Abstract
Rationale
Atherosclerosis is a major cause of death in patients with chronic kidney disease. Chronic inflammation of the arterial wall including invasion, proliferation and differentiation of leukocytes is important in atherosclerotic lesion development. How atherosclerotic inflammation is altered in renal impairment is incompletely understood.
Objective
This study analyzed leukocytes of the atherosclerotic aorta in mice with impaired and normal renal function and studied a mechanism for the alteration in aortic myeloid leukocytes.
Methods and Results
Unilateral nephrectomy significantly decreased glomerular filtration rate and increased atherosclerotic lesion size and aortic leukocyte numbers in two murine atherosclerosis models, Apolipoprotein E (Apoe−/−) and LDL receptor (LDLr−/−) deficient mice. The number of aortic myeloid cells increased significantly. They took up less oxidized LDL, while CD11c expression, interaction with T cells and aortic T cell proliferation were significantly enhanced in renal impairment. In human PBMC cultures, chronic kidney disease serum decreased lipid uptake and increased HLAII expression. Supplementation with Interleukin (IL)-17A similarly increased HLAII and CD11c expression and impaired oxLDL uptake. IL-17A expression was increased in atherosclerotic mice with renal impairment. Ablation of IL-17A in LDLr−/− mice by lethal irradiation and reconstitution with Il17a−/− bone marrow abolished the effect of renal impairment on aortic CD11b+ myeloid cell accumulation, CD11c expression and cell proliferation. Atherosclerotic lesion size was decreased to levels observed in normal kidney function.
Conclusions
Kidney function modifies arterial myeloid cell accumulation and phenotype in atherosclerosis. Our results suggest a central role for IL-17A in aggravation of vascular inflammation and atherosclerosis in renal impairment.
Keywords: Atherosclerosis, vascular inflammation, leukocyte, renal insufficiency, Interleukin 17
INTRODUCTION
In patients with impaired renal function, risk of death, mostly from cardiovascular events, is significantly elevated.1-5 Acute hospital admissions for cardiac causes are common, patients with kidney disease presenting with less specific symptoms than the general population.6 At most stages of chronic kidney disease, patients are more likely to die from cardiovascular disease than to proceed to terminal renal failure and require renal replacement therapy.4 At a 50% decrease in renal function (glomerular filtration rate (GFR) of 60 ml/min or less) a significant association with cardiovascular mortality was consistently observed after correction for other risk factors.1, 3 This degree of chronic kidney disease (CKD stages III and IV) affects about 8% of the adult US population.7
Unilateral nephrectomy significantly increased atherosclerotic lesion size in Apolipoprotein E (Apoe−/−) mice,8-11 where, different from wild-type (wt) mice, serum creatinine was significantly elevated after unilateral nephrectomy.9, 11 Renal glomerular number was decreased in Apoe−/− compared to wt mice, but there was no evidence for accelerated progression of kidney disease.11 Blood pressure was unaffected by unilateral,9, 10, 12 and even 5/6 nephrectomy in Apoe−/− mice.9, 12, 13 Blood pressure treatment with the Angiotensin II blocker losartan and the vasodilator hydralazine was compared in Apoe−/− mice after unilateral nephrectomy.10 Losartan was more anti-atherogenic despite similar blood pressure levels and despite higher cholesterol.
Vascular calcification is prominent in end stage renal disease.14, 15 In contrast, histology of the atherosclerotic lesions at CKDIII-IV or Apoe−/− mice after unilateral nephrectomy very much resembles lesions in normal kidney function.8-10 Specific histopathologic features such as medial calcification that are observed in dialysis-dependent patients’ arteries14, 15 and severely uremic murine atherosclerosis models8, 9, 13, 16 are not found. The mechanism of increased atherosclerosis in patients with mild to moderate renal impairment is incompletely understood.
Vascular wall infiltration by both innate and adaptive immune cells contributes to atherosclerotic lesion progression.17 Alterations of the immune system by e.g. splenectomy, ablation of B cells or specific T helper cell subsets can change the size, and also the composition of atherosclerotic lesions and alter collagen, lipid or smooth muscle cell contents.17, 18 Monocyte derived myeloid cells that give rise to foam cell-forming macrophages and also fully functional antigen presenting cells are central in plaque development.17, 18 Their number within the arterial wall increases markedly during atherosclerosis development due to both immigration and local proliferation. Oxidized LDL up-regulates the expression of the β2 integrin subunit CD11c, a marker of antigen presenting cells in mice, on monocytes.19 CD11c-deficiency decreases atherosclerotic lesion size.19 Indeed, in atherosclerosis, CD11b+CD11c+ cells are capable of both lipid phagocytosis and antigen presentation to CD4+ T cells.20-24 This occurs in lymphatic organs,21, 22 however, T cells are also located in close proximity to antigen presenting cells in the atherosclerotic arteries of mice and humans25, 26 and interact with each other in the vascular wall.27 Close interaction with CD11b+CD11c+ cells in the vascular wall was limited to Apoe−/−CD4+ T cells and did not occur with wtCD4+ T cells suggesting an antigen specific process.27 The number of T lymphocytes increases during the atherosclerosis progression in the vessel.17, 18 T cells are differentially regulated by myeloid and plasmacytoid dendritic cells24 and can modulate atherosclerotic inflammation by cytotoxicity and cytokine secretion. While some T cell cytokines such as interferon gamma (IFNγ) have a proven proatherogenic role in diverse settings,17, 18 the effect of others such as interleukin (IL)-17A is controversial.28, 29 Enhancement of atherosclerotic lesion formation by IL-17A has been reported in some30-34 but not all models.35-38 While some studies were limited by available reagents, the role of IL-17A may also differ depending on the environment, e.g. lesion localization34, 35 or regulation of other immune mediators.39 We found an induction of atherosclerosis by IL-17A in pharmacologic immunosuppression39 and also in IL-17A overproduction in the absence of the IL-27 receptor.40
In patients with renal impairment, some serum inflammatory markers are increased and correlate with mortality, among them IL-6, that can enhance IL-17 production and be induced by IL-17 itself.41 Increased IL-17A in serum in a cohort of patients receiving hemodialysis for renal replacement was recently reported.42 Cellular immune alterations in renal failure include, among others, peripheral blood lymphopenia and neutrophilia.43 Circulating dendritic cells are depleted.44 However, data from the atherosclerotic vascular wall are required for a better understanding of the inflammatory disease process.45
Here, we examined the aortic leukocyte infiltrate in atherosclerotic Apoe−/− and LDL receptor (LDLr−/−) deficient mice with surgically induced impaired renal function (unilateral nephrectomy). Our initial screening revealed a significant increase in aortic leukocytes. We investigated myeloid cell phenotype, function and mechanism of accumulation in renal impairment.
METHODS
Detailed methods are available online.
Animals
Wild-type (wt) C57Bl/6, LDLr−/−, Apoe−/− mice (both on C57Bl/6 background)(Jackson Labs, Bar Harbor, ME), CD11cYFP, kindly provided by Dr. M. Nussenzweig, Rockfeller University, NY and crossed with Apoe−/−, and mice lacking IL-17A (Il17a−/−), 96% C57Bl/6 background, kindly provided by Dr. Y. Iwakura, University of Tokio, were genotyped by PCR and used in age- and sex-matched groups. Mice were kept in specific-pathogen-free conditions. Animal experiments were approved by the Animal Care Committee at LIAI and Landesamt für Verbraucherschutz und Lebensmittelsicherheit, Lower Saxony, Germany. High fat diet contained 40% of kcal from fat, 1.5% Cholesterol.
Bone marrow transplantation and unilateral nephrectomy
Experiments were commenced at 6 weeks of age. Bone marrow transplantations were performed as described.46 Unilateral nephrectomy or de-capsulation of the kidney were performed one week before high fat diet was started.
Quantification of atherosclerosis, histologic and aortic leukocyte analysis
Aortic en face staining and histologic assessment of aortic roots, plasma lipid FPLC and measurement of GFR by FITC-Inulin are described in the supplement. Preparation and flow cytometry of aortic leukocytes39 and two-photon-imaging of aortic CD11c+/T-cell interaction were performed as described.27
Cell culture experiments
Cell culture procedures are described online, serum for stimulation experiments was obtained after written informed consent from patients with non-diabetic chronic kidney disease described in the online supplement.
Statistical analysis
Data are expressed as mean ±SEM. Two-tailed student t-test and Mann Whitney test were used to compare two conditions. One-way-ANOVA and post-hoc tests as indicated were used if more than 2 conditions were compared. P-values <0.05 were considered significant. P values are indicated as follows: *p<0.05, **p<0.01, ***p<0.001.
RESULTS
Impaired renal function increases atherosclerotic lesion size and aortic leukocyte infiltration
Unilateral nephrectomy significantly increased serum creatinine (suppl. table I) and decreased glomerular filtration rate (GFR) measured by inulin clearance in Apoe−/− mice (suppl. figure I). This degree of renal impairment significantly increased aortic atherosclerotic lesion size in Apoe−/− mice (figure 1A,B) with very similar affection of both genders (suppl. figure IIA). Aortic root lesion size also increased (figure 1C,D). Relative collagen contents and composition was essentially un-altered (suppl. figure IIB,C). No significant differences in body or spleen weight occurred, serum calcium, phosphorus, total leukocytes as a marker of systemic inflammation, thrombocyte and erythrocyte counts were un-altered. Total serum cholesterol was increased, due to LDL, but not HDL levels (table 1). No alteration was observed in renal leukocytes (data not shown).
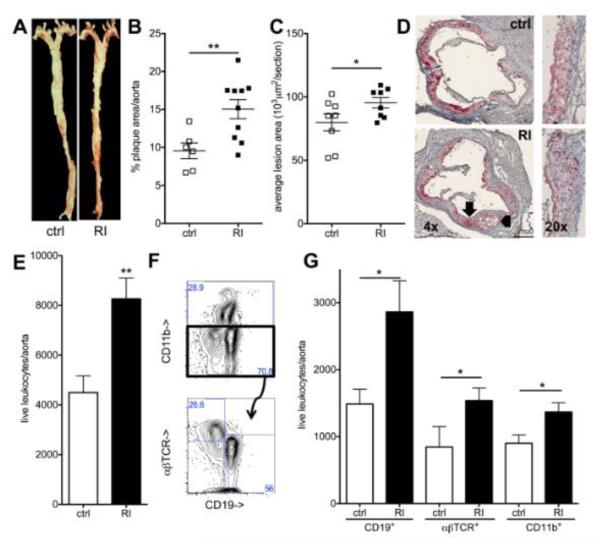
Figure 1. Renal impairment increases atherosclerotic lesion size and leukocyte infiltration.
(A,B) En face aortic atherosclerotic lesion size was assessed in Apoe−/− mice (12 weeks high fat diet) (A, ctrl: sham operated, RI, renal impairment) and quantified as percent of aortic surface area (B)(n=6-10, 3 independent experiments). (C,D) Aortic root lesion size in the 300 μm following the aortic valve (C, n=8, 4 independent experiments, D examples, 4x and 20x original magnification, arrows mark the same areas as in figure 2). (E-G) Aortic leukocyte numbers were analyzed by flow-cytometry (gated for live, CD45+ cells; E, n=9-11, 4 independent experiments). Staining for CD11b (myeloid cells), CD19 (B cells) and αβTCR (T cells) (example in F), was used for quantification of these subgroups (G, n=5-11 per group, 4 independent experiments).
In Apoe−/− mice with renal impairment, significantly more leukocytes were recovered from the aorta by flow cytometry analysis as described39 (figure 1E). All investigated leukocyte types, myeloid cells (CD11b+), B cells (CD19+) and αβTCR+ T cells were significantly more abundant (figure 1F,G).
These data show that the increase in atherosclerotic lesion size in Apoe−/− mice with renal impairment is accompanied by an extended inflammatory vascular infiltrate.
Enhanced accumulation of aortic myeloid cells and CD11c expression in renal impairment
We further investigated aortic accumulation of myeloid leukocytes in renal impairment. Numbers of CD11b+ myeloid cells in the aorta were significantly elevated already early in atherosclerosis development in Apoe−/− mice after three weeks of high fat diet (figure 2A). Aortic cell proliferation is highly elevated at this stage in mice with normal renal function.20, 39 We labeled proliferating cells with BrdU and measured incorporation into CD11b+ leukocytes. Renal impairment significantly increased proliferation of aortic myeloid leukocytes (figure 2B). Expression of the antigen presenting cell marker CD11c on aortic CD11b+ myeloid cells19,20, 39 was enhanced in renal impairment (figure 2A).
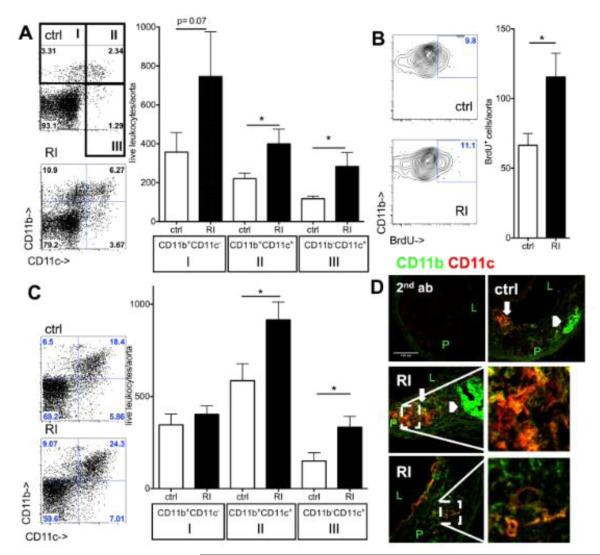
Figure 2. Myeloid cell accumulation in the aorta during atherosclerosis development in renal impairment.
(A) CD11b and CD11c expression on aortic leukocytes in Apoe−/− mice after three weeks high fat diet by flow cytometric analysis (ctrl=sham-operated, RI=renal impairment, examples and n=11-12, 4 independent experiments). (B) Proliferation of aortic myeloid cells by BrdU incorporation at the same timepoint (cell number/aorta, n=8-10 from 3 independent experiments). (C) Aortic flow cytometry after twelve weeks high fat diet showed increased CD11b+CD11c+ and CD11c+ cell numbers in renal impairment (n=9-11, 4 independent experiments). (D) Localization in established atherosclerotic lesions (12 weeks high fat diet) by confocal imaging revealed CD11c+ (red) cells in the neo-intima. Most of these cells were also CD11b+ (green) in both control and renal impairment (RI) Apoe−/− mice. CD11b+CD11c+ cells were present in foam cell (thick arrows; compare to lipid (figure 1D)) and highly cellular regions. CD11b was also found in a-cellular intimal areas (arrowheads compare to fig. 1D) (secondary antibodies only as negative control, P=plaque, L=lumen, 40x original magnification).
Both, myeloid cell numbers and the proportion that expressed CD11c further increased in developed lesions after 12 weeks of high fat diet (figure 2C). Very similar results were observed in male and female mice (suppl. figure III). Cell localization in the vascular wall by confocal imaging showed large CD11c+ and CD11b+ neo-intimal areas (figure 2D) in both lipid rich and cellular plaque areas in the animals with renal impairment.
Renal impairment alters myeloid cell function in the atherosclerotic aortic wall
We next investigated lipid uptake and antigen presenting function of myeloid aortic cells.
Uptake of labeled exogenous oxidized (ox)LDL in aortic leukocytes was measured by flow cytometry (figure 3A). Uptake into total CD11b+ and the CD11b+CD11c+ subgroup of aortic leukocytes was significantly lower in Apoe−/− mice with impaired renal function (figure 3B).
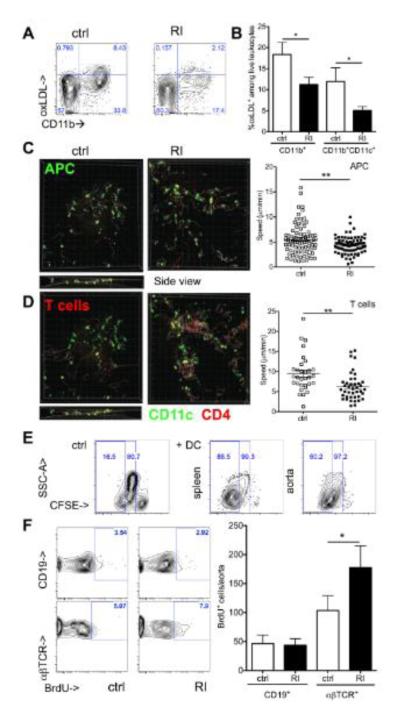
Figure 3. Altered lipid uptake and interaction with T cells of aortic myeloid leukocytes in renal impairment.
(A,B) Uptake of DiI-oxLDL by aortic leukocytes was assessed 24h after injection into Apoe−/− mice with and without renal impairment (RI) by flow cytometry (A shown in all live leukocytes, B quantified as proportion of CD11b+ and CD11b+CD11c+ cells, n=4-7, 2 independent experiments). (C,D) Aortic antigen presentation was assessed by live cell 2-photon microscopy after co-incubation of explanted aortas from CD11cYFPApoe−/− mice (ctrl and RI) with SNARF labeled Apoe−/− CD4+ T cells (red). CD11c+ antigen presenting cell (APC, C) and T cell (D) velocities were assessed in control and RI aortas in three dimensions (z axis shown in ctrl, stills from suppl. movies 1-4, available online, average cell velocities given, 1 of 2 independent experiments, unpaired t-tests). (E) Proliferation of BALB/c lymphocytes in mixed lymphocyte reaction with splenic and aortic CD11b+CD11c+ cells from atherosclerotic Apoe−/− mice was assessed by CFSE dilution (1 of 2 independent experiments with similar results, 4-5 aorta donors per group). (F) Proliferation of Apoe−/− aortic lymphocytes after three weeks high fat diet (BrdU+ cells/aorta, n=8-10 from 3 independent experiments).
We studied CD11c+ cell/CD4+ T cell movement and interactions in the aortic wall of mice with normal and impaired renal function. First, antigen presentation involves prolonged interactions with T cells and slowing of cell speed. We used a recently developed method27 (suppl. figure IV) to investigate this in the atherosclerotic aorta of mice with normal and impaired renal function. CD4+ T cells interacted with CD11c+ cells in aortas of mice with renal impairment for an increased proportion of the observation time (11±1% in ctrl, 19±1% in RI, n=2, p=0.02). Second, productive T cell interactions with antigen presenting cells decrease the speed of both cell types.47 The average speed of both CD11c+ cells and CD4+ T cells was significantly lower in aortas from animals with renal impairment than controls (CD11c+ in figure 3C, suppl. movies 1,2 and CD4+ in figure 3D, suppl. movies 3,4). Third, T cell stimulation by antigen presenting cells induces proliferation. Indeed, aortic CD11b+CD11c+ enhanced CD4+ T cell proliferation during mixed lymphocyte reaction in vitro similar to spleen cells (figure 3E). Renal impairment significantly increased proliferation of aortic αβTCR+ lymphocytes, but not CD19+ B lymphocytes (figure 3F).
Renal impairment and IL-17A alter myeloid cell differentiation in vitro
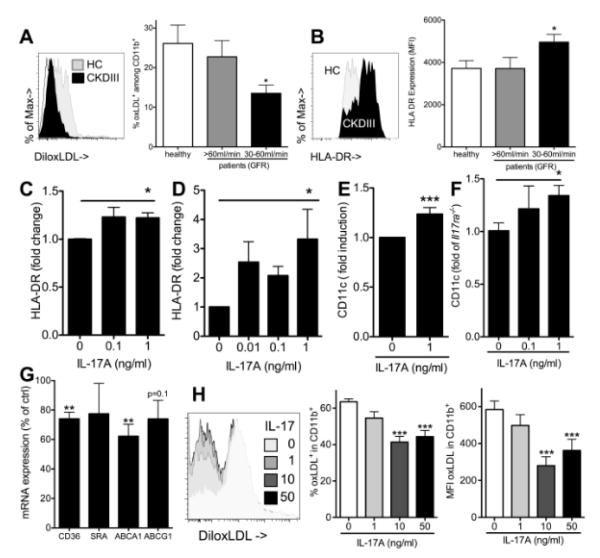
To test whether the altered aortic myeloid cell phenotype in mice with renal impairment could be replicated in human cells, human adherent PBMC cultures were supplemented with 10% serum from patients with mildly (CKDI,II, GFR>60 ml/min) and moderately (CKDIII, GFR=30-60 ml/min) impaired renal function or healthy controls. While lipid levels were elevated in both patient groups (suppl. figure VA,B), uptake of oxLDL was decreased in cells cultured in CKDIII, but not milder stages of chronic kidney disease (figure 4A). In contrast, HLAII expression as marker of antigen presenting cells was increased compared to healthy controls (figure 4B).
Figure 4. Renal impairment and IL-17A enhance antigen presenting cell marker expression and decrease oxLDL uptake in myeloid cell differentiation.
(A) oxLDL uptake by human PBMC derived macrophages differentiated in the presence of serum from patients with stable chronic kidney disease (GFR above 60 ml/min=CKDI,II, n=5, GFR 30-60ml/min=CKDIII, n=11 and healthy controls (HC), n=9, 3 independent experiments, Bonferroni after One-way-ANOVA) (B) HLAII surface expression on human PBMC derived macrophages assessed by flow cytometry (CKDI,II, n=11, CKDIII, n=19, healthy controls, n=8, 6 independent experiments, Bonferroni after One-way-ANOVA). (C,D) HLAII expression on PBMC derived macrophages cultured with and without recombinant IL-17A (C,n=5, linear trend after One-way-ANOVA) and dendritic cells (D, promoted by culture with GM-CSF and IL-4, n=4, linear trend after One-way-ANOVA). (E,F) Murine macrophages were generated by culture of adherent bone marrow cells. The effect of recombinant mouse IL-17A on CD11c expression was investigated in both wt (E, 4 independent experiments) and Il17a−/− compared to Il17ra−/− mice (F, t-test after One-way-ANOVA, 3 independent experiments). (G) Il17a−/− macrophage mRNA expression was assessed on day three of culture in the presence of recombinant IL-17A (1ng/ml) and compared to control cells (t-tests, 3 independent experiments). (H) Wt macrophage oxLDL uptake on day 7 after culture with different concentrations of recombinant IL-17A (Dunnetts after One-way-ANOVA, n=12, 3 independent experiments).
We previously found that IL-17A enhanced accumulation of CD11b+CD11c+ cells in peritonitis,39 and therefore tested whether IL-17A also up-regulates HLAII as an antigen presenting cell marker of human cells. HLAII expression was significantly higher if cells were exposed to IL-17A during macrophage culture (figure 4C) and, more markedly, dendritic cell polarization by GM-CSF and IL-4 (figure 4D). Similarly, IL-17A supplementation during mouse myeloid derived macrophage differentiation significantly increased CD11c expression (figure 4E). The same result was found when IL-17A effects were tested in Il17a−/− and Il17ra−/− cells in parallel to correct for potential unspecific effects of recombinant IL-17A (figure 4F). mRNA expression of genes implicated in cholesterol transport was assessed in Il17a−/− bone marrow derived macrophages in the presence of IL-17A during differentiation (figure 4G). Both CD36 and ABCA1 expression were significantly decreased. oxLDL uptake by wt macrophages was dose dependently decreased by IL-17A if cell were exposed during differentiation (figure 4H).
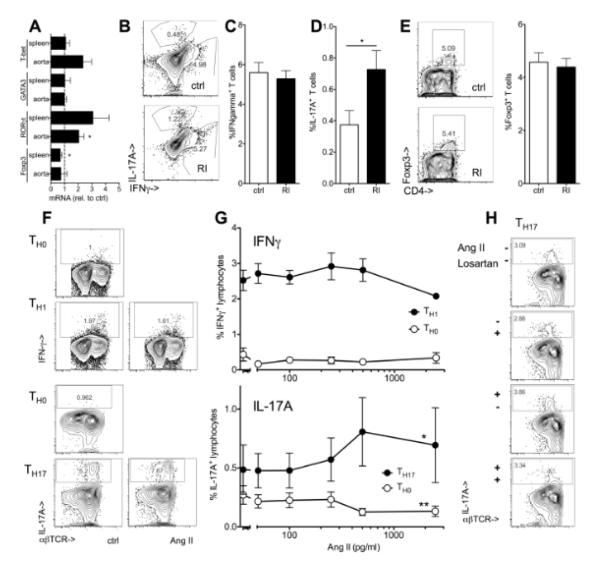
IL-17A expression in renal impairment
We next assessed T cell polarization in vivo. Among T cell lineage markers, the TH17-transcription factor RORγt was significantly higher expressed in aortas of atherosclerotic Apoe−/− mice with renal impairment (figure 5A) and the proportion of splenic IL-17A producers was significantly increased (figure 5B,D). No significant change was seen in T-bet and IFNγ, markers of TH1 cells and very well described pro-atherogenic mediators (figure 5A,B,C) or Foxp3+ regulatory T cells with known anti-atherogenic function (figure 5A,E). The increase in IL-17 production was not limited to CD4+ T cells but also observed in the CD4−CD3+ compartment that includes multiple lineages (data not shown).48 In serum from patients with impaired renal function (CKDIII), many IL-17A measurements were below detection limit, similar to cohorts of patients with hypertension and the acute coronary syndrome.49, 50 However, in 16/32 patients but only 3/9 of healthy volunteers, serum IL-17A was detectable (>6pg/ml) and IL-17A effects on macrophage differentiation started at low concentrations (figure 4C,D). Similarly, most other cytokine concentrations were below detection limit, however, there was a trend towards elevated TNF TH17-related IL-6 levels (data not shown).
Figure 5. Renal impairment and Angiotensin II enhance TH17 polarization.
(A) mRNA expression of markers of T cell lineages (T-bet for the TH1, GATA-3 for TH2, RORγt for TH17 and Foxp3 for Treg) was assessed in spleens and aortas of Apoe−/− mice after 12 weeks high fat diet (renal impairment relative to controls, n=5, t-tests). (D-E) Interferon gamma (IFNγ)(B,C, n=6-8) and IL-17A producing (B,D, n=8-13) T cells and Foxp3+ regulatory T cells (E, n=4-5) in spleens from Apoe−/− mice with normal and impaired renal function (RI) (5h PMA/ionomycin, % of CD3+ cells, Il17a−/− spleens used to define IL-17A positivity (<0.1%)). (F,G) Mouse splenocytes were cultured under TH0, TH1 and TH17 polarizing conditions with and without exogenous Angiotensin II (AngII, 250 pg/ml unless indicated) and intracellular cytokine expression was assessed after re-stimulation as described in methods (F, typical examples and G, n=3 independent experiments, One-way ANOVA, *indicates significant slope). (H) TH17 polarization was carried out in the presence or absence of Angiotensin II and Losartan (1nM) or solvent control (one of 2 independent experiments).
Recent data have shown that Angiotensin-induced increase in blood pressure49, 51 and tissue damage52, 53 was mediated by IL-17A. Blockade of Angiotensin signaling is among the most successful pharmacologic interventions in renal impairment.54, 55 Angiotensin II serum concentration was increased in Apoe−/− mice with renal impairment (135±47 pg/ml (RI), 48±14 (ctrl), n=6-7, p=0.02). We investigated the effect of Angiotensin II on T cell cytokines implicated in atherosclerosis development. No significant effect on IFNγ production was observed, but Angiotensin II significantly increased the number of IL-17A producing T cells under TH17 polarizing conditions (figure 5F,G). No effect on the proportion of IL-17A was observed if Angiotensin II was applied either without TH17 favoring conditions (TH0, figure 5F,G) or during re-stimulation of T cells (data not shown). The effect on TH17 polarization was reverted by the Angiotensin receptor blocker Losartan (figure 5H).
Increased aortic lesion size and CD11b+CD11c+ leukocyte accumulation in impaired renal function is IL-17A dependent
To directly investigate the role of IL-17A in atherosclerosis in renal impairment, we reconstituted LDLr−/− mice with either wt or Il17a−/− bone marrow. Unilateral nephrectomy significantly decreased renal function as measured GFR also in this mouse model of atherosclerosis (suppl. figure I). Suppl. table II shows that no significant differences occurred in body weight, circulating leukocytes and serum triglyceride and total cholesterol levels. In renal impairment, there was a tendency towards an increase in LDL and vLDL levels as determined by FPLC (suppl. figure VI). Aortic lesion size was significantly increased in impaired renal function similar to the Apoe−/− atherosclerosis model (figure 7A-C).
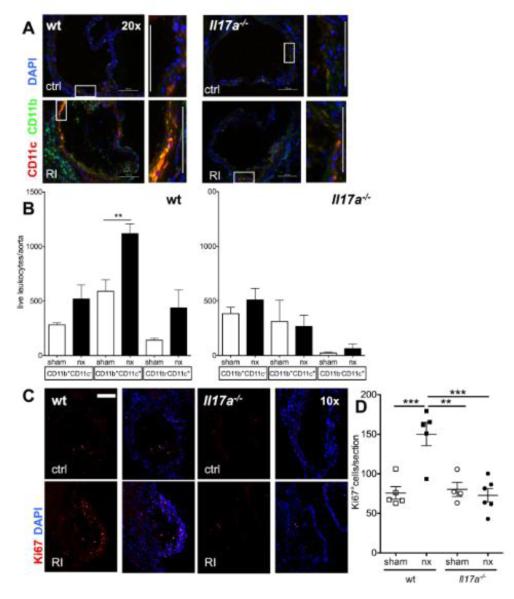
Figure 7. IL-17A ablation abolishes enhanced aortic macrophage accumulation in renal impairment.
The aortic leukocyte infiltrate was analyzed in LDLr−/− mice reconstituted with either wt or Il17a−/− bone marrow and normal and impaired renal function. (A) Immunofluorescence to assess CD11b (green) and CD11c (red) in the aortic root (typical examples of aortic valves, rectangles mark the cell rich intimal regions shown in zoom. 20x orig. magn., bars=100μm). (B) Number of total aortic CD11b+ and CD11c+ cells determined by flow cytometry (n=5-8, 4 independent experiments). (C,D) Aortic cell proliferation was assessed by Ki67 staining (E, examples of aortic valve area plaques, 10x orig. magn., bar=200μm, F: mean Ki67+ cell numbers/aortic section from n=4 sections/mouse, n=4-6 mice/group, One-way-ANOVA and Bonferroni post-hoc test).
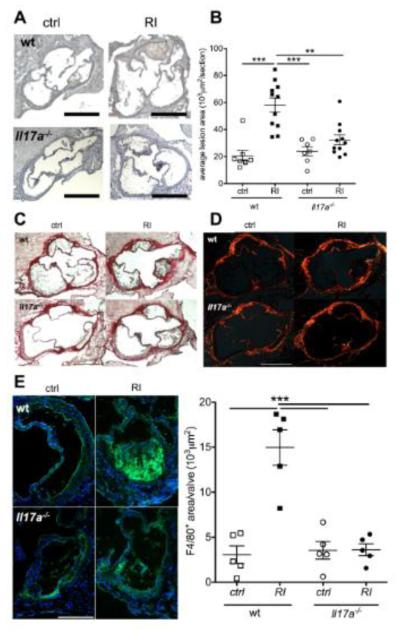
Reconstitution with Il17a−/− bone marrow abolished IL-17A producing cells in the spleen (suppl. figure VIIA). Comparing control LDLr−/− mice transplanted with wt and Il17a−/− bone marrow, aortic root lesion size was very similar. However, in the absence of IL-17A, renal impairment no longer increased atherosclerotic lesion size (figure 6A,B, suppl. figure VIIB,C). Lesional collagen content was very similar in all four experimental groups (figure 6C,D), similar to what was observed in Apoe−/− mice (suppl. figure III). However, lesional macrophage accumulation was significantly enhanced in renal impairment in wt mice (figure 6E). This was abolished in the absence of IL-17A.
Figure 6. IL-17A ablation abolishes atherosclerosis enhancement in renal impairment.
Atherosclerosis development was studied in LDLr−/− mice reconstituted with either wt or Il17a−/− bone marrow and normal and impaired renal function. (A,B) Aortic root lesion size (A, examples, 5x orig. magn., bars=500μm, B means±SEM, n=7-11/group, 4 independent experiments, Bonferroni after One-way-ANOVA. (C,D) Picrosirius-red stain of collagen contents as translucent image (D) and with the use of polarized light (E, examples of at least 4 mice/group). (F) F4/80-macrophage staining (examples and statistical analysis of n=5 mice per group, F4/80 positive area/valvular lesion, Bonferroni after One-way-ANOVA).
Renal impairment increased CD11b and more markedly CD11c immunofluorescence staining in the aortic root of LDLr−/− mice with wt bone marrow (figure 7A). This was abrogated in the absence of IL-17A. Also by flow cytometry, significantly more CD11b+CD11c+ leukocytes were detected in the aortas of wt LDLr−/− mice with renal impairment compared to controls with normal renal function (figure 7B). This increase was completely abolished in Il17a−/−-reconstituted LDLr−/− mice. Similar to the Apoe−/− model (figure 2), renal impairment significantly increased aortic cell proliferation in control LDLr−/− mice (figure 7C,D). The proliferation increase was also abolished in the absence of IL-17A.
Collectively, these results suggest a central role for IL-17A in aggravation of atherosclerosis and vascular leukocyte accumulation in renal impairment.
DISCUSSION
Kidney disease is a frequent and an independent risk factor for the development of atherosclerosis and its complications. The inflammatory response is increased, but dysfunctional in patients receiving renal replacement therapy.41, 56 However, inflammatory leukocytes within the vascular wall have not been systematically explored in patients on renal replacement therapy or with lesser degrees of renal impairment. Our study shows a significant increase of total aortic leukocytes and CD11b+CD11c+ myeloid cells in two independent murine atherosclerosis models, Apoe−/− and LDLr−/− mice, with a 50% decrease in renal glomerular number and significantly decreased GFR. Our study also identifies IL-17A as a critical mediator of atherosclerosis enhancement in renal impairment.
The role of IL-17A in atherosclerosis in general is controversial.28, 29 A pro-atherogenic role is suggested by anti-atherogenic effects of IL-17-receptor-blockade and IL-17A-blockade in some, albeit not all models28, 29 and pro-atherogenic IL-17A effects in immunosuppressed Apoe−/− mice where IL-17A participated in myeloid cell accumulation in the arterial wall.39 The expression of IL-17A and CD11b+ myeloid cell accumulation in the aorta of mice with impaired kidney function were enhanced. Published data suggest that IL-17A can enhance antigen presenting cell functions30, 31 and macrophage cytokine secretion57 in fully differentiated cells. In our in vitro model, IL-17A supplementation during differentiation significantly increased dendritic cell marker expression on mouse and human myeloid cells. It was demonstrated that CD11b+CD11c+ cells can function as fully functional antigen presenting cells within the aortic wall.24, 27 We confirmed their ability to induce CD4+ T cell proliferation.24 We also demonstrate that renal impairment not only enhanced accumulation of CD11b+CD11c+ cells, but also increased interactions with T cells. Productive interactions could locally promote T cell cytotoxicity and cytokine production further increasing atherosclerotic inflammation, including activation of other cell types such as smooth muscle cells and endothelium. In addition, activated lymphocytes and antigen presenting cells migrating from the vascular wall to secondary and tertiary lymphatic organs19, 22 could amplify systemic immune response.
Not only the recruitment but also the lipid scavenging function of aortic CD11b+CD11c+ cells was significantly affected by renal impairment. An earlier report has described impaired efflux of cholesterol from peritoneal macrophages from mice with impaired renal function due to a decrease in ABCA1 gene expression.58 The authors concluded that decreased cholesterol efflux would promote foam cell formation and thereby the enhancement of atherosclerosis in renal impairment. We are able to expand this finding to the aorta in vivo. We also observed a decrease in lipid uptake in human macrophages exposed to serum from patients with chronic kidney disease in vitro. While multiple cytokines and other plasma components lipids may be differentially present in patients with renal impairment, IL-17A by itself was sufficient for similar effects on human macrophages. IL-17A also decreased oxLDL uptake in murine bone marrow derived macrophages. Both CD36 and ABCA1 cholesterol trafficking molecules gene expression was diminished by IL-17A. This change in lipid uptake may contribute to the altered lipid profile in Apoe−/− mice with renal impairment as observed here and by others.8, 9, 13, 16, 58 The changes in circulating lipid levels were significant in Apoe−/−, but not LDLr−/− mice in our study. It is possible that the extreme lipid overload in Apoe−/− mice may make the changes in transport molecules more visible in systemic lipid levels. On the other hand, circulating lipid levels do not always correlate with disease severity. For example, in double ABCA1−/−/ABCG1−/− mice, a decrease in cholesterol efflux decreased macrophage lipid clearance abilities and increased atherosclerosis levels despite lower circulating lipid levels.59 Also in impaired renal function, Angiotensin II blockade was more anti-atherogenic than a vasodilator despite higher cholesterol levels.10 IL-17A influenced molecules responsible for both, cholesterol influx and efflux and therefore may have local pro-atherogenic actions by increasing lipid deposition in the vascular wall that are not necessarily reflected in plasma levels.
We found more IL-17A producing T cells in Apoe−/− mice with decreased kidney function. IL-17A serum levels were also increased in a cohort of patients on hemodialysis.42 Most importantly, renal impairment had no effect on atherosclerotic lesion size and aortic CD11b+CD11c+ leukocytes of LDLr−/− mice reconstituted with Il17a−/− bone marrow, suggesting a mechanistic role rather than an association of IL-17A in enhanced atherogenesis in renal impairment. Several factors may confer atherosclerosis-promoting function to IL-17A in renal impairment not observed under baseline conditions in LDLr−/− mice here and by others.37 First, our results show enhancement of IL-17A production by Angiotensin II under TH17 polarizing conditions. Angiotensin II blockade is a major disease-modifying factor in patients with kidney disease54, 55 and more effective than blood pressure treatment with other agents in 5/651 and unilaterally nephrectomized mice.10 Indeed, Angiotensin II levels were elevated in mice with renal impairment. The TH17-regulating cytokine TGFβ induced by Angiotensin II in several forms of vascular inflammation.60 Possibly, an enhancement by Angiotensin II increases IL-17A levels enough to become a determinant of atherosclerosis severity. On the other hand, an amplification or alteration of the IL-17A signal in renal impairment may also occur by other yet to be determined factors, e.g. cytokines on IL-17A responsive cells such as leukocytes.
In conclusion, our data show that renal impairment increases atherosclerotic inflammation, alters aortic myeloid cell phenotype and their interaction with T lymphocytes and suggest IL-17A as a key mediator for this increase in disease severity.
Supplementary Material
Novelty and Significance.
What Is Known?
Chronic kidney disease increases the risk of atherosclerosis and its complications.
During atherogenesis, leukocytes accumulate in the arterial wall and contribute to plaque growth.
Apoe−/− mice after unilateral nephrectomy can serve as a mouse model of atherosclerosis with moderate renal impairment.
What New Information Does This Article Contribute?
Unilateral nephrectomy in Ldlr−/− mice significantly decreases kidney function and aggravates atherosclerosis.
During renal impairment, more myeloid cells, T and B lymphocytes accumulate and antigen presenting cells interact more with T cells in the arterial wall.
Absence of interleukin 17 abrogates enhancement of myeloid cell accumulation and atherosclerosis that accompany renal impairment.
Large population-based studies have shown that chronic kidney disease increases morbidity and mortality in patients with cardiovascular disease, however; the effect of kidney disease on atherosclerosis has not been systematically investigated. We examined inflammatory cells inflammation in atherosclerotic lesions in moderate renal impairment using a combination of histology, qPCR, flow cytometry and multi-photon imaging. Our data show in two mouse models that arterial inflammation is markedly increased. We characterized myeloid cell phenotype and demonstrate increased antigen-presenting-cell function. Our data show activation of the IL-17 pathway and indicate to its mechanistic importance in leukocyte accumulation and plaque growth in moderate renal impairment, indicating that -IL-17 therapy may be a therapeutic anti-inflammatory target in impaired renal function.
ACKNOWLEDGEMENTS
We thank Dr. J. Witztum and Jennifer Pattison for performance of FPLC.
SOURCES OF FUNDING This work was supported by National Science Foundation of China (#81200531 to S.G.), American Heart Association (#10POST4160142-01 to E.K.K.), NIH (HL58108, HL55798 to K.L.), Deutsche Forschungsgemeinschaft (VI508/4-1) and TUI-Stiftung to S.v.V..
Nonstandard Abbreviation and Acronyms
- Apoe−/−
Apolipoprotein E deficient mice
- LDLr−/−
LDL receptor deficient mice
- RI
renal impairment
- IL-17A
Interleukin 17A
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: A systematic review. Journal of the American Society of Nephrology : JASN. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 2.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the us population. American journal of epidemiology. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Herzog CA, Asinger RW, Berger AK, Charytan DM, Diez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E. Cardiovascular disease in chronic kidney disease. A clinical update from kidney disease: Improving global outcomes (kdigo) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 5.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: Effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 6.Shroff GR, Frederick PD, Herzog CA. Renal failure and acute myocardial infarction: Clinical characteristics in patients with advanced chronic kidney disease, on dialysis, and without chronic kidney disease. A collaborative project of the united states renal data system/national institutes of health and the national registry of myocardial infarction. American heart journal. 2012;163:399–406. doi: 10.1016/j.ahj.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the united states. JAMA : the journal of the American Medical Association. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 8.Bro S, Bentzon JF, Falk E, Andersen CB, Olgaard K, Nielsen LB. Chronic renal failure accelerates atherogenesis in apolipoprotein e-deficient mice. Journal of the American Society of Nephrology : JASN. 2003;14:2466–2474. doi: 10.1097/01.asn.0000088024.72216.2e. [DOI] [PubMed] [Google Scholar]
- 9.Buzello M, Tornig J, Faulhaber J, Ehmke H, Ritz E, Amann K. The apolipoprotein e knockout mouse: A model documenting accelerated atherogenesis in uremia. Journal of the American Society of Nephrology : JASN. 2003;14:311–316. doi: 10.1097/01.asn.0000045048.71975.fc. [DOI] [PubMed] [Google Scholar]
- 10.Suganuma E, Zuo Y, Ayabe N, Ma J, Babaev VR, Linton MF, Fazio S, Ichikawa I, Fogo AB, Kon V. Antiatherogenic effects of angiotensin receptor antagonism in mild renal dysfunction. Journal of the American Society of Nephrology : JASN. 2006;17:433–441. doi: 10.1681/ASN.2005080883. [DOI] [PubMed] [Google Scholar]
- 11.Buzello M, Haas CS, Hauptmann F, Gross ML, Faulhaber J, Schultze-Mosgau S, Ehmke H, Ritz E, Amann K. No aggravation of renal injury in apolipoprotein e knockout mice (apoe(-/-)) after subtotal nephrectomy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19:566–573. doi: 10.1093/ndt/gfg578. [DOI] [PubMed] [Google Scholar]
- 12.Bro S, Moeller F, Andersen CB, Olgaard K, Nielsen LB. Increased expression of adhesion molecules in uremic atherosclerosis in apolipoprotein-e-deficient mice. Journal of the American Society of Nephrology : JASN. 2004;15:1495–1503. doi: 10.1097/01.asn.0000128371.33195.7b. [DOI] [PubMed] [Google Scholar]
- 13.Massy ZA, Ivanovski O, Nguyen-Khoa T, Angulo J, Szumilak D, Mothu N, Phan O, Daudon M, Lacour B, Drueke TB, Muntzel MS. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein e knockout mice. J Am Soc Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- 14.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 15.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: The killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 16.Westenfeld R, Schafer C, Kruger T, Haarmann C, Schurgers LJ, Reutelingsperger C, Ivanovski O, Drueke T, Massy ZA, Ketteler M, Floege J, Jahnen-Dechent W. Fetuin-a protects against atherosclerotic calcification in ckd. J Am Soc Nephrol. 2009;20:1264–1274. doi: 10.1681/ASN.2008060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: Lessons from mouse models. Nature reviews. Immunology. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 18.Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nature reviews. Immunology. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of cd11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. Gm-csf regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautier EL, Huby T, Saint-Charles F, Ouzilleau B, Pirault J, Deswaerte V, Ginhoux F, Miller ER, Witztum JL, Chapman MJ, Lesnik P. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation. 2009;119:2367–2375. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 22.Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, Lichtman AH. Cd11c(+) dendritic cells maintain antigen processing, presentation capabilities, and cd4(+) t-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rekhter MD, Gordon D. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. The American journal of pathology. 1995;147:668–677. [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JH, Cheong C, Dandamudi DB, Park CG, Rodriguez A, Mehandru S, Velinzon K, Jung IH, Yoo JY, Oh GT, Steinman RM. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Bobryshev YV. Dendritic cells in atherosclerosis: Current status of the problem and clinical relevance. Eur Heart J. 2005;26:1700–1704. doi: 10.1093/eurheartj/ehi282. [DOI] [PubMed] [Google Scholar]
- 26.Wick G, Romen M, Amberger A, Metzler B, Mayr M, Falkensammer G, Xu Q. Atherosclerosis, autoimmunity, and vascular-associated lymphoid tissue. FASEB J. 1997;11:1199–1207. doi: 10.1096/fasebj.11.13.9367355. [DOI] [PubMed] [Google Scholar]
- 27.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic t cell-apc interactions sustain chronic inflammation in atherosclerosis. The Journal of clinical investigation. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 29.von Vietinghoff S, Ley K. Interleukin 17 in vascular inflammation. Cytokine Growth Factor Rev. 2010;21:463–469. doi: 10.1016/j.cytogfr.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ. Inhibition of il-17a attenuates atherosclerotic lesion development in apoe-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 31.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17a results in reduced atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon il-17r signaling disruption in ldlr deficient mice. Biochemical and biophysical research communications. 2009;388:261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Shimada K, Zhang W, Huang G, Crother TR, Arditi M. Il-17a is proatherogenic in high-fat diet-induced and chlamydia pneumoniae infection-accelerated atherosclerosis in mice. J Immunol. 2010;185:5619–5627. doi: 10.4049/jimmunol.1001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The il-17a/il-17ra axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. Loss of socs3 expression in t cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, Yan XX, Nie SF, Liao MY, Cheng Y, Mallat Z, Liao YH. Inhibition of il-17a in atherosclerosis. Atherosclerosis. 2011;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 38.Danzaki K, Matsui Y, Ikesue M, Ohta D, Ito K, Kanayama M, Kurotaki D, Morimoto J, Iwakura Y, Yagita H, Tsutsui H, Uede T. Interleukin-17a deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:273–280. doi: 10.1161/ATVBAHA.111.229997. [DOI] [PubMed] [Google Scholar]
- 39.von Vietinghoff S, Koltsova EK, Mestas J, Diehl CJ, Witztum JL, Ley K. Mycophenolate mofetil decreases atherosclerotic lesion size by depression of aortic t-lymphocyte and interleukin-17-mediated macrophage accumulation. Journal of the American College of Cardiology. 2011;57:2194–2204. doi: 10.1016/j.jacc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koltsova EK, Kim G, Lloyd KM, Saris CJ, von Vietinghoff S, Kronenberg M, Ley K. Il-27 receptor limits atherosclerosis in ldlr-/- mice. Circulation research. 2012 doi: 10.1161/CIRCRESAHA.112.277525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zoccali C, Tripepi G, Mallamaci F. Dissecting inflammation in esrd: Do cytokines and c-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol. 2006;17:S169–173. doi: 10.1681/ASN.2006080910. [DOI] [PubMed] [Google Scholar]
- 42.Chung BH, Kim KW, Sun IO, Choi SR, Park HS, Jeon EJ, Kim BM, Choi BS, Park CW, Kim YS, Cho ML, Yang CW. Increased interleukin-17 producing effector memory t cells in the end-stage renal disease patients. Immunology letters. 2012;141:181–189. doi: 10.1016/j.imlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nature reviews. Nephrology. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 44.Hesselink DA, Betjes MG, Verkade MA, Athanassopoulos P, Baan CC, Weimar W. The effects of chronic kidney disease and renal replacement therapy on circulating dendritic cells. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2005;20:1868–1873. doi: 10.1093/ndt/gfh897. [DOI] [PubMed] [Google Scholar]
- 45.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Vietinghoff S, Asagiri M, Azar D, Hoffmann A, Ley K. Defective regulation of cxcr2 facilitates neutrophil release from bone marrow causing spontaneous inflammation in severely nf-kappa b-deficient mice. Journal of immunology. 2010;185:670–678. doi: 10.4049/jimmunol.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 48.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin ii-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang J, Zheng Z, Wang M, Han L, Peng J, Liu Z, Wei Y. Myeloperoxidase (mpo) and interleukin-17 (il-17) plasma levels are increased in patients with acute coronary syndromes. J Int Med Res. 2009;37:862–866. doi: 10.1177/147323000903700331. [DOI] [PubMed] [Google Scholar]
- 51.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, Sobel RA, Steinman L. Blocking angiotensin-converting enzyme induces potent regulatory t cells and modulates th1- and th17-mediated autoimmunity. Proc Natl Acad Sci U S A. 2009;106:14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stegbauer J, Lee DH, Seubert S, Ellrichmann G, Manzel A, Kvakan H, Muller DN, Gaupp S, Rump LC, Gold R, Linker RA. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci U S A. 2009;106:14942–14947. doi: 10.1073/pnas.0903602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kdoqi clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 56.Yoon JW, Gollapudi S, Pahl MV, Vaziri ND. Naive and central memory t-cell lymphopenia in end-stage renal disease. Kidney international. 2006;70:371–376. doi: 10.1038/sj.ki.5001550. [DOI] [PubMed] [Google Scholar]
- 57.Barin JG, Christian Baldeviano G, Talor MV, Wu L, Ong S, Quader F, Chen P, Zheng D, Caturegli P, Rose NR, Cihakova D. Macrophages participate in il-17-mediated inflammation. Eur J Immunol. 2011 doi: 10.1002/eji.201141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuo Y, Yancey P, Castro I, Khan WN, Motojima M, Ichikawa I, Fogo AB, Linton MF, Fazio S, Kon V. Renal dysfunction potentiates foam cell formation by repressing abca1. Arterioscler Thromb Vasc Biol. 2009;29:1277–1282. doi: 10.1161/ATVBAHA.109.188995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch CL, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of abca1 and abcg1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circulation research. 2013 doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X, Lu H, Rateri DL, Cassis LA, Daugherty A. Conundrum of angiotensin ii and tgf-beta interactions in aortic aneurysms. Current opinion in pharmacology. 2013;13:180–185. doi: 10.1016/j.coph.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.