Abstract
Background
The development of genetically modified pigs which lack the expression of alpha 1–3 galactosyl transferase, (GalT-KO pigs) has facilitated the xenogeneic transplantation of porcine organs and tissues into primates by avoiding hyperacute rejection due to pre-existing antibodies against the Gal epitope. However, antibodies against other antigens (anti-non-Gal antibodies), are found at varying levels in the pre-transplant sera of most primates. We have previously found that baboons with high levels of pre-transplant anti-non-Gal IgG, conditioned with a non-myeloablative conditioning regimen, failed to engraft following pig-to-baboon bone marrow transplantation [8]. Two baboons with low levels of pre-transplant anti-non-Gal IgG, conditioned with the same regimen, showed porcine bone marrow progenitors at 28 days following transplantation, suggesting engraftment. These baboons also showed evidence of donor-specific hypo-responsiveness. This observation led us to investigate the hypothesis that selecting for baboon recipients with low pre-transplant anti-non-Gal IgG levels might improve engraftment levels following GalT-KO pig-to-baboon bone marrow transplantation.
Methods
Five baboons, with low pre-transplant anti-non-Gal IgG levels, received transplantation of bone marrow cells (1–5 × 10^9/kg of recipient weight) from GalT-KO pigs. They received a non-myeloablative conditioning regimen consisting of low-dose total body irradiation (150cGy), thymic irradiation (700cGy), anti-thymocyte globulin (ATG) and tacrolimus. In addition, two baboons received Rituximab and Bortezomib (Velcade) treatment as well as extra-corporeal immunoadsorption using GalT-KO pig livers. Bone marrow engraftment was assessed by porcine-specific PCR on colony forming units (CFU) of day 28 bone marrow aspirates. Anti-non-Gal antibody levels were assessed by serum binding towards GalT-KO PBMC using flow cytometry (FACS). Peripheral macro-chimerism was measured by FACS using pig and baboon-specific antibodies and baboon anti-pig cellular responses were assessed by mixed lymphocyte reactions (MLR).
Results
As previously reported, two of five baboons demonstrated detectable bone marrow engraftment at four weeks after transplantation. Engraftment was associated with lack of an increase in anti–non-Gal IgG levels as well as cellular hypo-responsiveness towards pig. Three subsequent baboons with similarly low levels of pre-existing anti-non-Gal IgG showed no engraftment and an increase in anti-non-Gal IgG antibody levels following transplantation. Peripheral macrochimerism was only seen for a few days following transplantation regardless of antibody development.
Conclusions
Selecting for baboon recipients with low levels of pre-transplant anti-non-Gal IgG did not ensure bone marrow engraftment. Failure to engraft was associated with an increase in anti-non-Gal IgG levels following transplantation. These results suggest that anti-non-Gal-IgG is likely involved in early bone marrow rejection and that successful strategies for combating anti-non-Gal IgG development may allow better engraftment. Since engraftment was only low and transient regardless of antibody development, innate immune, or species compatibility mechanisms will likely also need to be addressed in order to achieve long term engraftment.
Keywords: xenotransplantation, bone marrow, anti-non-Gal, antibody, miniature swine, baboons
Introduction
There remains a large discrepancy between the number of patients awaiting transplantation and the available donor organs. Because of their size, favorable breeding characteristics and the similarity of many of their organ systems to those of humans, miniature swine are an attractive potential xenograft donor to overcome this limitation [17]. In addition, the strongest barrier to the transplantation of porcine organs into primates, due to pre-existing “natural” antibodies towards the galactosyl-α 1,3-galactose(Gal) epitopes present on pig but not primate cells [7], has now been overcome by the development of Gal transferase knockout (GalT-KO) pigs [11] Using GalT-KO pigs, hyperacute rejection has been avoided allowing graft survival of several months in baboons [12,24].
Nevertheless, xenogeneic transplantation continues to pose significant immunologic challenges compared to allotransplantation [25]. Mixed chimerism has been successfully used to induce immunologic tolerance in animal models of allotransplantation [1,19,20] and in a recent human clinical trial of kidney transplantation [10]. Mixed chimerism has also been used to induce transplant tolerance across concordant xenogenic barriers [3,18]. We have therefore investigated this approach as a potential means of inducing tolerance across the discordant pig-to-baboon barrier.
Our previous attempts to induce pig-to-baboon mixed chimerism using either whole bone marrow or mobilized peripheral blood progenitor cells (PBPC) resulted in transient chimerism with no evidence of a systemic effect on the anti-donor immunological response. [4,16,22]. In a recent study using an attenuated non-myeloablative conditioning regimen, we found that transient engraftment, associated with donor-specific hyporesponsiveness, was achieved in 2 baboons with low levels of pre-transplant non-Gal IgG but not in those with high pre-transplant IgG levels [8]. We therefore hypothesized that the high pre-transplant IgG levels precluded engraftment by clearing transplanted cells soon after infusion. In the present study we have tested whether selecting for recipients with low levels of pre-transplant anti-non-Gal IgG would allow engraftment, which would in turn maintain humoral unresponsiveness.
Methods
Animals
Recipients were Papio Hamadrayas baboons (n=5) of 5–10 kgs. (Manheimer Foundation, Homestead, FL, USA). In addition to the two baboons (B156 and B158) that were part of our previous study [7] we selected three baboons (B265, B270 and B284) with low pre-transplant anti-non-Gal IgG levels to make a total of five low pre-transplant IgG recipients. Bone marrow donors were SLA dd Massachusetts General Hospital (MGH) inbred GalT-KO miniature swine (N =5), weighing between 40 and 98 kg. All animal care was performed in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1996). The protocol was approved by the MGH subcommittee of research animal care.
Bone marrow harvest and transplantation
Under general anesthesia, GalT-KO miniature swine donors were sacrificed by exsanguination. Bone marrow was collected from long bones and vertebra, cut into fragments, agitated at room temperature to further release bone marrow cells, then filtered through 125u nylon mesh to remove bone fragments. Red cells were lysed with ACK lysing buffer and the resulting bone marrow cell pellet was washed and re-suspended in Hanks medium. The bone marrow cells were infused intravenously into conditioned baboons in two fractions on days 0 and 2. Bone marrow cells to be infused on day two were stored at 4° centigrade and washed one more time in Hanks buffer before infusion.
Conditioning regimen
Baboons were conditioned with a previously described non-myeloablative regimen [8]. The regimen consisted of T cell depletion with 150 cGy Total Body Irradiation (TBI), 700 cGy of Thymic Irradiation, 2 doses of a monoclonal anti-CD2 antibody (loCD2b), and one dose of 20 mg/kg rabbit anti-thymocyte globulin (ATG). Animals received a continuous infusion of tacrolimus maintenance therapy from day −5 to day 28, through indwelling intra-venous catheters, after which all immunosuppression was discontinued and the intravenous catheters removed. The dose of tacrolimus was 0.1–0.2 mg/kg/day to achieve a target blood level of 30–40 ng/ml. Splenectomy was performed on day −6, at the time of catheter placement. Two of five baboons (B265 and B284) underwent a modification of this regimen, receiving two doses of horse ATG (50 mg/kg/dose) instead of rabbit ATG and treatment with one dose of rituximab, 4 doses of Velcade on days −9, −6, 2 and 5 and extra-corporeal immunoadsorption on day 0, using the liver of the GalT-KO pig donor prior to bone marrow transplantation. In baboons receiving extra-corporeal immunoadsorption, splenectomy was performed on day 0 instead of day −6, to minimize the number of operative procedures conducted. All baboons received prostacyclin and heparin to prevent thrombotic microangiopathy, CMV prophylaxis with Ganciclovir and prophylactic cefazolin and levofloxacin.
Extra-corporeal immunoadsorption
Two baboons underwent extra-corporeal immunoadsorption using the donor liver to remove pre-existing anti-non-Gal antibodies on day 0 before bone marrow transplantation. A donor hepatectomy was performed, the liver flushed with Ringer’s lactate and connected to the recipient circulation, with the recipient blood routed from the abdominal aorta to the pig liver and then back through the donor infrahepatic vena cava to the recipient vena cava. Extra-corporeal immunoadsorption was performed for one hour. Splenectomy was performed during the same procedure, immediately prior to immunoadsorption.
Assessment of bone marrow engraftment
Bone marrow engraftment was assessed on day 28 bone marrow aspirates by pig-specific cytochrome B PCR of CFU growing on pig-specific media as previously described [8]. Briefly, mononuclear cells from bone marrow aspirates were plated at concentrations of 50,000 cells in 1.5 ml of methylcellulose-based medium (Methocult H4230; Stem Cell Technologies, Vancouver, British Columbia, Canada). To select for porcine cell-specific proliferation, the methylcellulose-based media was enriched with porcine-specific cytokines as described previously [8]. Following 10–14 days of incubation in 5% CO2 at 37°C, single colony forming units were individually picked for PCR analysis to detect the presence of porcine cytochrome B DNA, which is a reliable, pig-specific marker. PCR reactions using baboon GAPDH primers were performed as well, to control for quantity and quality of DNA template.
Assessment of peripheral blood and bone marrow chimerism
Pig cells were assessed in recipient peripheral blood as previously described [8,21] using biotinylated pig-specific antibodies which do not cross react with baboon followed by detection with Phycoerythrin StreptAvidin. They included: 1030H1-19 mouse anti-pig leukocyte (pan pig), 2.27.3a mouse anti-pig class I [16], 2.12.3 allele-specific, anti-pig class Id [8], anti-pig CD1, CD5, CD9, CD16, and CD172 [15] Double staining was performed using a FITC conjugated mouse anti-HLA-ABC at 1/5 dilution (BD Pharmigen/clone 46-2.6)
Assessment of anti-non-Gal antibody levels
Serum levels of anti-non-Gal baboon IgG and IgM antibodies were measured by Flow Cytometry. Briefly, 1×10^6 GalT-KO pig PBMC within 1–3 days of isolation, suspended in 100 ul of FACS media (Hanks Balanced Salt Solution in 0.1% Bovine Serum Albumin and 0.1% sodium azide), were incubated with 1/10 diluted baboon sera for 30 minutes at four degrees centigrade. Cells were then washed twice with FACS wash media and incubated with goat anti-human IgG or IgM secondary antibodies conjugated to FITC (invitrogen zymed) for 30 minutes at four degrees centigrade. After staining with secondary antibodies, cells were washed twice with FACS media, acquired on FACS Caliber, and analyzed using WinList analysis software for detection of mode fluorescence intensity in the FITC channel. Preliminary studies were done using several undiluted sera, 1/10 diluted sera and 1/100 diluted sera to give final sera dilutions of 1/10, 1/100 and 1/1000. We found the 1/100 final sera dilutions to give the most reliable and consistent estimation of overall antibody levels when used to stain 1 × 10^6 GalT KO PBMC suspended in 100 ul of media. This dilution was therefore used for all subsequent serum antibody analyses
Peripheral blood cell counts
Daily counts of lymphocyte numbers and cell phenotyping were performed by incubation of whole heparinized blood with anti-human CD3 (sp34 clone), CD20, CD4, CD8 antibodies (all from BD biosciences) that were previously validated for cross reaction with baboon. Stained whole blood was then lysed with ACK lysis buffer to remove red cells and data were acquired on a FACS caliber. Cell analysis was performed using FloJo software.
Cellular responses towards donor
Mixed lymphocyte reactions were set up against allogenic and GalT-KO pig stimulators. One week or two weeks after discontinuation of immunosuppression, cellular responses were tested against the same allogeneic stimulator and a donor matched GalT KO pig. Briefly, triplicates of responder PBMC at a concentration of 400 K/well were cultured together with equal numbers of irradiated (2500 cGy) baboon or GalT-KO stimulators in Aim V media (Invitrogen) for five days. Responders were then pulsed with 1 uCi/well of thymidine and incubated for 5 hours to allow proliferating cells to incorporate the thymidine. Responder cells were then harvested on a filter paper and the incorporated thymidine per well quantified using a beta counter to assess the levels of proliferation.
Results
All baboons had low pre-transplant IgG levels
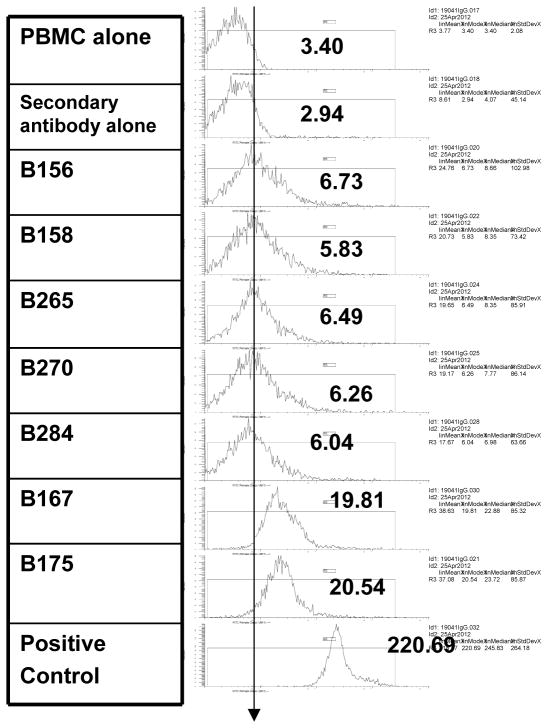
All baboons tested had detectable pre-transplant anti-non-Gal IgG above background (Figure 1). As previously reported [8] we found that two baboons with low pre-transplant anti-non-Gal IgG exhibited detectable CFUs suggestive of BM engraftment 28 days post xeno BMT. We selected three additional baboons with low pre-transplant anti-non-Gal IgG in an attempt to confirm these findings. A “low” anti-non-Gal IgG level was considered to be below a fluorescence intensity mode of 10, with all higher values considered “high”. (Figure 1). Neither the levels of pre-transplant anti-non-Gal IgM nor antibody-mediated cytotoxicity were considered in baboon selection, as these parameters had not shown a difference in the previous study in terms of engraftment or non-engraftment.
Figure 1.
All five baboons (B158, B156, B265, B270 and B284) used in this study, had low levels of anti- non-Gal IgG pre-transplant. Pre transplant anti-non-Gal IgG levels from baboons 167 and 175, are shown to demonstrate the varying levels of pre-transplant IgG in baboons available to us. The positive control sera were from a baboon that was known to be sensitized to pig and the background is shown using secondary goat anti-human IgG.
Bone Marrow Engraftment
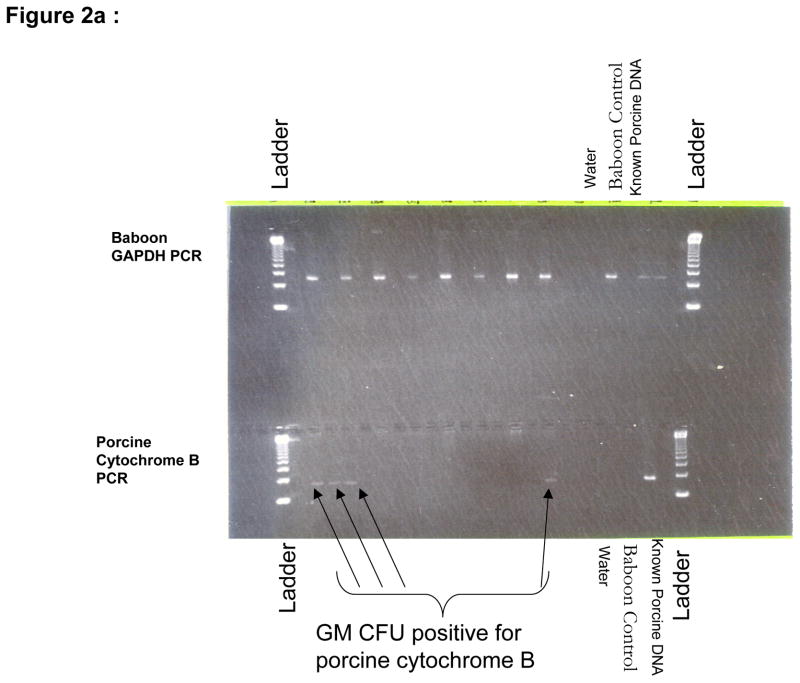
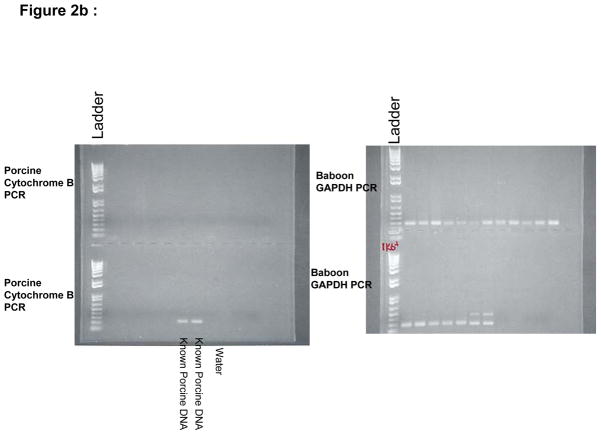
No porcine cells were detectable in day 28 bulk bone marrow aspirates by FACS. However, the CFU assays on growth media enriched with porcine-specific cytokines detected porcine colony forming units (i.e. early progenitors) in two of five baboons (B156 and B158) at day 28 (Figure 2a). The levels of porcine progenitors constituted a very low percentage of the bone marrow and swine CFU were not detectable in subsequent analyses on day 58 of surviving baboons (B158, B265, B284, and B270).
Figure 2.
a: Cytochrome B PCR of B158 day 28 bone marrow aspirate showing the presence of porcine DNA positive colony forming units (arrows) and b: Analysis of B270 day 28 bone marrow aspirate showing lack of porcine colony forming units. DNA grade water and a naïve baboon DNA are used as controls to ensure lack of contamination and known porcine DNA is used a positive control. Baboon GAPDH PCR is run alongside to ensure adequate DNA in the samples.
Anti-non-Gal antibody levels
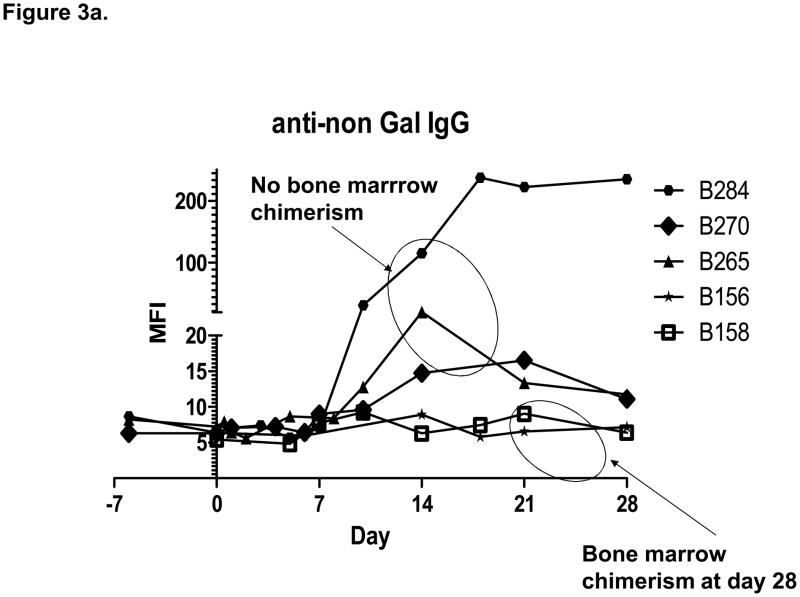
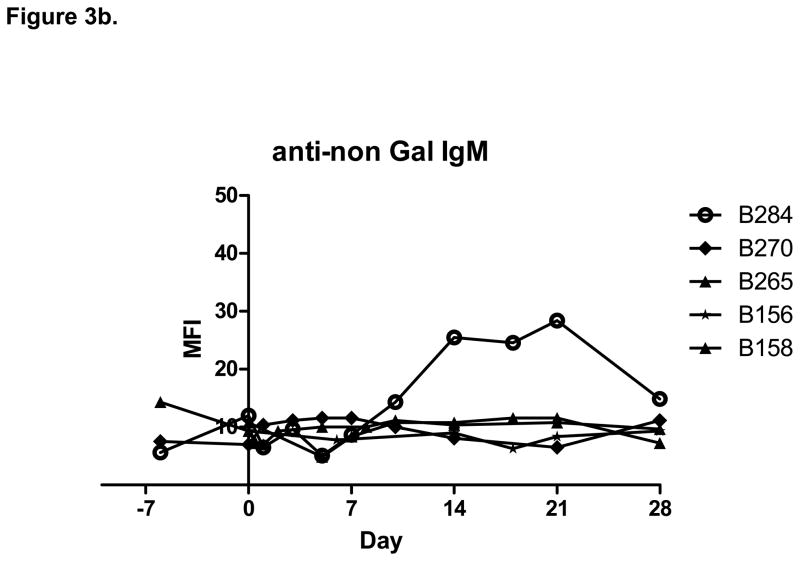
Starting at the same low pre-transplant level, two of five animals (B156 and B158) showed no increase in anti-non-Gal IgG following bone marrow transplantation, while three animals (B270, B265 and B284) showed an increase in anti-non-Gal IgG levels starting 10 to 14 days following bone marrow transplantation (Figure 3). Only one animal (B284) showed an increase in anti-non-Gal IgM following transplantation. Tacrolimus maintenance immunosuppression did not prevent the formation of anti-non-Gal antibodies. Two baboons (B265 and B284) developed increased anti-non-Gal IgG despite treatment with one dose of Rituximab and a course of peri-transplant Velcade.
Figure 3.
a. The increase in anti-non-Gal IgG following transplantation was associated with failure of bone marrow engraftment. b. Only one animal showed increase in anti-non-Gal IgM following bone marrow transplantation.
Peripheral chimerism
Peripheral donor chimerism was observed for up to four days following bone marrow transplantation. A dose effect per recipient weight was observed in the duration of donor chimerism (Table 1). Peripheral chimerism did not reappear during subsequent follow-up and was not a predictor of bone marrow engraftment. Chimeric pig cells circulating in baboon blood in the early post-transplant period expressed monocyte and granulocyte but not lymphocyte lineage markers.
Table 1.
Shows a summary of the experiment including conditioning regimen, amount of bone marrow received, as well as the immunological response to donor.
| Conditioning regimen received | Total number of Bone marrow cells/Kg recipient weight | Duration of detectable peripheral chimerism | Increase in anti-non-Gal IgG levels | Increase in anti-non-Gal IgM levels | Bone marrow Chimerism at day 28 | Invitro cellular response by MLR to GalT-KO pig PBMC after stopping immunosupression. | |
|---|---|---|---|---|---|---|---|
| B156 | Basic | 1 × 10^ 9 | Minutes | No | No | Detected | Not Tested |
| B158 | Basic | 7.5 × 10^ 8 | Minutes | No | No | Detected | Specific hyporesponse to donor |
| B270 | Basic | 1.6 × 10 ^ 9 | Minutes | Yes | No | Not detectable | Proliferation to GalT-KO pig donor |
| B265 | Enhanced | 4.56 × 10 ^ 9 | 2 days | Yes | No | Not detectable | Proliferation to GalT-KO pig donor |
| B284 | Enhanced | 5.3 × 10^9 | 4 days | Yes | Yes | Not detectable | Proliferation to GalT-KO pig donor |
Basic conditioning regimen: Total Body Irradiation (150 cGy), Thymic irradiation (700 cGy), 2 doses of loCD2b (4mg/kg/dose), 1 dose of Rabbit ATG (20 mg/kg/dose), Splenectomy and continuous tacrolimus to day 28.
Enhanced Conditioning regimen: [Total Body Irradiation (150cGy), Thymic irradiation, (700cGy) 2 doses of loCD2b (4mg/kg/dose), 2 doses of Horse ATG (50 mg/kg/dose), Splenectomy,] plus Rituximab (20 mg/kg/dose), Velcade (1.3mg/kg/m2 BSA), 1 hour Immuno-adsorption and continuous tacrolimus to day 28.
Peripheral blood counts
Some baboons experienced transient thrombo-cytopenia and mild anemia one to two weeks following transplantation, which resolved with minimal platelet and pRBC transfusions.
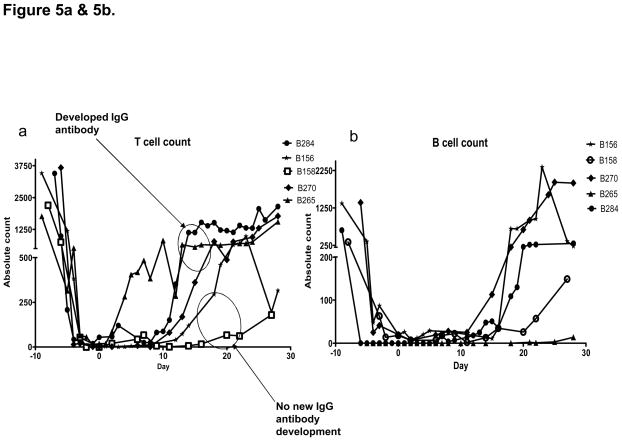
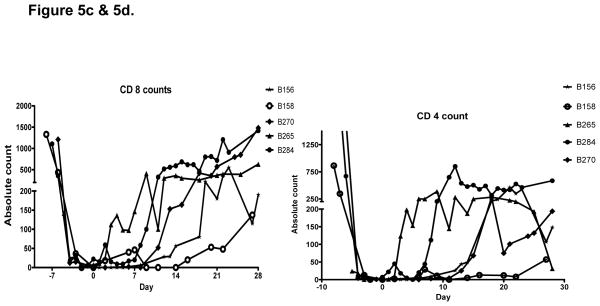
Baboon T cells (CD3 positive lymphocytes) were depleted prior to transplantation to below 100 cells/ul in all recipients. Recovery to pre-transplant levels was variable, starting as early as day 3 and as late as day 20. Engrafted baboons had slower T cell recovery compared to baboons which did not engraft. (Figure 5a). This difference was not sufficient to indicate a clear relationship of T cell numbers to bone marrow engraftment. Further phenotyping of T cells showed no significant difference in CD4 or CD 8 T cell numbers between engrafted and non-engrafted baboons. B cells (CD 20 positive lymphocytes) were also depleted in all animals to below 100 cells/ul and started to return to baseline between days 14 and 30 (Figure 5b). Little difference in peripheral B lymphocyte kinetics was observed among animals treated with or not treated with Rituximab. However, both groups received TBI (total body irradiation) and splenectomy which undoubtedly contributed to B cell depletion. Plasma cell numbers, which might potentially have been able to detect an effect of Velcade, were not monitored because peripheral CD138 positive plasma cell numbers were exceedingly low, even in untreated baboons (data not shown).
Figure 5.
Figure 5a. T cells and B cells were well depleted in all animals. T cells started to recover between days three and twenty with engrafted baboons showing slower T cell return. b. B cell recovery was between days 14 and 20 and showed no difference in rate of recovery between engrafted and non-engrafted animals.. Figure 5c and 5d. CD4 and CD 8 lymphocyte subsets showed similar kinetics among all animals.
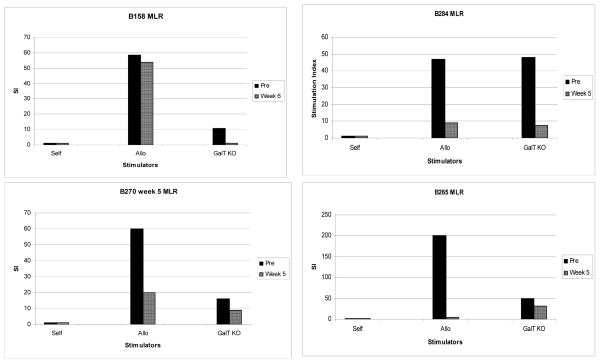
Cellular responses towards donor
All baboons demonstrated cellular proliferative responses by MLR to GalT-KO donor cells prior to transplantation. Following transplantation, after discontinuation of all immunosuppression on day 28, baboon anti-GalT-KO pig proliferative responses could be detected in non-engrafted animals (B265, B270 and B284) but not in the one engrafted animal tested (B158). The second engrafted animal (B156) died on day 28 and therefore MLR assay could not be performed.
Discussion
Mixed hematopoietic chimerism has successfully been used to induce allograft tolerance in animal studies as well in a recent human clinical trial [9,10,19,20]. Across the concordant xenogeneic barrier, mixed chimerism has been used to achieve tolerance in rat-to-mouse and cynomolgus monkey- to-baboon models [3,18]. We have therefore attempted to use of this modality for inducing tolerance across the pig-to-baboon model, which is likely the most relevant model for possible future human clinical xenotransplantation.
In our previous studies, we have reported evidence of porcine bone marrow microchimerism for up to 300 days using Gal positive pigs [16]. Evidence of microchimerism was however not associated with immune hypo-responsiveness towards pig. Using GalT-KO pig bone marrow and mobilized peripheral progenitor cells, we have achieved varying lengths of transient peripheral and bone marrow chimerism but again without showing donor-specific hyporesponsiveness [4,21].
More recently using a modified non-myeloablative conditioning regimen, we achieved transient bone marrow engraftment and donor-specific hyporesponsiveness in two baboons that had low levels of pre-transplant anti-non-Gal IgG but not in those with high pre-transplant anti-non-Gal IgG levels. [8]. We therefore tested the hypothesis that selecting for baboons with low levels of pre-existing anti-non-Gal IgG using this regimen would provide a reproducible way of achieving bone marrow engraftment and donor-specific hyporesponsiveness. In addition, we tested strategies to block anti-non-Gal antibody production following bone marrow transplantation.
Three baboons received the basic non-myeloablative regimen while two baboons received further treatment with Rituximab, Velcade and extra-corporeal immunoadsorption to further control humoral responses to donor. Two of three recipients with similarly low pre-transplant anti-non-Gal IgG levels, treated with the basic conditioning regimen, and having similar levels of T cell depletion, showed engraftment and donor-specific hyporesponsiveness at day 28. In one of three however, we observed a rise in anti-non-Gal IgG levels within the first two weeks following transplantation and a failure of bone marrow engraftment at day 28. This disproved the hypothesis that simply selecting for the levels of anti-non-Gal IgG would allow uniform engraftment. The reasons for this difference remain unclear.
We next tested strategies to avoid the production of anti-non-Gal antibodies following pig to baboon bone marrow transplantation. Rituximab has been used clinically in combination with IVIG or plasmapheresis to treat antibody-mediated rejection (AMR) [13]. In addition, a recently available drug (Velcade) has shown promising results in reversing anti-HLA antibodies in allotransplantation [6,14]. However, these strategies combined with extra-corporeal immunoadsorption were likewise unsuccessful in preventing the increase in anti-non-Gal IgG following transplantation. In fact, the only animal that developed increased anti-non-Gal IgM (B284) was one of the two that received treatment with rituximab and Velcade. This is the first time that an attempt to use Rituximab and Velcade to prevent anti-non-Gal IgG formation has been reported. It appears that, at least at the doses used, these drugs were not adequate to prevent anti-non-Gal IgG formation.
It might be suggested that extra-corporeal immunoadsorption failed to improve engraftment because it resulted in non-specific removal of thymoglobulin, thus allowing early return of T cells. However, we did not find any apparent association between the rates of T cell return among the animals that were and were not exposed to immunoadsorption. On the other hand, increased IgG was detected in the sera at the same time as B and T cell numbers began to return, suggesting that returning xeno-reactive cells might play a role in anti-non-Gal IgG development. The return of cellular proliferative responses towards GalT-KO cells at the five-week time-point in the animals that developed increased anti-non-Gal IgG further supports this possibility. Future studies to isolate and characterize xeno-reactive cells from the total B cells and T cells are warranted.
Furthermore, we have found in this study that the duration and level of the initial peripheral chimerism was not correlated to subsequent engraftment or anti-non-Gal IgG development. On the other hand, day 28 bone marrow engraftment was well correlated with donor-specific hyporesponsiveness. Unfortunately, we found that even in the animals that engrafted, chimerism was low and not detectable at subsequent time points tested. Other barriers to engraftment, for example the innate immune response, may be at play and need to be explored in order to improve the level and duration of engraftment.
Our anti-non-Gal antibody measurements represent the sum of the primate antibodies towards GalT-KO pig PBMC and we have not assessed the possibility that among these antibodies, antibodies to SLA might be of particular importance in preventing bone marrow transplantation. Proteomic studies by Byrne et al in baboons sensitized by Gal-KO pig heart transplants, identified several antigenic targets for anti-non-Gal antibodies but did not examine their individual immunological relevance [5] Previous studies from our laboratory have demonstrated no apparent relationship between anti-HLA titers and anti-swine titers in sera of high and low PRA individuals [2,23]. However, only alloantigenic specificities of HLA and not the actual molecules were examined in those studies, so the relevance of antibodies against constant portions of HLA molecules would have been missed. In future studies, we intend to assess the potential relevance of antibodies to constant portions of SLA molecules in preventing engraftment.
In conclusion, we have shown a consistent association between the rise of anti-non-Gal IgG and the failure to engraft following bone marrow transplantation across the pig-to-baboon barrier. A rise in anti-non-Gal IgG may be seen despite pre-selecting for low levels of pre-transplant anti-non-Gal IgG and despite treatment with rituximab and Velcade. Of course, this association does not prove a causative role of increased anti-non-Gal antibody in failure of engraftment since it is possible that failure of engraftment is caused by other species incompatibilities and that the rise of anti-non-Gal antibodies is secondary to sensitization concomitant with graft loss. Nevertheless, our work suggests the potential clinical relevance of anti-non-Gal IgG and raises the question of whether it is one of the major factors limiting the durability of bone marrow engraftment, even in the animals in which engraftment is successfully achieved.
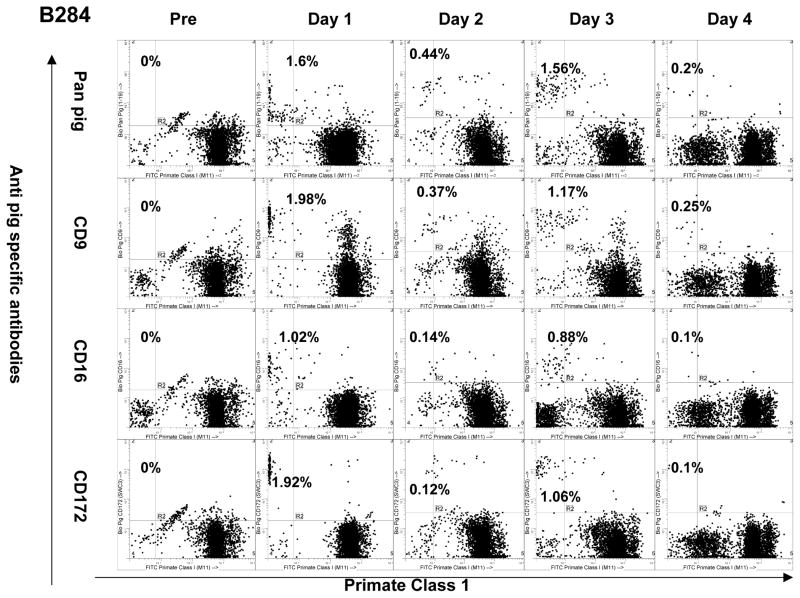
Figure 4.
Peripheral macrochimerism was detectable up to 4 days in B284. Circulating cells expressed porcine CD9, CD 16 and CD 172.
Figure 6.
The return of cellular responses towards GalT KO pig donor is seen in B265, B270 and B284 who did not show bone marrow engraftment but not in B158 who showed transient bone marrow engraftment at day 28.
Acknowledgments
The authors would like to thank Genentech for Rituxamib, Millennium Pharmaceuticals for Velcade, Dr. David Leonard and Abraham Matar for critical review of this paper and Rebecca A. Wark for expert editorial assistance. The authors would also like to acknowledge CO6RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine and NIH 5P01AI45897.
Footnotes
AUTHOR CONTRIBUTIONS
Fan Liang and Isaac Wamala contributed equally to the research design, performance of experiments, and writing of the paper.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Abe M. Mixed chimerism induces donor-specific T-cell tolerance across a highly disparate xenogeneic barrier. Blood. 2002;99:3823–3829. doi: 10.1182/blood.v99.10.3823. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomew A, Latinne D, Sachs DH. Utility of xenografts: lack of correlation between PRA and natural antibodies to swine. 1997 [Google Scholar]
- 3.Bartholomew AM, Powelson J, Sachs DH, et al. Tolerance in a concordant nonhuman primate model. Transplantation. 1999;68:1708–1716. doi: 10.1097/00007890-199912150-00014. [DOI] [PubMed] [Google Scholar]
- 4.Bühler L, Awwad M, Treter S, et al. Pig hematopoietic cell chimerism in baboons conditioned with a nonmyeloablative regimen and CD154 blockade. Transplantation. 2002;73:12–22. doi: 10.1097/00007890-200201150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008;15:268–276. doi: 10.1111/j.1399-3089.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 7.Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480–482. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]
- 8.Griesemer A, Liang F, Hirakata A, et al. Occurrence of specific humoral non-responsiveness to swine antigens following administration of GalT-KO bone marrow to baboons. Xenotransplantation. 2010;17:300–312. doi: 10.1111/j.1399-3089.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 10.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwaki K, Tseng Y-L, Dor FJMF, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery RA, Cozzi E, West LJ, Warren DS. Humoral immunity and antibody-mediated rejection in solid organ transplantation. Semin Immunol. 2011;23:224–234. doi: 10.1016/j.smim.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Morrow WR, Frazier EA, Mahle WT, et al. Rapid reduction in donor-specific anti-human leukocyte antigen antibodies and reversal of antibody-mediated rejection with bortezomib in pediatric heart transplant patients. Transplantation. 2012;93:319–324. doi: 10.1097/TP.0b013e31823f7eea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pescovitz MD, Book BK, Aasted B, et al. Summary of workshop findings for antibodies reacting with porcine T-cells and activation antigens: results from the Second International Swine CD Workshop; 1998. pp. 251–260. [DOI] [PubMed] [Google Scholar]
- 16.Sablinski T, Emery DW, Monroy R, et al. Long-term discordant xenogeneic (porcine-to-primate) bone marrow engraftment in a monkey treated with porcine-specific growth factors. Transplantation. 1999;67:972–977. doi: 10.1097/00007890-199904150-00007. [DOI] [PubMed] [Google Scholar]
- 17.Sachs DH. The pig as a potential xenograft donor. Vet Immunol Immunopathol. 1994;43:185–191. doi: 10.1016/0165-2427(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 18.Sharabi Y, Aksentijevich I, Sundt TM, Sachs DH, Sykes M. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J Exp Med. 1990;172:195–202. doi: 10.1084/jem.172.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sykes M, Shimizu I, Kawahara T. Mixed hematopoietic chimerism for the simultaneous induction of T and B cell tolerance. Transplantation. 2005;79:S28–9. doi: 10.1097/01.tp.0000153296.80385.e7. [DOI] [PubMed] [Google Scholar]
- 21.Tseng Y-L, Dor FJMF, Kuwaki K, et al. Bone marrow transplantation from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Xenotransplantation. 2004;11:361–370. doi: 10.1111/j.1399-3089.2004.00151.x. [DOI] [PubMed] [Google Scholar]
- 22.Tseng Y-L, Tseng Y-L, Sachs DH, Cooper DKC. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation. 2005;79:1–9. doi: 10.1097/01.tp.0000146504.73727.13. [DOI] [PubMed] [Google Scholar]
- 23.Wong BS, Yamada K, Okumi M, et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82:314–319. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 24.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2004;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y-G, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007;7:519–531. doi: 10.1038/nri2099. [DOI] [PubMed] [Google Scholar]