Abstract
Synthetic agonists of the growth hormone secretagogue receptor (GHSR) rejuvenate the pulsatile pattern of GH-release in the elderly, and increase lean but not fat mass in obese subjects. Screening of tissue extracts in a cell line engineered to overexpress the GHSR led to the identification of a natural agonist called ghrelin. Paradoxically, this hormone was linked to obesity. However, it had not been directly shown that the GHSR is a physiologically relevant ghrelin receptor. Furthermore, ghrelin's structure is significantly different from the synthetic agonist (MK-0677) used to expression-clone the GHSR. To address whether the GHSR mediates ghrelin's stimulatory effects on GH release and appetite, we generated Ghsr-null mice. In contrast to wild-type mice, acute treatment of Ghsr-null mice with ghrelin stimulated neither GH release nor food intake, showing that the GHSR is a biologically relevant ghrelin receptor. Nevertheless, Ghsr-null mice are not dwarfs; their appetite and body composition are comparable to that of wild-type littermates. Furthermore, in contrast to suggestions that ghrelin regulates leptin and insulin secretion, fasting-induced changes in serum levels of leptin and insulin are identical in wild-type and null mice. Serum insulin-like growth factor 1 levels and body weights of mature Ghsr-null mice are modestly reduced compared to wild-type littermates, which is consistent with ghrelin's property as an amplifier of GH pulsatility and its speculated role in establishing an insulin-like growth factor 1 set-point for maintaining anabolic metabolism. Our results suggest that chronic treatment with ghrelin antagonists will have little effect on growth or appetite.
In 1988, a reverse pharmacology approach was initiated to identify small molecules that would restore the amplitude of growth hormone (GH) pulsatility in the elderly (1). We elucidated the mechanism of action of a class of small, synthetic, GH-releasing peptides, and used this knowledge to develop nonpeptide mimetics (2-5). The mimetic MK-0677, when administered chronically to elderly subjects, resulted in sustained rejuvenation of the physiological profile of the growth hormone axis, and increased lean but not fat mass in obese subjects (6, 7). MK-0677 was also exploited to expression-clone the receptor involved (8); this orphan G protein-coupled receptor was named the GH secretagogue receptor (GHSR) (8). Besides the pituitary gland and hypothalamic areas that regulate GH release, the GHSR is expressed in brain centers that control appetite, pleasure, mood, biological rhythms, memory, and cognition (6, 9, 10).
Ghrelin and adenosine were identified as naturally occurring agonists for the orphan GHSR by fractionating and assaying animal tissue extracts in cell lines engineered to express the GHSR (11-13). Administration of ghrelin and adenosine to rats stimulates feeding, but only ghrelin stimulates GH release (12, 14). Accordingly, ghrelin more closely mimics MK-0677, and it was assumed that the GHSR is the ghrelin receptor. However, evidence has been presented to suggest the existence of receptor subtypes (15). Furthermore, as a 28-aa peptide containing a unique octanoyl modification (11), ghrelin is structurally different from MK-0677. Although molecular modeling studies that compared structural features assigned from proton NMR of MK-0677 and other synthetic GHSR ligands illustrated certain similarities with ghrelin, these studies did not precisely predict the receptor-ligand binding characteristics (16). To directly investigate a potentially significant physiological relationship between ghrelin and GHSR, we generated Ghsr-null (-/-) mice.
Materials and Methods
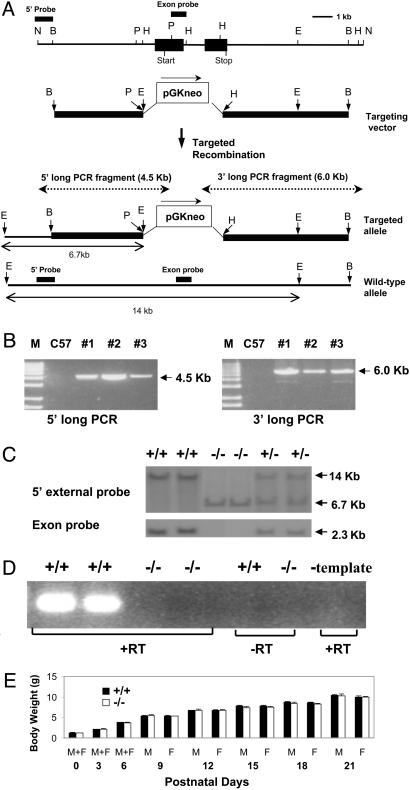
Generation of Ghsr Null Mice. In the targeting vector, a pGKneo cassette was used to replace a region from the PstI site at 5′ of the coding exon-1 to the HindIII site in the coding exon-2 (Fig. 1). The targeting vector was linearized by BamHI digestion and transfected into 129Sv embryonic stem (ES) cells by electroporation. The 1.1-kb NotI/BamHI fragment at the 5′ end of the genomic clone was used as the 5′ external probe to select positive ES clones in Southern analysis. The appropriately targeted ES cells were injected into blastocysts derived from C57BL/6J. Southern analysis was later used in genotyping the offspring of heterozygous parents. Ten micrograms of mouse DNA was digested with either EcoRI or HindIII, electrophoresed on 0.8% agarose gel, transferred to the membrane, and hybridized with either the 5′ external probe or the exon probe. When the 5′ external probe was hybridized with EcoRI-digested DNA, a 14-kb fragment was produced from the wild-type allele. Because of the presence of an EcoRI site in the PGKneo cassette, a 6.7-kb fragment was produced from the mutant allele. To ensure the deletion of the coding region, an exon probe (a 1.1-kb PstI/HindIII fragment encoding part of the first coding exon) was used. When HindIII-digested DNA was hybridized with the exon probe, the 2.3-kb HindIII fragment corresponding to the Ghsr exon was detected in Ghsr wild type (+/+) and heterozygote (+/-), but not in homozygote (-/-) mice. To confirm the precise integration of mutant fragment at both insertion sites, the long-template PCR was performed by using Expand Long Template PCR System (Boehringer Mannheim). For the 5′ long PCR fragment (4.5-kb): forward primer, 5′-GGGATGGGCACATGAATCTTTCTGGAAAGGGGG; reverse primer, 5′-GGAAAAGCGCCTCCCCTACCCGGTAGAATTC. For the 3′ long PCR fragment (6.0-kb): forward primer, 5′-CTTCTATCGCCTTCTTGACGAGTTCTTCTGAGG; reverse primer, 5′-GACCATCAGAGAGGATACACAGATTGGAAGC.
Fig. 1.
(A) Restriction enzyme map of a mouse Ghsr genomic DNA clone, and the strategy for deriving Ghsr-/- mice by homologous recombination. The filled boxes represent the two coding exons. Restriction enzyme sites: N, NotI; B, BamHI; P, PstI; H, HindIII; E, EcoRI. The dotted lines show the long-template PCR products. (B) Long-template PCR analysis of F1 founder mice. M, Marker; C57, C57BL/6J mice for control; 1, 2, and 3 are the three founder mice. (C) Southern blot analysis of the offspring from heterozygous mating. (Upper) DNA was digested with EcoRI then hybridized with 5′ probe. (Lower) DNA was digested with HindIII then hybridized with exon probe. The 5′ probe and exon probe are shown in A. +/+, wild-type; -/-, homozygote; +/-, heterozygote. (D) GHSR mRNA expression in pituitary as determined by RT-PCR. +RT, with reverse transcriptase; -RT, without reverse transcriptase; -template, with reverse transcriptase but without RNA template. (E) Body weights of pups at birth and postnatal days. Before sex can be distinguished (from day 0 to day 6), n = 48 for -/- and 51 for +/+. From day 9 to weaning, body weight data from male and female pups were collected separately.
RT-PCR Analysis. Total RNA was isolated from individual mice. Twenty nanograms of total RNA was used in semiquantitative RT-PCR. The intron flanking primers are: forward, 5′-TATGGGTGTCGAGCGTCTT (in coding exon 1); reverse, 5′-GAGAATGGGGTTGATGGC (in coding exon 2).
Hormone Assays. All hormone assays were done in mature male mice. In fasting experiments, the fasting time was 48 h from 8 a.m. to 8 a.m. To measure GH, mice were injected i.p. with pentobarbital (50 mg/kg body weight); 15 min later, 100 μl of physiologic saline, either with or without 10 μg of ghrelin (Phoenix Pharmaceuticals, St. Joseph, MO), was injected i.p. Blood was collected by retro-orbital bleeding at 0, 5, and 15 min after saline/ghrelin. GH was measured in plasma samples by using rat GH EIA kit (American Laboratory Products, Windham, NH). Ten micrograms of MK-0677 (Merck Research Laboratories) and 10 μg of human GH-releasing hormone (GHRH, Phoenix Pharmaceuticals) were also tested in similar experimental setup. For other assays, blood was collected by either retro-orbital bleeding or tail vein bleeding. Serum was collected for measurements of: ghrelin (rat ghrelin RIA kit, Phoenix Pharmaceuticals); leptin (mouse leptin RIA kit, Linco Research Immunoassays, St. Joseph, MO); insulin (sensitive rat insulin RIA kit, Linco Research Immunoasays); insulin-like growth factor 1 (IGF-1) (rat IGF-1 RIA kit, Diagnostic Systems Laboratories, Webster, TX).
Effects of Acute Administration of Ghrelin on Appetite. Mice were injected i.p. with 100 μl of physiologic saline first and food intake was measured at 0.5 h after the saline injection (0-0.5 h). Later, the same mice were injected with 100 μl of physiologic saline containing 10 μg of ghrelin. Food intake was measured at 0.5 h and 1.0 h after the ghrelin injection to get the food intake of the first 0.5 h (0-0.5 h) and the second 0.5 h (0.5-1.0 h). One hour after the first ghrelin injection, ghrelin was reinjected, and the food intake during the next 0.5 h (0-0.5 h) was measured.
Body Composition. Bone density (bone mineral density and bone mineral content) and body composition (fat %) were measured by using the noninvasive technique of dual energy-x-ray absorptiometry (Lunar PIXI Mouse densitometer, Lunarcorp, Madison, WI). Fat and lean body mass were also measured by using a Minispec mq benchtop NMR spectrometer (Bruker Instruments) at the Yale Mouse Metabolic Phenotyping Center. The fat represents total fat, independent of where it is localized. The intensities of the fat, muscle, and free fluid were calculated automatically from the time domain [1H]NMR signals by the instrument software and expressed in units of grams.
Body Weight and Food Intake Under Ad Libitum Condition. The experimental mice were individually caged and provided with ad libitum access to water and regular chow. Body weight and food intake were measured every other week at the same time of the day.
The Evaluation of Appetite During Fasting and Refeeding. The mice (12 weeks old) were weighed and chow was removed. Twenty-four hours later, the animals were weighed, then provided with a weighed amount of chow, and food intake was measured at 1, 2, 4, 6, 24, and 48 h; body weights were measured at 24 and 48 h.
Animals and Data Analysis. All experiments were conducted on N3 mice by backcrossing F1 mice onto C57BL/6J mice for two generations. In all experiments, Ghsr-null mice (Ghsr-/-) were compared to wild-type littermates (Ghsr+/+). Mice were kept in a standard 7 a.m. to 7 p.m. light cycle (light off at 7 p.m.) facility, and fed with regular mouse chow. Mice were housed one per cage during the experiments. Data are presented as mean ± SEM in all figures. The number of subjects is indicated by n. Significant differences between the groups were evaluated by different ANOVA tests using sigmastat 3.0 software. Two-way ANOVA test was used for Figs. 1E, 2, 3, 4, and 5. Figs. 1E and 4 A and B were also evaluated by two-way repeated-measures ANOVA. P < 0.05 was considered as statistical significance.
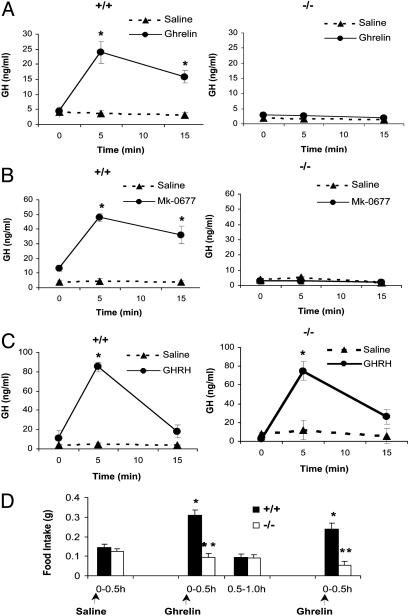
Fig. 2.
(A) The effect of ghrelin administration on GH-release in Ghsr+/+ and Ghsr-/- mice (n = 10 for ghrelin and n = 4 for physiologic saline). At 5 min and 15 min after ghrelin injection, an asterisk shows P < 0.001 saline vs. ghrelin in +/+, but P > 0.05 in -/-. At 5 min and 15 min, P < 0.001 ghrelin-injected +/+ vs. -/-.(B) The effect of MK-0677 administration on GH-release in Ghsr+/+ and Ghsr-/- mice (n = 5 for MK-0677 and n = 3 for physiologic saline). At 5 min and 15 min after MK-0677 injection, an asterisk shows P < 0.001 saline vs. MK-0677 in +/+, but P > 0.05 in -/-. At 5 min and 15 min, P < 0.001 MK-0677-injected +/+ vs. -/-.(C) The effect of hGHRH administration on GH-release in Ghsr+/+ and Ghsr-/- mice (n = 3 for both hGHRH and physiologic saline). At 5 min after hGHRH injection, an asterisk shows P < 0.001 saline vs. hGHRH in both +/+ and -/-. GH release was stimulated in both +/+ and -/-. (D) Effects of ghrelin administration on food intake in Ghsr+/+ and Ghsr-/- mice. Mice were injected i.p. with 100 μl of physiologic saline first and food intake was measured at 0.5 h after the saline injection (0-0.5 h). Later, the same mice were injected with 100 μl of physiologic saline containing 10 μg of ghrelin (arrow in the middle). Food intake was measured at 0.5 h and 1.0 h after the ghrelin injection to get the food intake of the first 0.5 h (0-0.5 h) and the second 0.5h (0.5-1.0 h). One hour after the first ghrelin injection, ghrelin was reinjected (arrow on the right) and the food intake of the first 0.5 h (0-0.5 h) was remeasured. Food intake was significantly increased in the first 0.5 h after each ghrelin injection in +/+ (*, P < 0.001 ghrelin vs. saline). There were no changes in food intake in -/- after ghrelin injection. For the second 0.5 h (0.5-1.0 h) of ghrelin injection, P > 0.05 saline vs. ghrelin for both +/+ and -/-. Arrows show the saline or ghrelin injections. n = 10. Double asterisk indicates P < 0.001 comparing +/+ vs. -/- during the first 0.5 h after each ghrelin injection. The same experiment was repeated three times on different days between 9 a.m. and 12 a.m. under ad libitum condition (n = 10 in each experiment).
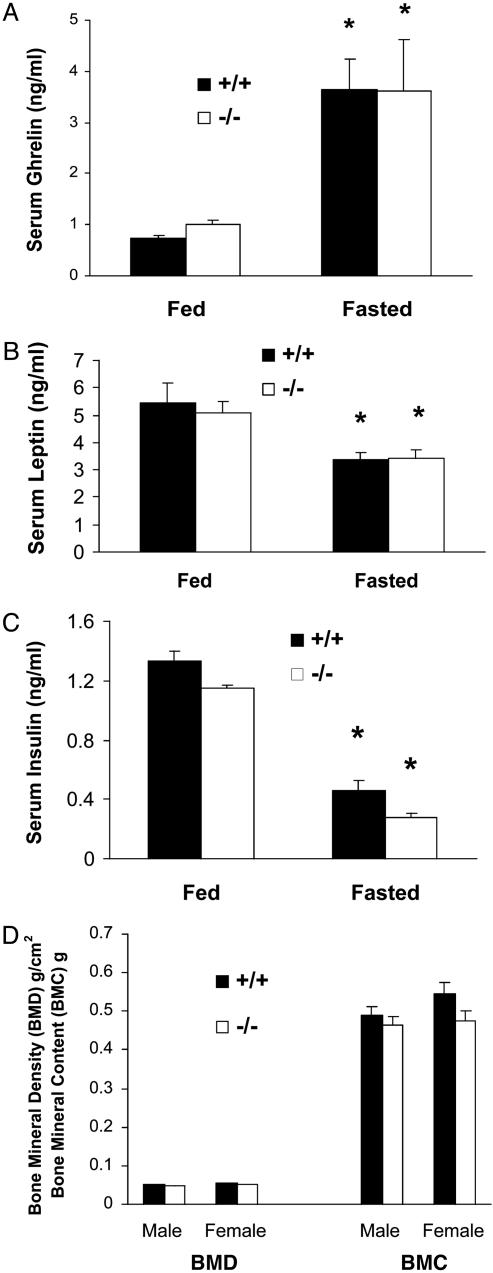
Fig. 3.
(A) Fed and fasted serum ghrelin in 24-week-old male mice (n = 7, asterisk indicates P < 0.05 fed vs. fasted; P > 0.05 +/+ vs. -/- in both fed and fasted states). (B and C) Fed and fasted serum leptin and insulin of 20-week-old male mice (n = 7, asterisk indicates P < 0.05 fed vs. fasted for both leptin and insulin; P > 0.05 +/+ vs. -/- in both fed and fasted states). (D) Bone density and content of 24-week-old mice (n = 7, P > 0.05 +/+ vs. -/-). BMD, bone mineral density; BMC, bone mineral content.
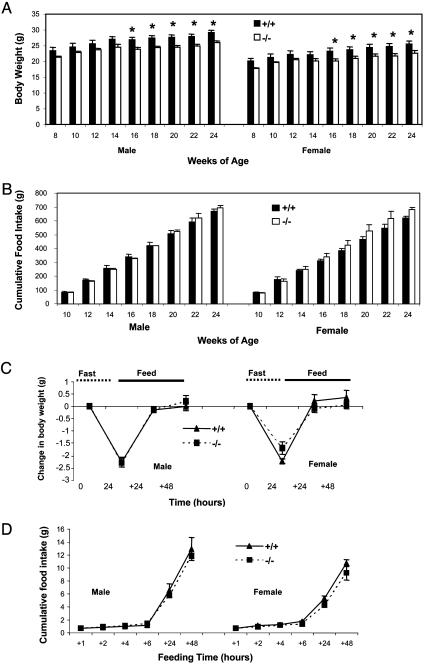
Fig. 4.
(A) Body weights of 8- to 24-week-old mice. The data were collected every other week (n = 7, asterisk indicates P < 0.05 +/+ vs. -/- at 16-24 weeks). (B) Cumulative food intakes from 8 to 24 weeks of age (n = 7, P > 0.05 +/+ vs. -/- at all data points). (C and D) Changes in body weight and food intake during fasting and refeeding. Twelve-week-old mice were fasted for 24 h and then allowed to eat. Body weight was measured before and after the fasting, and at 24 h and 48 h after the food was given. Cumulative food intake was measured at 1, 2, 4, 6, 24, and 48 h after food was given (n = 6, P > 0.05 +/+ vs. -/-).
Fig. 5.
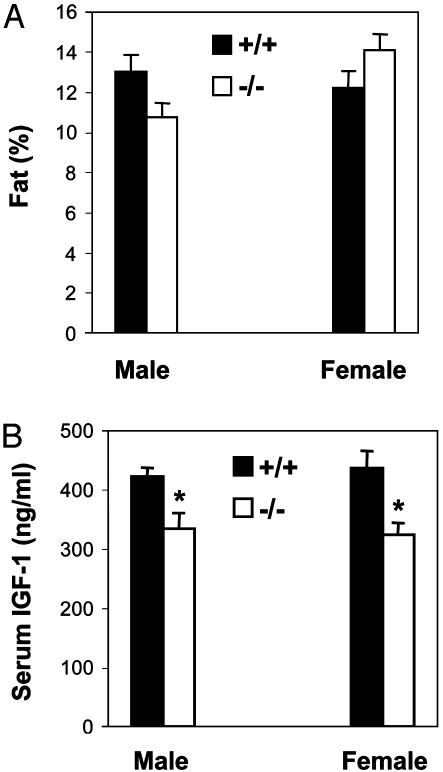
Body composition (fat %) and serum IGF-1 of the mice in Fig. 4 A and B at 24 weeks of age. (A) Fat %, n = 7, P > 0.05 +/+ vs. -/-.(B) Serum IGF-1, n = 7, an asterisk indicates P < 0.05 +/+ vs. -/-.
Results and Discussion
To generate Ghsr-null mice, a 15.3-kb Ghsr mouse genomic phage clone was used to characterize the Ghsr locus. A pGKneo cassette was inserted into the Ghsr locus to replace the entire coding exon 1 and part of the coding exon 2. The targeting vector consisted of 3.6- and 5.7-kb homologous regions of genomic DNA at 5′ and 3′ of the selection cassette, respectively (Fig. 1 A). After initial PCR analysis of all agouti mice, three positive F1 founder mice were identified. To ensure the precise integration of the targeting fragment at targeted allele, long-template PCR was next performed to amplify PCR products from the upstream of 5′ insertion site to the neo cassette (4.5 kb) and the downstream of 3′ insertion site to the neo cassette (6.0 kb) (Fig. 1B). Mating of heterozygous mice produced progeny of all three genotypes. Southern blots of EcoRI-digested tail DNA are shown in Fig. 1C, illustrating the predicted-sized fragments in Ghsr+/+, +/-, and -/- mice. Deletion of the Ghsr was also confirmed by the lack of hybridization to a probe selective for Ghsr-coding exon 1. Confirmation of the genotype was also provided by RT-PCR analysis of RNA isolated from mouse pituitary glands using oligonucleotide primers selected to prime in Ghsr coding exons 1 and 2. Fig. 1D illustrates the predicted-sized RT-PCR product (326 bp) in mRNA isolated from Ghsr+/+, but not in Ghsr -/- mice.
The well characterized properties of acute ghrelin administration are its stimulatory effects on GH release, appetite, and fat deposition (11, 14, 17). Therefore, if the GHSR is the biologically relevant ghrelin receptor, we might anticipate that the Ghsr-null mice would exhibit an anorexic dwarf phenotype. However, the appearance of Ghsr-null mice cannot be distinguished from that of their wild-type littermates. RT-PCR analysis indicated that ghrelin is expressed broadly in peripheral tissues (18); however, total necropsy of null- and wild-type mice and evaluation of hematoxylin/eosin-stained paraffin sections of individual tissues show no significant differences between the two genotypes. It has also been proposed that ghrelin plays a role in testicular (19) and placental function (20), but breeding of Ghsr heterozygous mice produced normal size litters with normal Mendelian distribution in genotype and sex. Furthermore, the homozygous litters produced by null parents showed no difference in body weight compared to wild-type litters of wild-type parents at birth and postnatal days (Fig. 1E). Collectively, these observations suggest that, if the GHSR is the ghrelin receptor, the physiological role of ghrelin is subtle.
Acute administration of ghrelin to wild-type animals stimulates GH release (11). To test whether ghrelin's effect on GH was mediated by the GHSR, we compared the effects of exogenous ghrelin in wild-type and Ghsr-null mice. Serum GH levels were measured in each mouse before ghrelin treatment, and at 5 and 15 min after treatment with vehicle or ghrelin. It is clear from the results in Fig. 2A that, in contrast to the response in wild-type mice, ghrelin fails to stimulate GH release in Ghsr-null mice, which shows unambiguously that the stimulatory effect of ghrelin on GH release is mediated by the GHSR. The GH-stimulatory effect of MK-0677 was also tested. Similar to that of ghrelin, GH release was only detected in wild-type mice, but not in Ghsr-null mice (Fig. 2B); therefore, the biological effects of ghrelin and MK-0677 on GH release are mediated by the GHSR.
Both GHSR agonists and GHRH stimulate GH release, but it is unknown whether these two signal pathways are dependent or independent. To test whether the GHRH-GH pathway remains functional in the absence of the Ghsr, we tested the stimulatory effect of GHRH in Ghsr-null mice. In contrast to treatment with ghrelin and MK-0677, GHRH stimulated GH release in both wild-type and null mice (Fig. 2C). These data show that the activity of GHRH does not depend on Ghsr expression.
Another well characterized property of ghrelin is its acute stimulatory effect on appetite (14, 17). To investigate whether the GHSR is indeed the ghrelin receptor that controls appetite, we compared food intake after ghrelin administration. Fig. 2D illustrated food intake in Ghsr-null and wild-type littermates that were treated in parallel with vehicle or 10 μg of ghrelin. We selected a dose of 10 μg per mouse because this dose produced serum ghrelin levels in the range observed in fasted mice (data not shown). Food intake was measured 30 min and 60 min after the first ghrelin injection. In wild-type mice, the 30-min food intake was unchanged after i.p. saline (0-0.5 h), but increased during the 30 min (0-0.5 h) immediately after i.p. ghrelin treatment (P < 0.001). During the second 30 min (0.5-1 h), food intake returned to control levels, which reflects the short half-life of ghrelin. After a second injection of ghrelin, feeding was again stimulated. The duration and level of response to this dose of ghrelin was similar to that reported previously (17). In contrast to wild-type mice, ghrelin treatment did not influence food intake in Ghsr-null mice. These results were confirmed by experiments repeated 24 h later, and then 7 days later. Hence, stimulation of appetite by ghrelin is reproducible and depends on expression of the Ghsr.
Having established that the GHSR is the ghrelin receptor involved in the regulation of GH release and appetite, we investigated the metabolic characteristics of the Ghsr-null mice. It has been reported that reciprocal relationships exist between ghrelin and leptin and between ghrelin and insulin, during feeding and fasting (21, 22). Therefore, we measured the effects of fasting on ghrelin, leptin, and insulin levels in Ghsr-null mice and wild-type littermates. Remarkably, a similar increase of ghrelin was observed in both genotypes during fasting (Fig. 3A), illustrating that serum levels of ghrelin are not regulated by the GHSR. Fig. 3 B and C illustrates that fasting causes a parallel decline in leptin and insulin levels in both wild-type and null littermates, which suggests that ghrelin does not regulate leptin and insulin concentrations via the GHSR in both fed and fasted states.
Ghrelin is suggested to function as an antagonist of leptin on hypothalamic neurons (23), and because leptin action on the hypothalamus is reported to reduce bone density in rodents (24), we investigated whether Ghsr-null mice might exhibit reduced bone density. Fig. 3D shows that both bone mineral density and bone mass are comparable in Ghsr wild-type and null littermates, suggesting that the lack of a ghrelin receptor does not compromise significant bone growth. To more definitively evaluate the potential effects of ghrelin on bone during aging, comprehensive histological and morphological analysis will be carried out in isogenic strains of Ghsr-null mice.
A link between ghrelin and obesity has been made through the observations that, in obese humans who underwent gastric bypass surgery, ghrelin production declined in parallel with sustained weight loss and reduced appetite (25, 26). There is also a conflicting report that bypass surgery has no effect on ghrelin levels; the weight loss appeared to be obtained independently by the surgery (27). Recent studies show that ghrelin binds to terminals of neuropeptide Y (NPY)/Agouti-related protein (AGRP) neurons, and that a population of hypothalamic ghrelin-synthesizing neurons project to these terminals and modulate γ-aminobutyric acid (GABA) currents that are involved in appetite stimulation and corticotropin-releasing factor (CRF) release (28). To address whether a ghrelin/GHSR interaction is related to obesity, growth curves and food intake of Ghsr-null mice were monitored. The body weights of null mice were modestly lower than that of wild-type mice (P < 0.05) from 16 to 24 weeks of age (Fig. 4A). Although differences in body weight did not reach significance until the mice were 16 weeks old, the trend was present in younger animals. There was no significant difference in cumulative food intake (Fig. 4B) or biweekly food intake (data not shown) in 16- to 24-week-old Ghsr-/- mice compared to their wild-type littermates.
Ghrelin administration causes an acute increase in appetite, and serum ghrelin is up-regulated during fasting (17, 22), suggesting that ghrelin might be involved in fasting-induced hyperphagia. Interestingly, our data (Fig. 3A) showed that fasting increased serum ghrelin levels in Ghsr -/- mice as well. To further evaluate whether ghrelin is involved in reflex hyperphagia, we fasted the mice for 24 h, then refed them. The changes in body weight and food intake were identical in wild-type and Ghsr-/- littermates (Fig. 4 C and D). We also observed that there was no significant difference in short period (0.5 h, 1.0 h, and 2.0 h) food consumption after either 24 h or 48 h of fasting (data not shown). Our data show that the absence of the Ghsr has no effect on appetite, suggesting that ghrelin is not an essential orexigenic factor.
The reduced body weights of the Ghsr-null mice in Fig. 4A were not explained by either reduced bone density (Fig. 3D) or reduced food intake (Fig. 4B). Ghrelin has been suggested to be involved in fat utilization and deposition (17, 29), so we questioned whether the body composition of Ghsr-null mice is different from that of wild-type mice. Fig. 5A shows that, by peripheral instantaneous x-ray imager (PIXI) densitometry, there was no significant difference in fat ratio between the two genotypes. Fat and muscle mass were also determined by using a Minispec mq benchtop NMR spectrometer (Bruker Instruments). Although both fat and muscle content were found to be slightly less in Ghsr-null mice than in wild-type mice, these differences were not statistically significant (Table 1). In summary, our NMR data provided no clear explanation for the modestly lower weight exhibited by the Ghsr-null mice.
Table 1. Body composition analysis of Ghsr-null mice by NMR spectroscopy.
| Body weight | Fat | Muscle | Free fluid | |
|---|---|---|---|---|
| +/+ | ||||
| Mean, g | 31.33 | 4.35 | 22.54 | 0.52 |
| SEM, g | 1.2 | 0.59 | 0.61 | 0.05 |
| −/− | ||||
| Mean, g | 28.14 | 3.33 | 21.29 | 0.71 |
| SEM, g | 0.54 | 0.46 | 0.46 | 0.1 |
| P, n = 7 | 0.0451 | 0.1837 | 0.1209 | 0.1995 |
Because GHSR positively regulates levels of GH and IGF-1 (1), we predicted that the levels of these anabolic hormones would be lower in Ghsr-null mice. If GH and IGF-1 were lower, muscle mass and bone mass would be reduced; consequently, the modest reduction in body weights might be explained by subtle alterations in body composition caused by lower GH and IGF-1. The physiological profile of GH release is pulsatile; to make comparisons of the amplitude of GH pulses, sequential blood samples should be collected from a conscious animal at 10-min intervals for at least 12 h, which, to our knowledge, has never been accomplished in the mouse. However, serum IGF-1 does not exhibit pulsatility, and under conditions of similar nutritional status, reflects the basal GH profile. A comparison of IGF-1 levels showed that indeed IGF-1 was lower in the Ghsr-null mice (Fig. 5B, P < 0.05). Consequently, we speculate that the modestly lower body weight exhibited by the Ghsr-null mice is explained by subtle reductions in both muscle and bone mass, which when measured individually do not reach statistical significance.
It has been reported that long-lived Ames dwarf mice, which have reduced IGF-1 levels and are deficient in GH, prolactin, and thyroid stimulating hormone, have lower body temperature (30). We tested the rectal temperature of fed and 24- and 48-h fasted mice by using a temperature monitoring system from Indus Instruments (Houston, TX) and found no difference in core body temperature between null and wild-type mice, which suggests that, in contrast to the dwarf mice, the metabolic rate of the Ghsr-null mice is normal.
Our results with the Ghsr-null mice are consistent with earlier observations with long-acting ghrelin mimetics (1), but challenge the popular belief that ghrelin receptor null mice would have an anorexic dwarf phenotype. The anabolic effects of chronically stimulating this pathway were illustrated by increases in lean, but not fat, mass in obese subjects (7) and by the beneficial effects observed in treatment of a catabolic state (31). During aging, when ghrelin levels fall, the amplitude of GH pulsatility declines and serum IGF-1 levels drop (32). Restoration of depleted ghrelin levels would require either constant infusion of ghrelin or chronic treatment with a long-acting ghrelin mimetic. Indeed, chronic treatment of old animals with a ghrelin mimetic restores the physiology of the GH/IGF-1 axis to that of young adults (6). The observations that IGF-1 levels were lower and body weight was modestly reduced in Ghsr-null mice supports our early hypothesis that the GHSR is an enhancer of function (1), and is consistent with observations that ghrelin mimetics produce a sustained increase in the electrophysiological activity of hypothalamic arcuate neurons (33). We speculate that ghrelin enhances function of the GH/IGF-1 axis by modulating the “gain” or “set-point” of GHRH neurons.
The results of experiments in Ghsr-null mice show unambiguously that the GHSR is the physiologically relevant receptor controlling ghrelin's stimulatory effects on GH secretion and appetite. Because the appearance of Ghsr-null and wild-type mice is similar, it is unlikely that ghrelin plays a dominant role in determining growth and body composition. This conclusion is subject to the caveat that alternative pathways might compensate for the inability of the Ghsr-null mice to respond to ghrelin. Nevertheless, it seems unlikely that regulation of growth and appetite would be subject to equivalent compensation, and that ghrelin antagonists would be broadly efficacious antiobesity agents.
Acknowledgments
We thank Dr. Kevin Behar at Yale Mouse Metabolic Phenotyping Center for NMR body composition analysis (supported by National Institute of Diabetes and Digestive Kidney Diseases Grant U24 DK 59635) and Merck Research Laboratories for providing MK-0677. We thank Dr. Mark Asnicar for his valuable input, Adelina Gunawan for excellent technical assistance, Michael R. Honig for proofreading the manuscript, and Edith A. Gibson for preparing and editing the manuscript. We gratefully acknowledge the support of the National Institutes of Aging (Grants RO1AG18895 and RO1AG19230), the Hankamer Foundation, and the postdoctoral fellowship for Y.S. from Canadian Institutes of Health Research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GH, growth hormone; GHSR, GH secretagogue receptor; ES, embryonic stem; GHRH, GH-releasing hormone; IGF-1, insulin-like growth factor 1.
References
- 1.Smith, R. G., Van der Ploeg, L. H., Howard, A. D., Feighner, S. D., Cheng, K., Hickey, G. J., Wyvratt, M. J., Jr., Fisher, M. H., Nargund, R. P. & Patchett, A. A. (1997) Endocr. Rev. 18, 621-645. [DOI] [PubMed] [Google Scholar]
- 2.Momany, F. A., Bowers, C. Y., Reynolds, G. A., Chang, D., Hong, A. & Newlander, K. (1981) Endocrinology 108, 31-39. [DOI] [PubMed] [Google Scholar]
- 3.Smith, R. G., Cheng, K., Schoen, W. R., Pong, S.-S., Hickey, G. J., Jacks, T. M., Butler, B. S., Chan, W. W.-S., Chaung, L.-Y. P., Judith, F., et al. (1993) Science 260, 1640-1643. [DOI] [PubMed] [Google Scholar]
- 4.Smith, R. G., Pong, S.-S., Hickey, G. J., Jacks, T. M., Cheng, K., Leonard, R. J., Cohen, C. J., Arena, J. P., Chang, C. H., Drisko, J. E., et al. (1996) Rec. Prog. Horm. Res. 51, 261-286. [PubMed] [Google Scholar]
- 5.Patchett, A. A., Nargund, R. P., Tata, J. R., Chen, M.-H., Barakat, K. J., Johnston, D. B. R., Cheng, K., Chan, W. W.-S., Butler, B. S., Hickey, G. J., et al. (1995) Proc. Natl. Acad. Sci. USA 92, 7001-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman, I. M., Bach, M. A., Van, C. E., Farmer, M., Krupa, D. A., Taylor, A. M., Schilling, L. M., Cole, K. Y., Skiles, E. H., Pezzoli, S. S., et al. (1996) J. Clin. Endocrinol. Metab. 81, 4249-4257. [DOI] [PubMed] [Google Scholar]
- 7.Svensson, J., Lonn, L., Jansson, J.-O., Murphy, G., Wyss, D., Krupa, D., Cerchio, K., Polvino, W., Gertz, B., Boseaus, I., et al. (1998) J. Clin. Endocrinol. Metab. 83, 362-369. [DOI] [PubMed] [Google Scholar]
- 8.Howard, A. D., Feighner, S. D., Cully, D. F., Arena, J. P., Liberator, P. A., Rosenblum, C. I., Hamelin, M., Hreniuk, D. L., Palyha, O. C., Anderson, J., et al. (1996) Science 273, 974-977. [DOI] [PubMed] [Google Scholar]
- 9.Guan, X. M., Yu, H., Palyha, O. C., McKee, K. K., Feighner, S. D., Sirinathsinghji, D. J., Smith, R. G., Van der Ploeg, L. H. & Howard, A. D. (1997) Brain Res. Mol. Brain Res. 48, 23-29. [DOI] [PubMed] [Google Scholar]
- 10.Smith, R. G., Feighner, S., Prendergast, K., Guan, X. & Howard, A. (1999) Trends Endocrinol. Metab. 10, 128-135. [DOI] [PubMed] [Google Scholar]
- 11.Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H. & Kangawa, K. (1999) Nature 402, 656-660. [DOI] [PubMed] [Google Scholar]
- 12.Tullin, S., Hansen, B. S., Ankersen, M., Moller, J., Von Cappelen, K. A. & Thim, L. (2000) Endocrinology 141, 3397-3402. [DOI] [PubMed] [Google Scholar]
- 13.Smith, R. G., Griffin, P. R., Xu, Y., Smith, A. G., Liu, K., Calacay, J., Feighner, S. D., Pong, C., Leong, D., Pomes, A., et al. (2000) Biochem. Biophys. Res. Commun. 276, 1306-1313. [DOI] [PubMed] [Google Scholar]
- 14.Nakazato, M., Murakami, N., Date, Y., Kojima, M., Matsuo, H., Kangawa, K. & Matsukura, S. (2001) Nature 409, 194-198. [DOI] [PubMed] [Google Scholar]
- 15.Papotti, M., Ghe, C., Cassoni, P., Catapano, F., Deghenghi, R., Ghigo, E. & Muccioli, G. (2000) J. Clin. Endocrinol. Metab. 85, 3803-3807. [DOI] [PubMed] [Google Scholar]
- 16.Silva Elipe, M. V., Bednarek, M. A. & Gao, Y. D. (2001) Biopolymers 59, 489-501. [DOI] [PubMed] [Google Scholar]
- 17.Wren, A. M., Small, C. J., Abbott, C. R., Dhillo, W. S., Seal, L. J., Cohen, M. A., Batterham, R. L., Taheri, S., Stanley, S. A., Ghatei, M. A., et al. (2001) Diabetes 50, 2540-2547. [DOI] [PubMed] [Google Scholar]
- 18.Gnanapavan, S., Kola, B., Bustin, S. A., Morris, D. G., McGee, P., Fairclough, P., Bhattacharya, S., Carpenter, R., Grossman, A. B. & Korbonits, M. (2002) J. Clin. Endocrinol. Metab. 87, 2988. [DOI] [PubMed] [Google Scholar]
- 19.Tena-Sempere, M., Barreiro, M. L., Gonzalez, L. C., Gaytan, F., Zhang, F. P., Caminos, J. E., Pinilla, L., Casanueva, F. F., Dieguez, C. & Aguilar, E. (2002) Endocrinology 143, 717-725. [DOI] [PubMed] [Google Scholar]
- 20.Gualillo, O., Caminos, J., Blanco, M., Garcia-Caballero, T., Kojima, M., Kangawa, K., Dieguez, C. & Casanueva, F. (2001) Endocrinology 142, 788-794. [DOI] [PubMed] [Google Scholar]
- 21.Bagnasco, M., Kalra, P. S. & Kalra, S. P. (2002) Endocrinology 143, 726-729. [DOI] [PubMed] [Google Scholar]
- 22.Toshinai, K., Mondal, M. S., Nakazato, M., Date, Y., Murakami, N., Kojima, M., Kangawa, K. & Matsukura, S. (2001) Biochem. Biophys. Res. Commun. 281, 1220-1225. [DOI] [PubMed] [Google Scholar]
- 23.Traebert, M., Riediger, T., Whitebread, S., Scharrer, E. & Schmid, H. A. (2002) J. Neuroendocrinol. 14, 580-586. [DOI] [PubMed] [Google Scholar]
- 24.Ducy, P., Amling, M., Takeda, S., Priemel, M., Schilling, A. F., Beil, F. T., Shen, J., Vinson, C., Rueger, J. M. & Karsenty, G. (2000) Cell 100, 197-207. [DOI] [PubMed] [Google Scholar]
- 25.Cummings, D. E., Weigle, D. S., Frayo, R. S., Breen, P. A., Ma, M. K., Dellinger, E. P. & Purnell, J. Q. (2002) N. Engl. J. Med. 346, 1623-1630. [DOI] [PubMed] [Google Scholar]
- 26.Leonetti, F., Silecchia, G., Iacobellis, G., Ribaudo, M. C., Zappaterreno, A., Tiberti, C., Iannucci, C. V., Perrotta, N., Bacci, V., Basso, M. S., et al. (2003) J. Clin. Endocrinol. Metab. 88, 4227-4231. [DOI] [PubMed] [Google Scholar]
- 27.Holdstock, C., Engstrom, B. E., Ohrvall, M., Lind, L., Sundbom, M. & Karlsson, F. A. (2003) J. Clin. Endocrinol. Metab. 88, 3177-3183. [DOI] [PubMed] [Google Scholar]
- 28.Cowley, M. A., Smith, R. G., Diano, S., Tschop, M., Pronchuk, N., Grove, K. L., Strasburger, C. J., Bidlingmaier, M., Esterman, M., Heiman, M. L., et al. (2003) Neuron 37, 649-661. [DOI] [PubMed] [Google Scholar]
- 29.Tschop, M., Smiley, D. L. & Heiman, M. L. (2000) Nature 407, 908-913. [DOI] [PubMed] [Google Scholar]
- 30.Hunter, W. S., Croson, W. B., Bartke, A., Gentry, M. V. & Meliska, C. J. (1999) Physiol. Behav. 67, 433-437. [DOI] [PubMed] [Google Scholar]
- 31.Murphy, M. G., Plunkett, L. M., Gertz, B. J., He, W., Wittreich, J., Polvino, W. M. & Clemmons, D. R. (1998) J. Clin. Endocrinol. Metab. 83, 320-325. [DOI] [PubMed] [Google Scholar]
- 32.Rigamonti, A. E., Pincelli, A. I., Corra, B., Viarengo, R., Bonomo, S. M., Galimberti, D., Scacchi, M., Scarpini, E., Cavagnini, F. & Muller, E. E. (2002) J. Endocrinol. 175, R1-R5. [DOI] [PubMed] [Google Scholar]
- 33.Bailey, A. R. T., Smith, R. G. & Leng, G. (1998) J. Neuroendocrinol. 10, 111-118. [DOI] [PubMed] [Google Scholar]