment caused by postacquisition anisomycin is long-lasting. Freezing is shown in the conditioning context during a 12-min test session conducted 1 and 21 days after conditioning. Mice received injections of 50 mg/kg anisomycin (Ani) or saline (Sal) immediately, 2, 4, and 6 h after conditioning. Half of the mice received both the 1- and 21-day tests (Retest); the other half received only the 21-day test (Initial Test). Error bars indicate SEM.
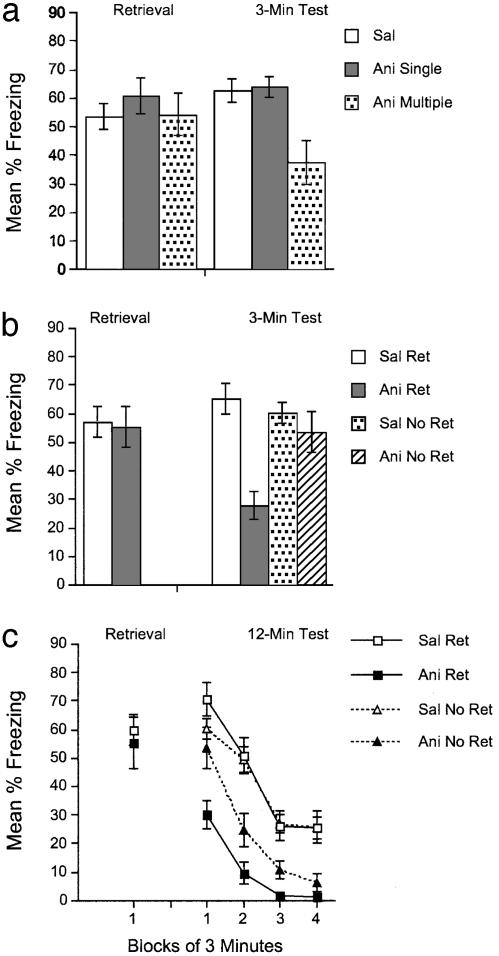
Fig. 3.
Multiple but not single anisomycin injections after context reexposure impair performance. (a) Freezing is shown during a 3-min retrieval session, which was followed either immediately (Ani Single) or immediately, 2, 4, and 6 h later (Ani Multiple) by 50 mg/kg anisomycin or saline (Sal). A 3-min test session occurred the following day. (b and c) During the retrieval session, different groups of mice were exposed to the conditioning context, followed immediately, 2, 4, and 6 h later by injections of 50 mg/kg anisomycin (Ani Ret) or saline (Sal Ret). Other mice did not receive the retrieval trial but received the same injections of anisomycin (Ani No Ret) or saline (Sal No Ret). Freezing is shown during retrieval and during a 3-min test session (b) and a 12-min test session (c) the day after retrieval. Error bars indicate SEM.
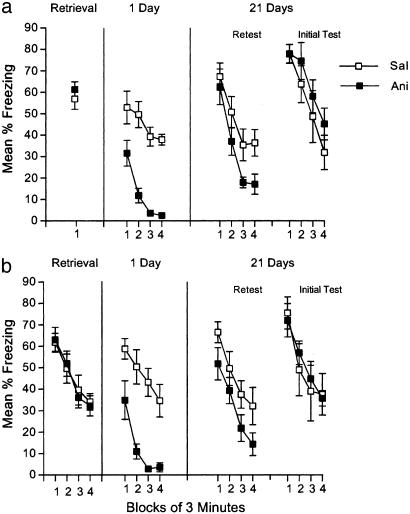
Fig. 4.
Performance impaired by anisomycin injections after retrieval recovers with time. Freezing is shown during the retrieval session, which was followed immediately, 2, 4, and 6 h later by injections of 50 mg/kg anisomycin (Ani) or saline (Sal). The retrieval trial was either 3 min (a) or 12 min (b). Mice were tested for 12 min at 1 or 21 days after retrieval. Half of the mice received both the 1- and 21-day tests (Retest); the other half received only the 21-day test (Initial Test). Error bars indicate SEM.
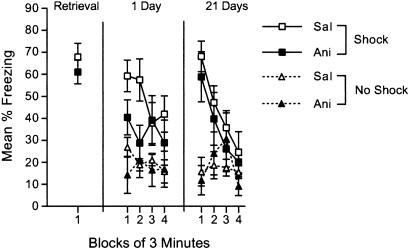
Fig. 5.
Recovery of the anisomycin-induced retrieval deficit is context-specific. Freezing is shown during the 3-min retrieval trial in the shocked (Shock) context and during 12-min test sessions conducted 1 and 21 days later in the shocked and unshocked (No Shock) contexts. Retrieval was followed immediately, 2, 4, and 6 h later by injections of 50 mg/kg anisomycin (Ani) or saline (Sal). Error bars indicate SEM.
Apparatus. The fear conditioning chamber consisted of Plexiglas walls (either a 23 × 23 × 23 cm cube or a 21.5 cm in diameter × 23 cm in height circular chamber) and a grid floor consisting of stainless steel grid rods 3.2 mm in diameter, spaced 0.5 cm apart (Med Associates, St. Albans, VT). Shock was delivered to the floor by a computer-controlled shock source and was scrambled by a Med Associates' solid-state grid floor scrambler. A fan provided background noise at 65-70 dB. The two contexts used in the context discrimination experiment are described in Supporting Methods, which is published as supporting information on the PNAS web site.
Procedure. On the day of acquisition in all but the context discrimination experiment, mice were placed into the conditioning chamber and received a 2-s 1.5-mA footshock at 2 min and 2.5 min after placement into the chamber. Mice were removed from the chamber 30 s after the second shock.
Acquisition: Short-term test (Fig. 1). Mice were removed from the chambers after conditioning and were immediately injected with saline (n = 32), 50 mg/kg anisomycin (n = 24), or 75 mg/kg anisomycin (n = 24). For half of the mice in each group, injections were repeated 2, 4, and 6 h later. During testing the next day, mice received a 3-min exposure to the context, during which conditioning was assessed by sampling the behavior of the mouse every 5 s and recording whether freezing occurred.
Acquisition: Long-term test (Fig. 2). Mice received conditioning, followed by repeated injections of 50 mg/kg anisomycin (n = 10) or saline (n = 10) as before. Half of the mice in the anisomycin and saline groups were tested both the next day and 21 days later. The other half of the mice were tested only 21 days later to make sure that the results of the 21-day test were not influenced by the experience of the earlier test (15, 16).
Reconsolidation: Short-term test (Fig. 3). Acquisition occurred as before, but there were no injections on the acquisition day. The next day, mice received a 3-min context exposure. Freezing during this exposure was used to match performance of mice in groups that received injections of 50 mg/kg anisomycin (n = 24) or saline (n = 24) immediately after the session. Half of the mice in each group received additional injections of 50 mg/kg anisomycin or saline 2, 4, and 6 h later (Fig. 3a). In the second reconsolidation experiment (Fig. 3 b and c), mice received acquisition as before. The next day, mice received a 3-min exposure to the conditioning context followed immediately, 2, 4, and 6 h later by injections of 50 mg/kg anisomycin (n = 16) or saline (n = 16). Other mice remained in their home cages during the context exposure but received injections of anisomycin (n = 12) or saline (n = 12) along with the other mice. All of the mice were tested in the conditioning context the next day for 3 min (Fig. 3b). Some mice (n = 12 per group) were tested an additional 9 min to look at the course of extinction (Fig. 3c).
Reconsolidation: Long-term test (Fig. 4). Acquisition occurred as before with no injections. The next day, half of the mice received a 3-min exposure to the conditioning context followed immediately, 2, 4, and 6 h later by injections of 50 mg/kg anisomycin (n = 14) or saline (n = 14). The other half of the mice received a 12-min exposure to the conditioning context followed immediately, 2, 4, and 6 h later by injections of 50 mg/kg anisomycin (n = 14) or saline (n = 14). Half of the mice from each group were tested the next day and 21 days later. The other half of the mice were tested only 21 days later. Test sessions were 12 min.
Context discrimination (Fig. 5). Mice received one exposure to Context Shock and one exposure to Context No Shock during acquisition, in counterbalanced order. In Context Shock, mice received a 1.5-mA shock 3.5, 7.5, and 11.5 min after placement into the context. They were removed from the context after a total of 12 min. In Context No Shock, mice received a 12-min exposure to the context in the absence of the shocks. The day after conditioning, mice received a 3-min exposure to Context Shock followed immediately, 2, 4, and 6 h later by injections of 50 mg/kg anisomycin (n = 8) or saline (n = 8). Mice were tested the next day and 21 days later in both contexts, in counterbalanced order. One set of four mice was tested in the wrong chambers at 21 days and was not included in the 21-day analysis. Test sessions were 12 min.
Statistical analysis. ANOVAs and simple t tests were performed in all experiments. α was set to 0.05. Bonferroni-adjusted α values were used where appropriate.
Results
Single or Multiple Anisomycin Injections After Acquisition Impair Contextual Fear Conditioning. Mice that received a single injection or multiple injections of 50 mg/kg or 75 mg/kg anisomycin froze less than did mice injected with saline (Fig. 1). Single and multiple injections of both doses of anisomycin resulted in less freezing than did saline injections [t(41) values >3.6, Bonferroni-adjusted P values <0.001], and multiple injections of anisomycin had larger effects on freezing compared to single injections [F(1,44) = 8.9, P < 0.01). These findings suggest that either single or multiple injections of anisomycin immediately after fear conditioning were sufficient to cause impairments in memory consolidation after contextual fear conditioning.
The Acquisition Impairment Caused by Anisomycin Is Long-Lasting. The impairment caused by repeated injections of 50 mg/kg anisomycin was observed both 1 and 21 days after conditioning [F(1,18) values >11.4, P values <0.01; Fig. 2]. During the 21-day test, saline-treated mice tested for the first time (Initial Test in Fig. 2) appeared to freeze more than did saline-treated mice that also received the 1-day test (Retest in Fig. 2), but they did not differ statistically [F(1,16) = 2.6]. The critical finding from this experiment is that anisomycin- and saline-treated mice differed 21 days after conditioning, demonstrating that deficits in contextual freezing do not reverse when anisomycin treatments follow initial acquisition.
Multiple but Not Single Anisomycin Injections After Context Reexposure Impair Performance. In contrast to the effects on acquisition, a single injection of 50 mg/kg anisomycin after context reexposure had no impact on freezing, but, consistent with the effects on acquisition, multiple injections of 50 mg/kg anisomycin impaired freezing [F(2,45) = 7.0, P < 0.005; Fig. 3a].
To look at the necessity of context reexposure, mice were conditioned as before and received repeated injections of anisomycin or saline immediately after reexposure or after no exposure (Fig. 3b). Mice that were exposed to the context and injected with anisomycin (group Ani Ret in Fig. 3b) froze much less during the test than did the other three groups [F(3,52) = 10.1, P < 0.001], which demonstrated similar levels of freezing. This suggests that context exposure was critical to the anisomycin-induced deficit.
The longer test session (Fig. 3c) revealed that group Ani Ret showed less freezing throughout the 12-min test compared with the other groups [t(22) values >3.6, Bonferroni-adjusted P values <0.05], but it also shows that after the first 3 min, group Ani No Ret, which received anisomycin injections without context reexposure, showed faster extinction than did the saline groups [t(22) values >3.3, Bonferroni-adjusted P values <0.05]. The lower freezing by group Ani Ret demonstrates that exposure to the conditioning context followed by multiple anisomycin injections selectively impaired performance. The moderate level of freezing in group Ani No Ret suggests that high doses of anisomycin may have some nonassociative effects in addition to those on memory. This pattern of context-dependent and context-independent effects of anisomycin is similar to that seen after lesions of the hippocampus, in which lesioned animals that do not receive reexposure to the context appear to show faster extinction compared to nonreexposed sham controls during repeated extinction testing (6). In general, longer or repeated test sessions may reveal additional nonassociative effects of postretrieval manipulations on learning and memory.
The critical finding from this experiment is the replication of the basic reconsolidation finding: a retrieval trial followed by multiple anisomycin injections impaired performance. The goal of the remaining experiments was to determine the long-term persistence of this effect.
Performance Impaired by Anisomycin Injections After Reexposure Recovers with Time. Anisomycin injections after reexposure caused a profound deficit in freezing compared with saline injections during the test 1 day after retrieval [F(1,14) = 44.5, P < 0.001; Fig. 4a]. As can be seen in Fig. 4a, spontaneous recovery of freezing occurred during the 21-day test, during which there were no overall differences between mice injected with saline or anisomycin [F(1,26) < 1.0]. This finding suggests that the deficit observed during the 1-day test did not reflect an impairment in the reconsolidation of the original memory.
These results were replicated with a longer reexposure trial, in which mice were exposed to the conditioning context for 12 min (Fig. 4b). Anisomycin injections after the reexposure trial resulted in decreased levels of freezing during the 1-day test compared with those caused by saline injections [F(1,14) = 22.8, P < 0.001]. As in Fig. 4a, however, the group differences disappeared during the 21-day test [F(1,26) = 1.2].
Although anisomycin-treated mice tested for the first time at 21 days showed complete recovery during the 21-day test (Initial Test in Fig. 4), anisomycin-treated mice tested for the second time (i.e., those that also received the 1-day test; Retest in Fig. 4) froze less than did saline-treated mice that also received that test [F(1,30) = 7.5, P < 0.05]. This finding suggests that multiple testing may weaken the recovery effect, perhaps because of differential experiences during the first test. Because the experience in the context by the mice in the Initial Test groups was identical before the 21-day test, we can be confident that performance during the 21-day test in these mice reflects the actions of the retention interval itself and is not an artifact of repeated testing.
These two experiments demonstrate that deficits in behavior induced by protein synthesis inhibition after retrieval are temporary. The data are not consistent with a reconsolidation view of retrieval, which suggests that performance deficits after retrieval reflect impairments in reconsolidating the original memory from acquisition
Recovery of the Anisomycin-Induced Retrieval Deficit Is Context-Specific. It is possible that the increase in performance at 21 days evident in Fig. 4 reflects different processes in anisomycin- and saline-treated groups. In the anisomycin group, recovery might reflect a general increase in freezing that would be evident in any context, regardless of its conditioning history, whereas in the saline group, the increase in behavior might reflect a specific increase in that particular context. This idea was tested by training mice to form a context discrimination in which one context was shocked and the other was not. This discrimination was followed by a retrieval trial in the shocked context, which was followed by repeated anisomycin or saline injections. Mice then were tested 1 and 21 days later.
As can be seen in Fig. 5, during the 1-day test, mice that received anisomycin showed lower levels of freezing in the shocked context than did the saline-treated mice during the first half of the session [t(14) = 2.3, P < 0.05]. By the last half of the session, the freezing in saline-treated mice had extinguished to similar levels as those in anisomycin-treated mice [t(14) < 1.0]. The two groups showed similar performance in the unshocked context during the 1-day test. During the 21-day test, both groups showed high levels of freezing early in the session in the shocked context and low levels of freezing in the unshocked context. By the end of the session, freezing had decreased in the shocked context to levels seen in the unshocked context in both groups. There was neither a reliable main effect of group nor any reliable interactions involving group during the 21-day test, revealing that both groups showed recovery specifically in the shocked context and that the two groups did not differ in amount of freezing in that context. Taken together with the other recovery experiments, this experiment suggests that protein synthesis inhibition after retrieval causes short-term changes in behavior that do not reflect long-term changes in memory storage.
Discussion
The critical findings from these experiments are that deficits in behavior caused by systemic injections of the protein synthesis inhibitor anisomycin after acquisition of contextual freezing are long-lasting but that deficits caused by anisomycin after retrieval are not. The spontaneous recovery of conditioned freezing that occurred when a 21-day retention interval was inserted between initial retrieval and testing demonstrates that the memory from acquisition remained even though it was not evident in behavior during a test 1 day after retrieval. These findings do not support the idea that retrieval returns a reactivated memory to a labile state that requires protein synthesis to be reconsolidated into a permanent state.
At the most basic level, these findings suggest that consolidation and reconsolidation are qualitatively different processes. For a reconsolidation account of memory to be viable, it will have to incorporate a mechanism that allows the original memory to be unaffected by manipulations after retrieval, which may mean abandoning the fundamental assumption that initial acquisition and retrieval act on the same underlying process. At the very least, one needs to be cautious about using the term “reconsolidation,” which inherently implies that the processes that occur after retrieval affect the consolidation of the original memory through the same consolidation process that operates after acquisition. Our experiments suggest that this is not true for the specific case of protein synthesis inhibition and contextual freezing. On a more general level, it is important to emphasize that a number of experiments using different preparations and manipulations, including anisomycin injections, have found recovery (9, 11, 14-16). These findings, however, have not been central to the recent development of reconsolidation theories, as others have noted (18, 19). Certainly, spontaneous recovery effects and the general idea that the original memory is preserved after postretrieval manipulations need to be incorporated into future theorizing and experimentation on reconsolidation.
Some experiments have failed to observe spontaneous recovery after postretrieval manipulations (5, 6). However, the lack of spontaneous recovery in the experimental animals of those studies is not informative about the state of the acquisition memory because little to no recovery was observed in control animals (5, 6). There are different circumstances in which recovery may fail to occur, such as when the retention interval is too short given the amount of extinction that has occurred, or when the same animals are tested repeatedly. The effects of repeated testing were evident in our Fig. 4, in which the overall levels of freezing in anisomycin-treated mice 21 days after retrieval was less in those mice that were tested for the second time compared to mice tested for the first time. Multiple testing, therefore, may cause changes in behavior that obscure the effects of retention interval (2, 20).
At a theoretical level, the present results are consistent with the idea that postretrieval deficits reflect the animal's inability to retrieve a stored contextual memory (10-15, 19). The challenge for retrieval theories is to determine what mechanism would allow the original memory to be preserved while temporarily preventing the animal from having access to it. One possibility is that during retrieval new memories are formed about the absence of the expected shock in the conditioning context (21, 22). When testing occurs soon after retrieval, this new extinction memory may be more retrievable than is the acquisition memory, but as time is inserted between the end of extinction and testing, the extinction memory may become harder to retrieve, resulting in spontaneous recovery (23). The idea that protein synthesis inhibition would facilitate such an extinction process is a counterintuitive one that will clearly require more research, including the study of signal transduction molecules linked to the regulation of gene expression, such as protein kinase A (PKA). The recent demonstration that PKA inhibition in the prefrontal cortex can enhance memory formation (24) is particularly intriguing for the idea of an enhanced extinction process in our experiments because of the possible involvement of this brain region in extinction (25).
Although one can only speculate about the mechanisms underlying the anisomycin-induced retrieval deficit, our results are consistent with the idea that the processes that occur after initial acquisition and retrieval have different protein synthesis requirements (5). A single systemic injection of 50 mg/kg anisomycin was sufficient to impair acquisition when given immediately after conditioning but had no effect when given immediately after retrieval, which is consistent with previous work with higher doses of anisomycin (5). Multiple injections of anisomycin were effective when given after acquisition or retrieval but only the acquisition effect was long-lasting. Thus, increased levels of protein synthesis inhibition may be required to see an effect after retrieval (ref. 6 but see ref. 16), but such effects do not result in permanent changes to the original memory. It is possible that prolonging protein synthesis inhibition may result in multiple waves of protein synthesis being affected (26), which might be critical for the postretrieval anisomycin effect (27). The role of protein synthesis after retrieval and extinction remains unclear as there are now a number of demonstrations that protein synthesis inhibition may block, enhance, or have no effect on extinction (5-7, 27-29). It is possible that these effects may depend critically on the amount of extinction that occurs during a retrieval trial (30-32).
Effects of anisomycin are often interpreted in terms of de novo synthesis of proteins that might occur as a result of induced gene expression, such as immediate early genes. However, it is important to note that blocking protein synthesis, especially for long periods as in the present experiments, will deplete proteins with short half-lives, perhaps reducing the level of these proteins to a point where it impacts neuronal function (2). Prolonged protein synthesis inhibition also may greatly skew the relation between synthesis and degradation in favor of protein degradation. Such a relation could cause a facilitation of extinction if these degradation processes were particularly important during extinction. Similar effects on protein degradation may occur when particularly high doses of anisomycin are delivered locally into specific brain regions, such as the hippocampus or amygdala, because higher doses may take longer to be eliminated from the brain. Additionally, anisomycin is known to have effects on mitogen-activated protein kinase (33) and catecholamines (34), so one needs to exercise caution when interpreting these results as implicating de novo protein synthesis.
Although our results suggest that deficits caused by protein synthesis inhibition after memory retrieval reverse with time, deficits observed after initial acquisition appear to be long-lasting. This long-lasting impairment in acquisition has been observed in other studies with repeated anisomycin injections (35), and the difference in persistence between initial acquisition and retrieval deficits also has been observed with other manipulations (15, 16). Of course, it is possible that the original acquisition memory was intact, but our method of unmasking that memory was unable to detect it. Indeed, as with putative deficits in reconsolidation, it is important to realize that there is no simple isomorphic relation between behavior and memory, meaning that the absence of a behavior should not by itself be taken as evidence for the absence of a memory. What is clear from our experiments is that the 21-day retention interval, although long enough to reverse a deficit after context retrieval, did not reverse a deficit after initial acquisition. These findings suggest that, at the very least, the effects of protein synthesis inhibition after acquisition and retrieval are quite different (5, 32).
As a final point, these experiments reinforce the importance of investigating whether differences in behavior reflect differences in learning or in the expression of that learning (36). There are many instances in which performance in the presence of a stimulus belies the content of the learning about that stimulus (37-39). It is critical, therefore, to recognize the distinction between the absence of a behavior and the absence of a memory. The challenge for a complete account of learning and memory is to determine the circumstances in which differences in behavior correspond to differences in memory storage.
Note Added in Proof. Fischer et al. (40) found that anisomycin-induced deficits after contextual retrieval, but not after acquisition, could be reversed with a footshock reminder.
Supplementary Material
Acknowledgments
We thank Tom Gould for helpful comments on the manuscript. This research was supported by a National Research Service Award postdoctoral fellowship (to K.M.L.) and by grants from the Merck Foundation, the National Institute of Health, the Packard Foundation, the University of Pennsylvania Research Foundation, and the Whitehall Foundation (to T.A.).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Abel, T. & Lattal, K. M. (2001) Curr. Opin. Neurobiol. 11, 180-187. [DOI] [PubMed] [Google Scholar]
- 2.Davis, H. P. & Squire, L. R. (1984) Psychol. Bull. 96, 518-559. [PubMed] [Google Scholar]
- 3.Nader, K. (2003) Trends Neurosci. 26, 65-72. [DOI] [PubMed] [Google Scholar]
- 4.Abel, T., Nguyen, P. V., Barad, M., Deuel, T. A., Kandel, E. R. & Bourtchouladze, R. (1997) Cell 88, 615-626. [DOI] [PubMed] [Google Scholar]
- 5.Lattal, K. M. & Abel, T. (2001) J. Neurosci. 21, 5773-5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debiec, J., LeDoux, J. E. & Nader, K. (2002) Neuron 36, 527-538. [DOI] [PubMed] [Google Scholar]
- 7.Nader, K., Schafe, G. E. & LeDoux, J. E. (2000) Nature 406, 722-726. [DOI] [PubMed] [Google Scholar]
- 8.Misanin, J. R., Miller, R. R. & Lewis, D. J. (1968) Science 160, 554-555. [DOI] [PubMed] [Google Scholar]
- 9.Mactutus, C. F., Riccio, D. C. & Ferek, J. M. (1979) Science 204, 1319-1320. [DOI] [PubMed] [Google Scholar]
- 10.Miller, R. R. & Springer, A. D. (1973) Psychol. Rev. 80, 69-79. [DOI] [PubMed] [Google Scholar]
- 11.Miller, R. R. & Springer, A. D. (1974) Psychol. Rev. 81, 470-473. [DOI] [PubMed] [Google Scholar]
- 12.Hinderliter, C. F., Webster, T. & Riccio, D. C. (1975) Anim. Learn. Behav. 3, 257-263. [Google Scholar]
- 13.DeVietti, T. L. & Kirkpatrick, B. R. (1976) Science 194, 438-440. [DOI] [PubMed] [Google Scholar]
- 14.Mactutus, C. F., Ferek, J. M., George, C. A. & Riccio, D. C. (1982) Physiol. Psychol. 10, 79-95. [Google Scholar]
- 15.Judge, M. E. & Quartermain, D. (1982) Physiol. Behav. 28, 585-590. [DOI] [PubMed] [Google Scholar]
- 16.Anokhin, K. V., Tiunova, A. A. & Rose, S. P. (2002) Eur. J. Neurosci. 15, 1759-1765. [DOI] [PubMed] [Google Scholar]
- 17.Flood, J. F., Rosenzweig, M. R., Bennett, E. L. & Orme, A. E. (1973) Physiol. Behav. 10, 555-562. [DOI] [PubMed] [Google Scholar]
- 18.Cahill, L., McGaugh, J. L. & Weinberger, N. M. (2001) Trends Neurosci. 24, 578-581. [DOI] [PubMed] [Google Scholar]
- 19.Millin, P. M., Moody, E. W. & Riccio, D. C. (2001) Nat. Rev. Neurosci. 2, 68-70. [DOI] [PubMed] [Google Scholar]
- 20.Balogh, S. A., Radcliffe, R. A., Logue, S. F. & Wehner, J. M. (2002) Behav. Neurosci. 116, 947-957. [DOI] [PubMed] [Google Scholar]
- 21.Myers, K. M. & Davis, M. (2002) Neuron 36, 567-584. [DOI] [PubMed] [Google Scholar]
- 22.Rescorla, R. A. (2001) in Handbook of Contemporary Learning Theories, eds. Mowrer, R. R. & Klein, S. (Erlbaum, Mahwah, NJ), pp. 119-154.
- 23.Bouton, M. E. (1993) Psychol. Bull. 114, 80-99. [DOI] [PubMed] [Google Scholar]
- 24.Ramos, B. P., Birnbaum, S. G., Lindenmayer, I., Newton, S. S., Duman, R. S. & Arnsten, A. F. (2003) Neuron 40, 835-845. [DOI] [PubMed] [Google Scholar]
- 25.Quirk, G. J., Russo, G. K., Barron, J. L. & Lebron, K. (2000) J. Neurosci. 20, 6225-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourtchouladze, R., Abel, T., Berman, N., Gordon, R., Lapidus, K. & Kandel, E. R. (1998) Learn. Mem. 5, 365-374. [PMC free article] [PubMed] [Google Scholar]
- 27.Flood, J. F., Jarvik, M. E., Bennett, E. L., Orme, A. E. & Rosenzweig, M. R. (1977) Pharmacol. Biochem. Behav. 7, 71-77. [DOI] [PubMed] [Google Scholar]
- 28.Vianna, M. R., Szapiro, G., McGaugh, J. L., Medina, J. H. & Izquierdo, I. (2001) Proc. Natl. Acad. Sci. USA 98, 12251-12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szapiro, G., Vianna, M. R., McGaugh, J. L., Medina, J. H. & Izquierdo, I. (2003) Hippocampus 13, 53-58. [DOI] [PubMed] [Google Scholar]
- 30.Eisenberg, M., Kobilo, T., Berman, D. E. & Dudai, Y. (2003) Science 301, 1102-1104. [DOI] [PubMed] [Google Scholar]
- 31.Pedreira, M. E. & Maldonado, H. (2003) Neuron 38, 863-869. [DOI] [PubMed] [Google Scholar]
- 32.Lattal, K. M., Honarvar, S. & Abel, T. (2004) Behav. Brain. Res., in press. [DOI] [PubMed]
- 33.Hazzalin, C. A., Le Panse, R., Cano, E. & Mahadevan, L. C. (1998) Mol. Cell. Biol. 18, 1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman, L. S., Judge, M. E. & Quartermain, D. (1982) Pharmacol. Biochem. Behav. 17, 187-191. [DOI] [PubMed] [Google Scholar]
- 35.Davis, H. P. & Rosenzweig, M. R. (1978) Pharmacol. Biochem. Behav. 8, 701-710. [DOI] [PubMed] [Google Scholar]
- 36.Lattal, K. M. (1999) J. Exp. Psychol. Anim. Behav. Process. 25, 433-450. [DOI] [PubMed] [Google Scholar]
- 37.Davis, M. & Wagner, A. R. (1968) Psychonom. Sci. 12, 337-338. [Google Scholar]
- 38.Rescorla, R. A. (1996) Q. J. Exp. Psychol. B 49B, 245-258. [Google Scholar]
- 39.Gewirtz, J. C., McNish, K. A. & Davis, M. (2000) Behav. Brain. Res. 110, 83-95. [DOI] [PubMed] [Google Scholar]
- 40.Fischer, A., Sananbenesi, F., Schrick, C., Spiess, J. & Radulovic, J. (2004) J. Neurosci. 24, 1962-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.