Abstract
BACKGROUND
Pancreatic cancer ranks as the fourth leading cause of cancer death in the United States with five year survival ranging from 1-5%. Positron emission tomography (PET) is a metabolic imaging system that is widely used for the initial staging of cancer and detecting residual disease after treatment. There are limited data, however, on the use of this molecular imaging technique to assess early tumor response after treatment in pancreatic cancer.
METHODS
The objective of the study was to explore the relationship of early treatment response using the 18 F- fluorodeoxyglucose (FDG) PET with surgical outcome and overall survival in patients with locally advanced pancreatic cancer. FDG-PET measurements of maximum standardized uptake value (SUV) and kinetic parameters were compared to the clinical outcome.
RESULTS
Twenty patients were enrolled in the study evaluating neoadjuvant induction chemotherapy followed by concurrent chemoradiotherapy (chemo-RT) for locally advanced pancreatic cancer. All twenty patients had pre-study PET scans and a total of fifty PET scans were performed. Among patients who were PET responders (≥50% decrease in SUV after cycle 1), 100% (2/2) had complete surgical resection. Only 6% (1/16) had surgical resection in the PET non-responders (<50% decrease). Two patients did not have the second PET scan due to clinical progression or treatment toxicity. Mean survival was 23.2 months for PET responders and 11.3 months for non-responders (p=0.234). Similar differences in survival were also noted when response was measured using Patlak analysis.
CONCLUSION
FDG-PET can aid in monitoring the clinical outcome of patients with locally advanced pancreatic cancer treated with neoadjuvant chemo-RT. FDG-PET may be used to aid patients who could have complete surgical resection as well as prognosticate patients’ survival.
Keywords: Pancreatic cancer, Combined modality therapy, FDG-PET, treatment response
INTRODUCTION
Pancreatic adenocarcinoma is one of the most lethal cancers in the world. For all stages combined, the 1-year survival rate is only 20%, and the 5-year survival rate is less than 5%. It is estimated that 37,680 new pancreatic cancer patients will be diagnosed and 34,290 deaths are expected for 2008 in the United States. (1) Pancreatic resection offers the potential for long term survival in a select patient population. However, in patients who seemed resectable after extensive perioperative staging, up to 35% of patients are deemed unresectable due to operative findings at laparatomy. (2;3) In patients with locally advanced pancreatic cancers, combined modality treatment with radiation and chemotherapy (chemo-RT) increases median survival to approximately 10 to 13 months and offers the possibility of long term survival in a few patients. (4;5)
Since many pancreatic cancer patients do not respond to therapy, an early assessment of tumor response would be useful in making treatment decisions. Structural imaging has significant limitations in the evaluation of treatment with chemotherapy or chemo-RT. The computed tomography (CT) scan and magnetic resonance imaging (MRI) cannot distinguish residual or necrotic tumor from fibrosis and radiation changes after treatment. Therefore, there is a need for technological improvement in assessing response to treatment in pancreatic cancer. Such a development could be used to identify patients in whom the course of disease might be altered by implementation of alternative therapy earlier in the course of treatment.
Positron emission tomography (PET) utilizes positron emitting radioisotopes labeled to molecules that create different images depending on tissue concentrations. Fluorodeoxyglucose (FDG) is the most commonly used tracer that is an analog of glucose that accumulates more specifically in metabolically active cells like cancer. It has both high sensitivity and specificity making it a useful tool in the diagnosis and staging of cancer patients. (6-8) Its reproducibility for quantitative metabolic measurements has been validated in malignant tumors. (9;10) PET is approved for use in the United States for cancer patients with breast, esophageal, lung, head and neck, lymphoma, thyroid, colorectal, and melanoma. (11) Use of PET in the initial staging of pancreas cancer has recently been proposed for coverage under Medicare. While a growing number of studies have used PET in assessing treatment response for other tumors, there are only four small studies in the treatment of in pancreatic cancer. (12-15) This study explored the utilization of FDG-PET to monitor clinical outcome in locally advanced pancreatic cancer patients treated with neoadjuvant chemo-RT.
METHODS
All twenty patients had pathologically confirmed pancreatic adenocarcinoma that was initially considered unresectable, but that might be resected if there was a local response to therapy. Unresectable disease status was determined by exploratory laparotomy, or by CT or MR imaging, in the setting of a multidisciplinary team that involved surgeons, radiologists, medical and radiation oncologists, pathologists and gastroenterologists. All patients had MRI and PET scans done to follow tumor response. Neoadjuvant chemotherapy consisted of cisplatin 100 mg/m2 IV on day 1, cytarabine 2 g/m2 IV every 12 hours × 2 doses, and caffeine 400 mg/m2 subcutaneously after each cytarabine dose; and days 3 to 21, 5-fluorouracil (5FU) 250 mg/m2/day given by continuous infusion. Cycles were repeated every 28 days. After two cycles of induction chemotherapy, radiation therapy was given concurrently with 5FU at 200 mg/m2/day. Patients were to receive 39.6 Gy of photon radiation to the primary followed by a neutron boost of 8 NGy to the gross tumor volume (GTV). However, in 12 patients the volumes were deemed too large to be safely treated with neutrons. These patients were treated with photons only and received a median dose of 50.4 Gy. Photon therapy was delivered at 1.8 Gy per fraction and neutrons were delivered at 0.8 Gy per fraction, 5 days per week. Three-dimensional conformal planning was used, and the fields allowed a 2-cm margin around all aspects of the GTV as visualized by MRI or CT scan. Details on the patient eligibility, study design, response data and toxicity were previously reported. (16) The analysis of treatment response using the FDG-PET was planned before the initiation of the protocol and is reported separately here in this study. The human subject protocol for this study was reviewed and approved by the Human Investigation Committee of Wayne State University. All patients gave a signed informed consent before study entry.
PET Imaging Method
Patients were imaged with FDG PET before therapy (scan 1), at the end of the first cycle of chemotherapy (mean day 24.1, range 21-27 days) after the start of treatment (scan 2), and after the completion of radiation (scan 3) and prior to planned surgery (mean day 118.3, range 105-154 days). PET studies were performed using the Siemens EXACT/HR (Tennessee, USA) whole body tomograph located at Children’s Hospital of Michigan. This scanner has a 15 cm field of view and generates 47 image planes with a slice thickness of 3.25 mm. The reconstructed image in-plane resolution obtained is 5.5 + 0.35 mm at full-width-at-half-maximum (FWHM) and 6.0 + 0.49 mm in the axial direction (reconstruction parameters: Shepp-Logan filter with 0.3 cycles/pixel cutoff frequency). One venous catheter was placed for infusion of the FDG. An attenuation scan was done with a rotating Ge-68 source moving around the patient followed by injection of approximately 10 mCi of 18F-FDG (range 6.34 −10.9 mCi) and imaging for 60 minutes over the tumor. In six patients with diabetes, insulin was given prior to imaging to bring the glucose below 200 mg/dl. For quantitation, a 5.8 × 5.8 mm square region of interest was drawn over the hottest area of the tumor and the two adjacent planes. The image obtained from 20 to 60 minutes post injection was used to measure the maximum standardized uptake value (SUV). Dynamic images obtained at the following times: 20 sec × 4 images, 40 sec × 4, 1 min × 4, 3 min × 4, and then 5 min × 8 for a total of 60 min, were used to calculate the FDG metabolic rates using the graphical approach based on the work of Patlak (17) and Gjedde.(18) The standard three-compartment model was also used to calculate the FDG metabolic rate after fitting the dynamic curves using a parameter optimization program. For dynamic modeling the blood input function was obtained from the descending aorta.
Statistical Methods
The primary statistical objective of the study was to explore the relationship between the PET standardized uptake value (SUV), or the change in SUV from scan 1 to scan 2, with patient resectability and patient survival. Of the twenty patients, only one was still alive (at 83.6 months post-surgery). Since the resulting censoring rate was extremely low (5%), and since that one survival duration was the longest by far, we regarded the entire sample of twenty patients as uncensored. That permitted the use of simple, normal theory based statistical methods. Survival duration was transformed via the natural logarithm (ln) to achieve approximate Normality. The mean of ln(survival) was then compared by various dichotomous classification variables via the 2-sample t-test. Mean ln(survival) values were exponentiated to restore their original units of measurement (months) for ease of presentation and interpretation of results.
Proportions of patients who were completely resectable were compared by PET response (yes/no) category using Fisher’s exact test. To better illustrate one particular survival comparison, a standard Kaplan-Meier (K-M) graph was generated using survival from the time of the start of treatment. In the K-M plot, the one very long (and censored) survival time was truncated to 36 months to produce a more compact graph. This truncation had no effect on the K-M estimate of survivorship at any earlier time point. Due to the small sample sizes, survival statistics (e.g., 1-yr rate) were estimated more conservatively using linear interpolation (19) among successive event times on the K-M graph. Across sets of related statistical comparisons, the Type I error rate for that set of inferences was adjusted by the multiple comparisons procedure of Benjamini and Hochberg (20).
RESULTS
Twenty patients with locally advanced pancreatic cancer were enrolled in the clinical trial. Tumors were located in the head of the pancreas in sixteen patients, in the body in two patients, and in the tail in two patients. Prior to chemo-RT treatment, disease was considered unresectable in ten patients by CT or MRI. The ten remaining patients were unresectable at laparotomy and underwent biliary bypass surgery to relieve symptoms of obstructive jaundice before starting treatment. All twenty patients had a pre-study PET scan 1 done and the mean SUV was 6.6 (range 3.1-11.1). After the first cycle of chemotherapy, PET scan 2 was done to monitor disease response. There was a mean change of −22% (i.e., decrease) in the SUV from scan 1 to scan 2 and the mean scan 2 SUV was 5.2 (range 3.2-9.7). PET scan 3 was done after completion of chemotherapy and radiotherapy, prior to consideration of surgery. The mean scan 3 SUV was 4.07, a 39% decrease from scan 1 SUV. Eighteen patients had scan 2 completed, but only twelve patients had all three planned scans. Two patients did not have PET scan 2 due to toxicity from treatment (one patient) and disease progression (one patient) while six patients did not have PET scan 3 due to patient refusal (one patient) and disease progression (five patients). Overall, fifty PET scans were performed and analyzed in the study population. The SUV of scan 1 were varied, ranging from 3.1 to 11.1, but SUV was noted to have a narrower spectrum as the patients continued to have treatment in scan 2 and scan 3. Analysis of SUV at scan 1 had no prognostic significance. The individual patients’ SUV data, response by SUV or Patlak, and clinical outcome are illustrated in Table 1, along with mean values.
Table1.
Individual FDG-PET SUV Data and Clinical Outcome
| Patient | SCAN 1 SUV |
SCAN 2 SUV |
% change by SUV |
% change by Patlak |
SCAN 3 SUV |
Surgical Outcome | Survival (months) |
|---|---|---|---|---|---|---|---|
| 1 | 3.1 | na | na | na | na | unresectable | 13.4 |
| 2 | 7.1 | 7.0 | −1 | −7 | 7.0 | unresectable at laparatomy |
19.9 |
| 3 | 4.6 | 3.4 | −26 | −96 | na | unresectable | 20.3 |
| 4 | 9.6 | 7.5 | −21 | −25 | 4.6 | unresectable | 8.9 |
| 5 | 7.8 | na | na | na | na | unresectable | 4.5 |
| 6 | 6.9 | 6.9 | 1 | 108 | na | unresectable | 14.5 |
| 7 | 11.1 | 4.9 | −56 | −90 | 4.8 | Resected | 24.3 |
| 8 | 5.9 | 5.4 | −8 | −13 | na | unresectable | 7.4 |
| 9 | 4.6 | 3.8 | −18 | −88 | 3.9 | unresectable at laparatomy |
15.2 |
| 10 | 4.3 | 3.2 | −26 | 53 | 2.4 | unresectable at laparatomy |
8.8 |
| 11 | 8.0 | 6.2 | −23 | −27 | na | unresectable | 5.0 |
| 12 | 4.6 | 4.0 | −13 | −53 | 4.1 | Resected* | 83.6 |
| 13 | 5.3 | 4.8 | −11 | −39 | na | unresectable | 4.0 |
| 14 | 4.7 | 4.3 | −9 | −8 | 3.7 | unresectable | 14 |
| 15 | 9.4 | 4.4 | −53 | −68 | 3.3 | Resected | 22.2 |
| 16 | 5.5 | 4.1 | −25 | −36 | 4.0 | unresectable at laparatomy |
14.2 |
| 17 | 6.7 | 5.8 | −12 | −33 | 4.6 | unresectable | 7.3 |
| 18 | 10.7 | 9.7 | −10 | −25 | na | unresectable | 2.7 |
| 19 | 6.7 | 4.2 | −38 | −2 | 3.8 | unresectable at laparatomy |
8.8 |
| 20 | 6.3 | 3.4 | −46 | −83 | 2.7 | unresectable at laparatomy |
22.5 |
| Mean | 6.64 | 5.17 | −21.83 % | −29.68% | 4.07 |
All the patients are deceased except patient # 12 who is still alive at the time of manuscript submission.
na = not available (i.e., missing data).
Of the twenty patients, nine (45%) had post therapy imaging with CT-scan and MRI that indicated resection might be feasible as judged by the surgeon. In three of the patients explored surgically, complete resection was possible and carried out by the surgeon. No patients were noted to have a pathologic complete response on the surgical specimen. In six patients surgically explored, full tumor resection was not feasible and the tumor was left in place. Resection was precluded at laparotomy by extensive fibrosis with persistent disease by biopsy that was not resectable in three patients, vascular encasement with liver metastasis in two patients, and peritoneal carcinomatosis in one patient. PET was unable to detect small distant metastasis prior to surgery.
The percentage change in SUV from scan 1 to scan 2 was arbitrarily dichotomized into PET responders (≥ 50% decrease) versus non-responders (< 50% decrease) based on multiple similar study outcomes. (21;22) The result was that the mean survival of the two patients who had ≥ 50% decrease in SUV {23.2 months, 90% CL =( 17.5, 30.9 )} was longer, but not significantly so (p = 0.234) than the mean survival of the sixteen patients with < 50% decrease in SUV {(11.3 months, 90% CL =( 7.9, 16.0 )}. Similar analysis was performed using the compartmental and Patlak model. The Patlak graphical analysis showed that the six PET responders (≥50% decrease using glucose consumption kinetics) had a mean survival duration of 26.1 months as compared to a mean of 8.4 months in the twelve PET non-responders (< 50% decrease) (p < 0.05, even after adjustment for multiple comparisons). The overall survival curve is illustrated in Figure 1 using the Patlak analysis. Compartmental modeling showed a similar trend but it was not statistically significant. Eight of the unresected patients were subsequently treated with gemcitabine as was one of the three resected patients.
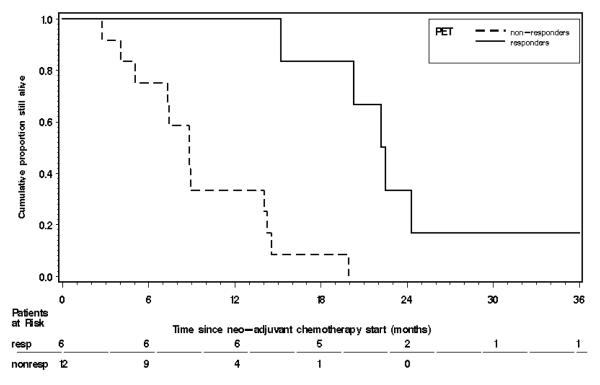
Figure 1.
Overall survival curve for PET responders vs. non-responders using the Patlak model. The one long (and censored) survival time of 83.6+ months was truncated to 36 months to produce a more compact graph. The 1-year survival rate for the six PET responders was 87% (with 90% CL = ( 0.67, 1.00), and for the twelve PET non-responders was 28% (with 90% CL = ( 0.07, 0.49).
Surgical resection rate was analyzed using different PET parameters. In the Patlak model, 3/6 (50%) PET responders completed surgical resection and 0/12 non-responders. Using SUV analysis, two PET responders underwent complete surgical resection for a resection rate of 100% as compared to 1/16 (6%) in PET non-responders. (p=0.020). An exploratory analysis was done to determine whether SUV change of at least 50% (i.e., SUV response) after completion of chemoradiation therapy was related to subsequent surgical resectability. SUV change between scan 3 and scan 1 revealed that 2/4 (50%) of the SUV responders later underwent surgical resection vs. 1/8 (12.5%) among the SUV non-responders, but this difference was not statistically significant (p = 0.236). All three patients (15%) who had a successful tumor resection were initially deemed unresectable by CT and MRI. An illustrative case of a PET responder using the PET is shown in Figure 2. The resected patients lived for 22.2, 24.3, and 83.6 months; the last patient is still alive and recently had a resection of a single metastatic pulmonary nodule. The median survival was 13.7 months for all twenty patients treated by neoadjuvant chemoradiotherapy.
Figure 2.
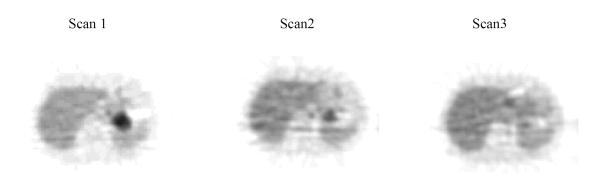
PET images of a PET responder (Patient 15)
PET images show high FDG uptake in the pancreatic bed before treatment (scan 1), and improvement after 1 cycle of chemotherapy (scan 2) and after completion of chemo-RT (scan 3).
DISCUSSION
FDG-PET is a functional imaging method that is specific to metabolically active cancer cells. Sperti et. al. reviewed sixty pancreatic cancer patients who had PET scans done and have shown that the initial PET scan predicted the clinical outcome when patients where dichotomized at an SUV of 4. (23) Smaller studies done by Nakata and Zimny also showed that higher initial SUV predicted worse outcomes at different SUV cutoffs of 3.0 and 6.1. (24;25) Since there is growing evidence that SUV is correlated to the aggressiveness of the tumors, it is hypothesized that it could predict the clinical outcome. However, our analysis of survival in twenty patients with pancreatic cancer did not distinguish patients via different SUV cutoff points. This could be affected by the neoadjuvant treatment or simply result from small sample size. One recent study done by Lyshchik et al. supports our finding; in sixty-five patients with pancreatic cancer who had FDG-PET, the initial SUV did not predict survival outcome but the retention index showed prognostic significance. (26)
Recent studies support the utilization of PET in early assessment of cancer treatment in esophageal, breast, and head and neck cancers. Swisher et al. evaluated eighty-three patients with esophageal cancer treated with chemo-RT and dichotomized the patients to SUV < or > 4 and have shown improved survival in patients who had lower post-treatment SUV values. (27) Similar studies in breast cancer patients who received neoadjuvant chemotherapy showed that patients who had > 50% reduction in SUV had a better clinical outcome. (21;22)
There are only four small studies that evaluated the usefulness of PET scans in pancreatic cancer patients who received treatment. Rose et al. studied nine patients with pancreatic cancer who received treatment with chemo-RT. Four patients achieved > 50% reduction in SUV and all were able to undergo resection of the tumor, while only 2/5 patients were able to undergo resection if they had < 50% PET response.(14) No survival data were presented in that study. In another study done by Maisey et al. (12), eleven patients with advanced pancreatic cancer received 5FU based chemotherapy and treatment was assessed with the PET scan. There was a difference in overall survival in patients who had no FDGPET uptake as compared to patients who had residual uptake after one month of treatment using qualitative measurements. Another small study done in ten pancreatic cancer patients in Japan showed that PET aided in assessing the effectiveness of treatment with arterial chemo infusion and external radiation therapy. (13) Bang et. al. reported a response evaluation using CT-scan and PET scan in 15 patients with pancreatic cancer who received concurrent chemoradiotherapy. There were six patients who had >50% reduction in FDG uptake and PET-responders had longer time to tumor progression. (15) It was also interesting to note a high discordance rate 9/15 patients using response noted in PET and CT-scan. In our study, the two patients who had > 50% PET response underwent surgical resection as compared to only 1/16 non-responders. Using conventional imaging with CT and MRI, nine patients were deemed resectable but only 3/9 underwent complete surgical resection. Although our study is limited by the small number of patients, it showed that FDG-PET scans may aid in the early monitoring clinical outcome. Further studies are needed to demonstrate that one can identify patients who could undergo complete surgical resection based on an evaluation as early as one month after the start of neoadjuvant treatment.
There are different sets of parameters that are obtained during the PET imaging acquisition. Glucose consumption rates can be measured by Patlak graphical analysis and by using compartmental modeling. Hoekstra et. al. showed that FDG-PET has prognostic relevance in patients treated with locally advanced lung cancer and was correlated with survival using the Patlak analysis. (28) Using the residual glucose consumption by Patlak, two distinct groups with different survival was identified after one cycle of chemotherapy. In PET-responders the survival was 26.1 months compared to 8.4 months in non-responders using the Patlak model. A similar outcome was noted using SUV analysis (23.2 months vs. 11.3 months) but was not statistically significant. Since complex mathematical modeling incorporates the glucose consumption rate for the whole tumor instead of a single area noted when calculating the SUV, it might be more sensitive when one is assessing treatment responses. However, for routine clinical practice, simpler semi-quantitative values like SUV would be easier to use. Hence larger studies comparing different parameters of PET data acquisition would be meaningful to address this hypothesis.
The needs for improved monitoring in cancer patients and personalized therapies have produced an increasing number of reports supporting the utilization of PET for early evaluation of treatment. Recent large studies in lymphoma have shown benefit of PET as a prognostic tool in early treatment response assessment and have incorporated PET in new clinical trial design. (29;30) Although our study is limited by small number of patients, it showed that FDG-PET scans may aid in monitoring clinical outcome such as the feasibility of complete surgical resection as early as one month after neoadjuvant treatment. There was also a suggestion of improved overall survival outcome in our PET responders. Larger studies are needed in pancreatic cancer to determine whether a SUV proportional change or a numerical cutoff can be used for initial (and subsequent) treatment assessment and survival prognosis. There is also an increasing need to standardize the use of SUV and its cutoff values for different cancers using larger patient populations.
Acknowledgments
Support: NIH Grants CA82645 and CA22453
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Dijkum EJ, Romjin MG, Terwee CB, et al. Laprascopic staging and subsequent palliatiion in patients with peripancreatic carcinoma. Ann Surg. 2003;237(1):66–73. doi: 10.1097/00000658-200301000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John T, Greig J, Carter D, et al. Carcinoma of the pancreatic head and periampullary regin. Ann. Surg. 1995;221:156–164. doi: 10.1097/00000658-199502000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd KE, Gloor B, Lane JS, Isacoff WH, Reber HA. Resection of locally advanced pancreatic cancer after downstaging with continuous-infusion 5-fluorouracil, mitomycin-C, leucovorin, and dipyridamole. J.Gastrointest.Surg. 1998;2:159–66. doi: 10.1016/s1091-255x(98)80008-5. [DOI] [PubMed] [Google Scholar]
- 5.Yeung RS, Weese JL, Hoffman JP, Solin LJ, Paul AR, Engstrom PF, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II Study. Cancer. 1993;72:2124–33. doi: 10.1002/1097-0142(19931001)72:7<2124::aid-cncr2820720711>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Zimny M, Schumpelick V. [Fluorodeoxyglucose positron emission tomography (FDGPET) in the differential diagnosis of pancreatic lesions] Chirurg. 2001;72:989–94. doi: 10.1007/pl00002602. [DOI] [PubMed] [Google Scholar]
- 7.Sperti C, Pasquali C, Chierichetti F, Liessi G, Ferlin G, Pedrazzoli S. Value of 18-fluorodeoxyglucose positron emission tomography in the management of patients with cystic tumors of the pancreas. Ann.Surg. 2001;234:675–80. doi: 10.1097/00000658-200111000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bombardieri E, Aliberti G, de GC, Pauwels E, Crippa F. Positron emission tomography (PET) and other nuclear medicine modalities in staging gastrointestinal cancer. Semin.Surg.Oncol. 2001;20:134–46. doi: 10.1002/ssu.1027. [DOI] [PubMed] [Google Scholar]
- 9.Weber W, Ziegler S, Thodtmann R, et al. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40:1771–1777. [PubMed] [Google Scholar]
- 10.Minn H, Zasadny K, Quint L, et al. Lung cancer; reproducibility of quantitative measurements for evaluating 2-Fluoro-2-deoxy-D-glucose uptake at PET. Radiology. 1995;196:167–173. doi: 10.1148/radiology.196.1.7784562. [DOI] [PubMed] [Google Scholar]
- 11.Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol.Clin.North Am. 2005;43:189–204. doi: 10.1016/j.rcl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Maisey NR, Webb A, Flux GD, Padhani A, Cunningham DC, Ott RJ, et al. FDG-PET in the prediction of survival of patients with cancer of the pancreas: a pilot study. Br.J.Cancer. 2000;83:287–93. doi: 10.1054/bjoc.2000.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshioka M, Sato T, Furuya T, Shibata S, Andoh H, Asanuma Y, et al. Role of positron emission tomography with 2-deoxy-2-[18F]fluoro-D-glucose in evaluating the effects of arterial infusion chemotherapy and radiotherapy on pancreatic cancer. J.Gastroenterol. 2004;39:50–5. doi: 10.1007/s00535-003-1244-2. [DOI] [PubMed] [Google Scholar]
- 14.Rose DM, Delbeke D, Beauchamp RD, Chapman WC, Sandler MP, Sharp KW, et al. 18Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann.Surg. 1999;229:729–37. doi: 10.1097/00000658-199905000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang S, Chung H, Park S, et al. The clinical usefulness of 18- Fluorodeoxyglucose positron emission tomography in differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol. 2006;40:923–929. doi: 10.1097/01.mcg.0000225672.68852.05. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sukhun S, Zalupski MM, Ben-Josef E, Vaitkevicius VK, Philip PA, Soulen R, et al. Chemoradiotherapy in the treatment of regional pancreatic carcinoma: a phase II study. Am.J.Clin.Oncol. 2003;26:543–9. doi: 10.1097/01.coc.0000037143.60502.54. [DOI] [PubMed] [Google Scholar]
- 17.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J.Cereb.Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 18.Gjedde A, Diemer NH. Autoradiographic determination of regional brain glucose content. J.Cereb.Blood Flow Metab. 1983;3:303–10. doi: 10.1038/jcbfm.1983.45. [DOI] [PubMed] [Google Scholar]
- 19.Lee E, Wang JW. Statistical Methods for Survival Data Analysis. 3rd Ed Wiley & Sons, Inc.; New York: 2003. [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. (Series B).J. Royal Stat. Soc. 1995;57:289–300. [Google Scholar]
- 21.Krak NC, Hoekstra OS, Lammertsma AA. Measuring response to chemotherapy in locally advanced breast cancer: methodological considerations. Eur.J.Nucl.Med.Mol.Imaging. 2004;31(Suppl 1):S103–S111. doi: 10.1007/s00259-004-1532-y. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Moore MO, Lehman CD, Mankoff DA, Lawton TJ, Peacock S, et al. Combined use of MRI and PET to monitor response and assess residual disease for locally advanced breast cancer treated with neoadjuvant chemotherapy. Acad.Radiol. 2004;11:1115–24. doi: 10.1016/j.acra.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Sperti C, Pasquali C, Chierichetti F, Ferronato A, Decet G, Pedrazzoli S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J.Gastrointest.Surg. 2003;7:953–9. doi: 10.1016/j.gassur.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Nakata B, Chung YS, Nishimura S, Nishihara T, Sakurai Y, Sawada T, et al. 18F-fluorodeoxyglucose positron emission tomography and the prognosis of patients with pancreatic adenocarcinoma. Cancer. 1997;79:695–9. [PubMed] [Google Scholar]
- 25.Zimny M, Fass J, Bares R, Cremerius U, Sabri O, Buechin P, et al. Fluorodeoxyglucose positron emission tomography and the prognosis of pancreatic carcinoma. Scand.J.Gastroenterol. 2000;35:883–8. doi: 10.1080/003655200750023273. [DOI] [PubMed] [Google Scholar]
- 26.Lyshchik A, Higashi T, Nakamoto Y, Fujimoto K, Doi R, Imamura M, et al. Dual-phase 18F-fluoro-2-deoxy-D-glucose positron emission tomography as a prognostic parameter in patients with pancreatic cancer. Eur.J.Nucl.Med.Mol.Imaging. 2005;32:389–97. doi: 10.1007/s00259-004-1656-0. [DOI] [PubMed] [Google Scholar]
- 27.Swisher SG, Erasmus J, Maish M, Correa AM, Macapinlac H, Ajani JA, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–85. doi: 10.1002/cncr.20585. [DOI] [PubMed] [Google Scholar]
- 28.Hoekstra CJ, Stroobants SG, Smit EF, Vansteenkiste J, van TH, Postmus PE, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J.Clin.Oncol. 2005;23:8362–70. doi: 10.1200/JCO.2005.01.1189. [DOI] [PubMed] [Google Scholar]
- 29.Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–81. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 30.Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann.Oncol. 2005;16:1160–8. doi: 10.1093/annonc/mdi200. [DOI] [PubMed] [Google Scholar]