Abstract
Despite the documented importance of the protein hormone relaxin in reproduction in various mammalian species, the role of relaxin in human reproduction is poorly understood, largely because of the lack of studies in women or in suitable non-human primate models. Here we describe the establishment of a non-human primate model of early human pregnancy and its use in defining the actions of relaxin. Results demonstrate that relaxin exerts dramatic uterine effects including pronounced increase in uterine weight and stimulation of endometrial angiogenesis and resident endometrial lymphocyte number. In addition, relaxin decreases endometrial levels of matrix metalloproteinases 1 and 3 and increases levels of their endogenous inhibitor, tissue inhibitor of metalloproteinase 1, resulting in maintenance of endometrial collagen content. Relaxin significantly inhibits endometrial levels of estrogen receptor α, but not β, and of progesterone receptor isoforms A and B. The findings that relaxin stimulates new blood vessel formation and increases cytokine-containing lymphocyte number while maintaining endometrial connective tissue integrity are consistent with a significant role of relaxin in the establishment and/or maintenance of early pregnancy.
Relaxin is a 6-kDa protein hormone member of the insulin-like growth factor family present in circulation in women during the latter part of the menstrual cycle and throughout pregnancy (1). In many mammalian species, relaxin exerts pronounced effects on the female reproductive tract that are involved in the maintenance of pregnancy and successful parturition (2, 3). Relaxin is important for normal delivery in several mammalian species because of its marked rearrangement of reproductive tract connective tissue (2-4). Despite the documented importance of relaxin in various mammalian species, the role of relaxin in human reproduction is, to date, an important, yet unanswered, question. Elucidation of the role of relaxin in women has been hampered by the inability to perform studies in women and by the lack of studies performed in suitable primate models of human pregnancy. Here we describe the establishment of a non-human primate model of early human pregnancy and its use in defining the actions of relaxin. Results demonstrate that relaxin exerts dramatic uterine effects including pronounced increase in uterine weight, stimulation of endometrial angiogenesis and resident lymphocyte number, maintenance of endometrial connective tissue integrity, and inhibition of endometrial estrogen and progesterone action. These effects are the developmental changes that occur in the human uterus during the late secretory phase of the menstrual cycle and early pregnancy. These findings support the thesis that relaxin acts as an important factor in uterine accommodation to and maintenance of early pregnancy in women.
The dramatic species differences in the sources, secretion patterns, and target organs of relaxin have contributed greatly to the lack of understanding of the role of relaxin in human reproductive physiology. Results from various studies demonstrate that in rodents the ovary is the source of circulating relaxin, which in these species is secreted only during the second half of pregnancy (5, 6). Several studies provide evidence that in rats there is a major prelabor surge in circulating relaxin levels, and relaxin appears to be critical for cervical dilation (4, 6). Relaxin is necessary for normal delivery in pigs, which also have a major prepartum relaxin surge not seen in primates (7-9). In horses and rabbits, the placenta is the major source of relaxin, not the ovary (2, 3, 10). In rodents and guinea pigs, relaxin significantly increases the intrapubic ligament to enlarge the diameter of the pubis (2-4). This is not seen in primates (2, 8, 9).
In women, the source of circulating relaxin is the ovarian corpus luteum (11). Relaxin is detectable in maternal circulation during the late luteal phase of the menstrual cycle. Relaxin in peripheral plasma is detected ≈9 days after ovulation in normal women in nonconception cycles. In conception cycles, circulating levels of relaxin continue to rise to reach levels of ≈1 ng/ml in the first trimester. Levels drop ≈20% at the end of the first trimester and are then maintained throughout the pregnancy (1, 12, 13).
Recent data demonstrate that, in women, the endometrium also synthesizes relaxin. Relaxin-specific mRNA is detected in human endometrial stromal and glandular epithelial cells, and relaxin protein is secreted into the medium taken from primary cultures of these cell types (14). A role for relaxin in human endometrial function is also supported by findings that relaxin binds specifically and with high affinity to human endometrial cells (15). In addition, a large body of evidence exists to demonstrate that relaxin has definitive effects on human endometrial cells in vitro (14, 16-27). Relaxin stimulates production of several endometrial products including prolactin, glycodelin, insulin-like growth factor binding protein 1 (IGFBP-1) and vascular endothelial growth factor in progestin-primed human endometrial cells in vitro. Despite these findings, to our knowledge, no in vivo physiological studies had been performed to determine the effects of relaxin in a non-human primate model of human pregnancy.
In this work, ovariectomized rhesus monkeys were given exogenous estradiol and progesterone in a manner that simulated a human menstrual cycle. Animals were then randomized to two groups. One group was given exogenous human relaxin in amounts that achieved physiological circulating levels equivalent to those detected in early human pregnancy; the other group was given vehicle only. This model insured that relaxin was the only independent variable. Relaxin significantly stimulated uterine weight, endometrial lymphocyte and arteriole number, and tissue inhibitor of metalloproteinase 1 (TIMP-1) levels. Relaxin significantly inhibited endometrial levels of estrogen receptor α, both isoforms of the progesterone receptor, and matrix metalloproteinases (MMPs) 1 and 3. Thus, relaxin acts as a significant factor in the establishment and/or maintenance of early pregnancy.
Materials and Methods
Animal Protocol. All animal work was performed at the Center for Research in Reproductive Physiology at the University of Pittsburgh School of Medicine. The protocol was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. A monkey model of early human pregnancy was constructed and used in this study. Mature, virgin, female rhesus monkeys (Macaca mulatta; n = 8) were ovariectomized during the mid-luteal phase of the menstrual cycle. Seven days after ovariectomy, animals were implanted s.c. with Silastic capsules containing crystalline estradiol; 14 days later, animals received Silastic capsules containing crystalline progesterone to achieve concentrations that simulated the steroidal environment of early pregnancy. Seven days after insertion of the progesterone implants, animals were randomized to two groups that received daily s.c. injections of 0.5 ml with either (i) 150 μg of human H2 relaxin (kindly provided by Genentech) in 50% (wt/vol) polyvinylpyrrolidone (PVP) (relaxin-treated group) or (ii) PVP (control, vehicle-treated group) starting on day 21 for 21 days. Blood samples were collected by femoral venipuncture while animals were sedated with ketamine hydrochloride on the days indicated in Table 1. Sera were separated and assayed for estradiol, progesterone, and relaxin levels by specific immunoassays. After 21 days of relaxin (or vehicle) treatment, animals were killed. The entire uterus was removed from each animal and weighed. Tissues were retained for histological and biochemical analyses.
Table 1. Circulating levels of estradiol, progesterone, and relaxin in study monkeys.
| Estradiol, pg/ml
|
Progesterone, ng/ml
|
Relaxin, ng/ml
|
|||
|---|---|---|---|---|---|
| Day | C | R | C | R | R |
| 7 | 96 ± 8 | 77 ± 12 | 2.3 ± 0.4 | 1.8 ± 0.3 | <0.05 |
| 21 | 70 ± 8 | 84 ± 12 | 21.1 ± 2.0 | 23.7 ± 2.9 | <0.05 |
| 24 | 53 ± 4 | 62 ± 13 | 16.7 ± 2.5 | 19.4 ± 2.6 | 0.71 ± 0.4 |
| 28 | 62 ± 8 | 61 ± 8 | 18.9 ± 2.5 | 19.6 ± 1.7 | 0.56 ± 0.1 |
| 31 | 53 ± 5 | 59 ± 11 | 17.3 ± 3.1 | 19.3 ± 2.8 | 0.64 ± 0.1 |
| 35 | 50 ± 2 | 57 ± 6 | 17.5 ± 3.6 | 17.2 ± 1.3 | 0.70 ± 0.2 |
| 38 | 60 ± 7 | 56 ± 18 | 16.7 ± 3.1 | 16.9 ± 1.0 | 0.90 ± 0.1 |
| 42 | 68 ± 7 | 62 ± 21 | 17.5 ± 2.3 | 17.9 ± 3.1 | 1.06 ± 0.1 |
Mean ± SEM values for the four monkeys in the control (C; i.e., vehicle-treated) and relaxin-treated (R) groups are shown for the days of the protocol indicated. Note that the values for days 7 and 21 are pre-relaxin treatment. Circulating levels for relaxin in the control group were <0.05 at each day shown.
Hormone Assays. Circulating estradiol and progesterone concentrations were measured by RIA using reagents obtained commercially (Coat-A-Count, Diagnostic Products, Los Angeles). The intra- and interassay coefficients of variation for the estradiol assay were 4.3% and 8.9%, respectively, and those for the progesterone assay were 4.0% and 5.6%, respectively. Concentrations of human relaxin in serum were measured by using a human relaxin-specific RIA described previously (28), which employs H2 human relaxin protein as standard, a rabbit polyclonal anti-human H2 relaxin antibody, and 125I-labeled H2 human relaxin as radioligand. The sensitivity of the assay was 10-25 pg per tube. The intra- and interassay coefficients of variation were 4.3% (n = 12 observations) and 11.8% (n = 13 assays), respectively. The assay was validated for accurate measurement of human relaxin in monkey serum.
Morphological Analyses. Tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections were deparaffinized and rehydrated in graded ethanols. Hematoxylin/eosin-stained sections were viewed under light microscopy by two independent investigators in a blinded and randomized fashion. Sections were stained with Masson's trichrome stain to assess the effect of relaxin on the abundance of collagen expression. At least three sections of uterus per animal were assessed; for each section at least five fields were evaluated. Quantitative morphometric determinations of lymphocyte number, arteriole number, epithelial cell number, stromal area, and stromal collagen abundance were performed by using a Leitz DMRB microscope and Leica QUANTIMET 500+ image analysis system using IMAGEPRO PLUS software as previously described.**
Western Blot Analyses. Western blot analyses employing specific, well characterized primary antibodies and semiquantitative methods described previously (29, 30) were used to determine expression of the steroid hormone receptors, MMPs, and TIMP-1. Mouse monoclonal antibodies to human procollagenase (proMMP-1) (Ab-1), human prostromelysin (proMMP-3) (Ab-1), and human TIMP-1 (Ab-1), human estrogen receptor α (GR17), human estrogen receptor β (GR39), pure proMMP-1, proMMP-3, and TIMP-1 proteins and peroxidase-conjugated goat anti-mouse IgG and IgM were from Calbiochem/Oncogene Research Products. Mouse monoclonal antibody to estrogen receptor α (1D5) was obtained from DAKO. Rabbit polyclonal anti-human estrogen receptor β antibody (PA1-311) was from Affinity Bioreagents (Golden, CO). Pure estrogen receptor α and β proteins were from Panvera (Madison, WI). Mouse monoclonal antibody 1294 and pure progesterone receptor B and A proteins were used (31). Anti-peroxidase-conjugated affinity-purified anti-rabbit IgG was from Rockland (Gilbertsville, PA). Poly(vinylidene fluoride) (PVDF) membranes and electrophoresis reagents were from Sigma-Aldrich. Enhanced chemiluminescence reagents were purchased from Amersham Pharmacia.
Tissues were homogenized in modified radioimmunoprecipitation assay (RIPA) buffer, 1% SDS, and protease-inhibitor cocktail (Boehringer Mannheim; 1 tablet per 10 ml), for 1 min by using a Polytron homogenizer; homogenates were clarified by centrifugation at 4°C at 16,000 × g for 15 min, and supernatants were electrophoresed on SDS/PAGE gels. For assessment of nuclear receptors, tissues were extracted with high-salt buffer to solubilize nuclear proteins. Broad-range kaleidoscope molecular weight markers were used to estimate molecular weights. Pure proteins were used as positive controls. Extracts from monkey negative control tissues were prepared in the same manner and used as negative controls. Proteins were electroblotted onto polyvinylidene fluoride (PVDF) membranes, which were then blocked, washed, and incubated with primary antibody. Blots were then washed and incubated with horseradish peroxidase-conjugated secondary antibody. Subsequently, the blots were washed three times with Tris-buffered saline Tween 20 over a 30-min period and developed by the enhanced chemiluminescence method. Intensities of the signals obtained on developed films were determined with a computing densitometer (model 300B, Molecular Dynamics) by using the volume-integration method with appropriate corrections for background absorption, as we have described previously (29, 30). Samples from all monkeys were run on the same blot. For each protein assessed, verification was made that the intensity of the signal in each band increased in the same linear fashion as the increase in the amount of sample protein loaded.
Statistical Analyses. Rank sum tests for group comparisons were performed with exact one-tailed P values computed. P values < 0.05 were considered significant.
Results
Hormone Levels. Mean circulating levels of the steroid hormones and relaxin are shown in Table 1. Circulating levels of estradiol and progesterone approximated those seen during early pregnancy. Injections of relaxin achieved circulating relaxin levels ranging from 0.56 to 1.06 ng/ml, approximately the same as levels previously detected during early human pregnancy (1).
Relaxin Stimulates Uterine Weight. A pronounced effect of relaxin on uterine weight was observed. The body and uterine weights of all animals are shown in Table 2. At the time of removal of the uteri (performed by investigators blinded to the treatment group), a markedly increased uterine size and weight were apparent in a set of animals later revealed to be the relaxin-treated group. The difference in uterine weights between the two groups was highly significant (P = 0.014). In contrast, body weights did not differ (P = 0.79).
Table 2. Body and uterine weights in study monkeys.
| Control group
|
Relaxin-treated group
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (±SEM) | Mean (±SEM) | |||||||||
| Animal | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Body weight, kg | 5.8 | 4.9 | 7.4 | 5.6 | 5.9 (0.53) | 9.1 | 6.5 | 5.7 | 4.6 | 6.5 (0.95) |
| Uterine weight, g | 12.36 | 12.81 | 9.87 | 12.86 | 11.98 (0.71) | 27.21 | 19.13 | 14.93 | 12.95 | 18.56 (3.15) |
Body weight, P = 0.79. Uterine weight, P = 0.014.
Relaxin Effects on Histology. The endometria of the control animals revealed the histological appearance of a secretory phase endometrium, as expected in view of the estradiol and progesterone levels achieved. Endometria of the relaxin-treated monkeys resembled a generally more decidualized morphology. To determine whether relaxin had any effect on endometrial and/or stromal cell proliferation, the number of epithelial cells and the stromal area were determined in hematoxylin/eosin-stained sections from each animal. No differences between the two groups of monkeys in either the number of epithelial cells per unit area or stromal area were detected (data not shown). Assessment of Masson's trichrome-stained sections revealed no differences in the relative stromal area stained for collagen in the relaxin-treated animals from those in the control group (data not shown).
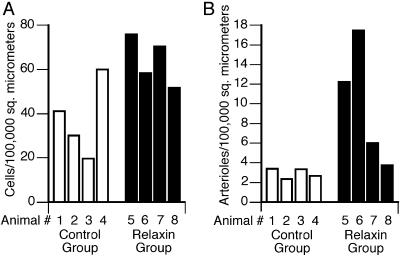
Relaxin Increases Endometrial Lymphocyte and Arteriole Number. The endometria of the relaxin-treated animals had a significantly greater number of lymphocytes (Fig. 1A). Quantitative assessment revealed that the number of endometrial lymphocytes for the relaxin-treated animals [mean = 64.1 ± 5.5 cells per 100,000 μm2 (±SEM)] was significantly higher than that for the control animals (mean = 34.7 ± 8.6) (P = 0.03). The endometria of the relaxin-treated animals had a greater number of arterioles (Fig. 1B). The number of endometrial arterioles for all animals in the relaxin-treated group [mean = 9.87 ± 3.12 per 100,000 μm2 (±SEM)] was significantly higher than that for all animals in the control group (mean = 2.94 ± 0.25) (P = 0.014).
Fig. 1.
The effects of relaxin on monkey endometrial lymphocyte (A) and arteriole (B) number. Morphological assessments were performed as described in Materials and Methods. Each bar shows the mean value for one animal in either the control (open bar) or relaxin-treated (filled bar) group; at least five fields for each of at least three uterine sections per animal were assessed.
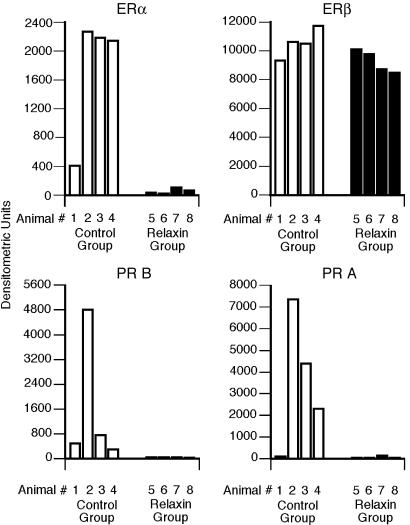
Relaxin Inhibits Endometrial Estrogen Receptor α but Does Not Affect Estrogen Receptor β Endometrial levels of estrogen receptor α in relaxin-treated monkeys were significantly lower (P = 0.01) than those in control monkeys (Fig. 2 Upper Left). Repeated Western analyses showed virtually identical inhibitory effects of relaxin. The use of three different estrogen receptor α antibodies showed similar results. In contrast, relaxin had no effect on endometrial estrogen receptor β levels (P = 0.11) (Fig. 2 Upper Right). The use of two different estrogen receptor β antibodies showed the same results.
Fig. 2.
The effects of relaxin on monkey endometrial estrogen receptor α (Upper Left) and β (Upper Right) and progesterone receptor B (Lower Left) and A (Lower Right) expression. Western blot analyses were performed by using endometrial tissue from each monkey as described in Materials and Methods. Densitometric values from a representative blot are shown. Each bar shows the value for one animal in either the control (open bar) or relaxin-treated (filled bar) group.
Relaxin Inhibits Endometrial Progesterone Receptors B and A. Endometrial levels of progesterone receptor B and A isoforms were significantly lower in the relaxin-treated animals (P = 0.01 and P = 0.03, respectively) than those in the control animals (Fig. 2 Lower).
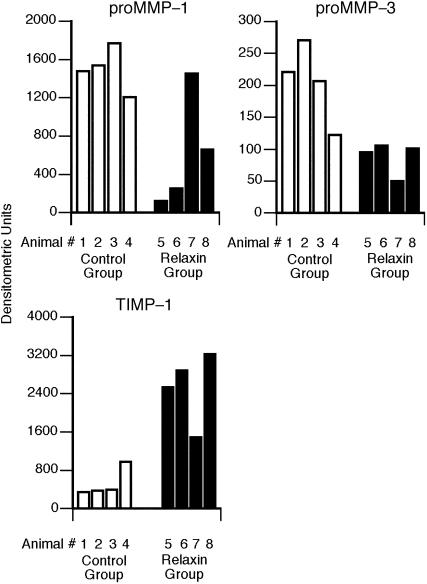
Relaxin Negatively Regulates Endometrial MMP Expression. Endometrial levels of proMMP-1 were significantly lower in the relaxin-treated animals (P = 0.01) (Fig. 3 Upper Left). Endometrial levels of proMMP-3 were also significantly lower in the relaxin-treated animals (P = 0.01) (Fig. 3 Upper Right). In contrast, levels of TIMP-1 were significantly increased in relaxin-treated animals (P = 0.01) (Fig. 3 Lower).
Fig. 3.
The effects of relaxin on proMMP-1 (Upper Left), proMMP-3 (Upper Right), and TIMP-1 (Lower) expression. Data are expressed as in Fig. 2.
Discussion
These results demonstrate that relaxin has pronounced in vivo effects on the primate uterus. The combination of effects demonstrated, consisting of increased uterine weight, stimulation of endometrial lymphocyte and arteriole number, and inhibition of steroid hormone receptor and MMP expression, suggests an important role for relaxin in the establishment and/or maintenance of early pregnancy and provides specific mechanisms responsible. The increased availability of cells that produce cytokines and other biochemical factors involved in pregnancy maintenance and labor induction, in concert with effects that cause a potential hyperemia, are mechanisms by which relaxin exerts its influence. Increased endometrial vascularization in association with the dramatic inhibition of MMP expression, allowing maintenance of connective tissue integrity, provides evidence for relaxin's important role in endometrial support.
Hisaw and coworkers (32, 33) performed the first studies to suggest that relaxin affects endometrial vascularization. The results of these earlier studies, also in the rhesus monkey, suggested that relaxin induces proliferation of endothelial cells of endometrial blood vessels and dilatation of superficial endometrial blood vessels (33). Animals in which administration of relaxin-containing extracts was discontinued but steroid treatment was maintained showed reduced endothelial proliferation and dilatation (32). Thus, relaxin seemed necessary to maintain endothelial proliferation and vascular dilatation in the endometrium of the rhesus monkey. These pioneering studies had severe limitations: relaxin-containing extracts were administered rather than pure hormone, which was not available at that time. To our knowledge, until the present investigations were performed, no additional studies to assess the effects of relaxin on non-human primate endometrium in vivo had been performed. Clinical trials performed in women to determine the efficacy of relaxin for treatment of systemic sclerosis (34) revealed that, as reported by Unemori and colleagues (27), administration of relaxin at doses that achieved circulating levels between 2.0 and 83.3 ng/ml was associated with menometrorrhagia in 72% of the subjects, compared with an incidence of 15% in the control group.
Results from various studies provide abundant evidence that relaxin is a potent regulator of the differentiated function of human endometrial cells in vitro (14, 16-27). Relaxin stimulates the production of prolactin (16, 17, 19), insulin-like growth factor (18), and insulin-like growth factor binding protein 1 (IGFBP-1) (19), in progestin-primed endometrial stromal cells. Because prolactin and IGFBP-1 are considered the major secretory proteins of decidual cells (21), induction of the expression of these secretory proteins has been widely used as a biochemical marker of decidualization of endometrial stromal cells in vitro (22). Detailed studies of the regulation of IGFBP-1 promoter activity in endometrial stromal cells demonstrate that relaxin, not progesterone, is the major inducer of IGFBP-1 gene transcription (20). Thus, relaxin seems to be a powerful regulator of human endometrial decidualization, at least as influential as progesterone, if not more so. No evidence of secretory activity was shown by cells treated with only progesterone and estradiol, although secretory activity appears to be a prominent feature of decidualized endometrial stromal cells in vivo (21). Cells in the stromal cultures that were treated with relaxin in addition to progestin exhibited ultrastructural features characteristic of secretory cells. Thus, in stromal cell cultures, progesterone alone seems to be inadequate for the induction of full cellular function; relaxin is necessary as well.
The present data demonstrate that relaxin significantly inhibits endometrial levels of estrogen receptor α, but not β, and of both progesterone receptor isoforms. Various studies demonstrate the decline in endometrial estrogen receptor α and progesterone receptor isoforms during the secretory phase of the human menstrual cycle (35, 36). Moreover, recent data suggest that the lack of this decline may be associated with an inability to become pregnant. Because relaxin levels rise during the late secretory phase, even in nonconceptive cycles (13), we now can hypothesize that relaxin may be responsible for the decline in endometrial expression of estrogen receptor α and progesterone receptors B and A.
Consistent with results from previous in vitro studies of human endometrial cells, relaxin negatively regulates endometrial MMP expression in the rhesus monkey in vivo. The present data demonstrate that relaxin significantly inhibits endometrial proMMP-1 and proMMP-3 and significantly stimulates levels of the endogenous inhibitor TIMP-1. Clearly, the effects of relaxin on MMP expression vary with cell/tissue type, negative regulation by relaxin in endometrial cells, in distinct contrast to the positive regulatory effect of relaxin on MMP expression in cervical and other types of fibroblasts (30, 37). Relaxin is an important agent in the remodeling of connective tissue in several reproductive tract tissues (2-4). Relaxin markedly modulates the connective tissue phenotype of human fibroblasts of several target organs. No previous studies have determined the effects of relaxin in the modulation of endometrial connective tissue in vivo despite considerable evidence that endometrial maturation involves remodeling of the interstitial extracellular matrix (38, 39).
Our previous studies (14) and those of Unemori et al. (27) have demonstrated that relaxin stimulates expression of endometrial vascular endothelial growth factor, suggesting a role for relaxin in endometrial angiogenesis. Our data shown here, demonstrating an increase in endometrial arteriole number, support this concept. The present demonstration that relaxin negatively regulates endometrial MMP levels suggests its role is in the maintenance of pregnancy, rather than menstruation, which could have been implied from the angiogenesis data alone. In concert with prior results, these data now suggest an important role for relaxin in early pregnancy maintenance. Other data demonstrate that progesterone also inhibits endometrial proMMP-1 expression (40, 41). However, because endometrial progesterone receptor levels are decreased in the late secretory phase, the action of relaxin may be critical to early pregnancy maintenance. It is clear that menstrual tissue breakdown is initiated by MMPs (42). In the endometrium, TIMPs, the endogenous inhibitors of MMP activity, are not regulated by ovarian steroids or cytokines (39, 40). Our finding that relaxin stimulates endometrial TIMP-1 points again to the importance of relaxin in early pregnancy maintenance.
In summary, the findings presented here support the thesis that relaxin causes the secretory phase remodeling of the primate endometrium, needed to support the establishment and maintenance of early pregnancy. Relaxin now can be considered an important regulator of endometrial development via mechanisms (in addition to the previously documented direct regulation of stromal cell differentiation) involving the stimulation of angiogenesis, maintenance of endometrial connective tissue integrity, and inhibition of the expression of estradiol and progesterone receptors. Relaxin is impressively uterotropic in the rhesus monkey, as it is in the pig (43, 44), an important mechanism of its role in uterine accommodation to primate pregnancy.
Acknowledgments
We thank the staff of the Primate Core of the Center for Research in Reproductive Physiology at the University of Pittsburgh for excellent assistance; Lisa Sherman for excellent technical assistance; Dr. Pam Maolli for valuable surgical assistance; and Dr. Geoffrey Greene for providing estrogen receptor α antibody H222. This work was supported by National Institutes of Health Grants HD 22338 (to G.W.) and HD 08610 (to T.M.P.).
Abbreviations: TIMP, tissue inhibitor of metalloproteinase; MMP, matrix metalloproteinase; proMMP-1, procollagenase; proMMP-3, prostromelysin.
Footnotes
Lambert, W. C., Lapidus, A. & Kuo, H.-R. (1997) Blood 91, 59 (abstr.).
References
- 1.Goldsmith, L. T., Weiss, G. & Steinetz, B. G. (1995) Endocrinol. Metab. Clin. North Am. 24, 171-186. [PubMed] [Google Scholar]
- 2.Sherwood, O. D. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. (Raven, New York), 2nd Ed., pp. 861-1009.
- 3.Schwabe, C., Steinetz, B., Weiss, G., Segaloff, A., McDonald, J. K., O'Byrne, E., Hochman, J., Carriere, B. & Goldsmith, L. (1978) Recent Prog. Horm. Res. 34, 123-211. [DOI] [PubMed] [Google Scholar]
- 4.Steinetz, B. G., O'Byrne, E. M. & Kroc, R. L. (1980) in Dilation of the Uterine Cervix, eds. Naftolin, F. & Stubblefield, P. G. (Raven, New York), pp. 157-177.
- 5.Goldsmith, L. T., Grob, H. S., Scherer, K. J., Surve, A., Steinetz, B. G. & Weiss, G. (1981) Endocrinology 109, 548-552. [DOI] [PubMed] [Google Scholar]
- 6.Golos, T. G. & Sherwood, O. D. (1982) Endocrinology 111, 872-878. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood, O. D., Chang, C. C., BeVier, G. W., Dial, J. R. & Dziuk, P. J. (1976) Endocrinology 98, 875-879. [DOI] [PubMed] [Google Scholar]
- 8.Weiss, G., Steinetz, B. G., Dierschke, D. J. & Fritz, G. (1981) Biol. Reprod. 24, 565-567. [DOI] [PubMed] [Google Scholar]
- 9.Stewart, D. R., Stouffer, R., Overstreet, J. W., Hendricks, A. & Lasley, B. L. (1993) Endocrinology 132, 6-12. [DOI] [PubMed] [Google Scholar]
- 10.Stewart, D. R., Addiego, L. A., Pascoe, D. R., Haluska, G. J., Pashen, R. (1992) Biol. Reprod. 46, 648-652. [DOI] [PubMed] [Google Scholar]
- 11.Weiss, G., O'Byrne, E. M. & Steinetz, B. G. (1976) Science 194, 948-949. [DOI] [PubMed] [Google Scholar]
- 12.Weiss, G. (1987) in The Primate Ovary, ed. R. L. Stouffer (Plenum, New York), pp. 223-236.
- 13.Stewart, D. R., Celniker, A. C., Taylor, C. A., Cragun, J. R., Overstreet, J. W. & Lasley, B. L. (1990) J. Clin. Endocrinol. Metab. 70, 1771-1775. [DOI] [PubMed] [Google Scholar]
- 14.Palejwala, S., Tseng, L., Wojtczuk, A., Weiss, G. & Goldsmith, L. T. (2002) Biol. Reprod. 66, 1743-1748. [DOI] [PubMed] [Google Scholar]
- 15.Osheroff, P. L. & King, K. L. (1995) Endocrinology 136, 4377-4381. [DOI] [PubMed] [Google Scholar]
- 16.Zhu, H. H., Huang, J. R., Mazella, J., Rosenberg, M. & Tseng, L. (1990) J. Clin. Endocrinol. Metab. 71, 889-899. [DOI] [PubMed] [Google Scholar]
- 17.Huang, J. R., Tseng, L., Bischof, P. & Janne, O. A. (1986) Endocrinology 121, 2011-2017. [DOI] [PubMed] [Google Scholar]
- 18.Bell, S. C., Jackson, J. A., Ashmore, J., Zhu, H. H. & Tseng, L. (1991) J. Clin. Endocrinol. Metab. 72, 1014-1024. [DOI] [PubMed] [Google Scholar]
- 19.Tseng, L., Gao, J. G., Chen, R., Zhu, H. H., Mazella, J. & Powell, D. R. (1992) Biol. Reprod. 47, 441-450. [DOI] [PubMed] [Google Scholar]
- 20.Gao, J. G., Mazella, J. & Tseng, L. (1994) Mol. Cell. Endocrinol. 104, 39-46. [DOI] [PubMed] [Google Scholar]
- 21.Lane, B., Oxberry, W., Mazella, J. & Tseng, L. (1994) Hum. Reprod. 9, 259-266. [DOI] [PubMed] [Google Scholar]
- 22.Irwin, J. C., Utian, W. H. & Eckert, R. L. (1991) Endocrinology 129, 2385-2392. [DOI] [PubMed] [Google Scholar]
- 23.Chen, G. A., Huang, J. R. & Tseng, L. (1988) Biol. Reprod. 39, 519-525. [DOI] [PubMed] [Google Scholar]
- 24.Tseng, L., Zhu, H. H., Mazella, J., Koistinen, H. & Seppala, M. (1999) Mol. Hum. Reprod. 5, 372-375. [DOI] [PubMed] [Google Scholar]
- 25.Thrailkill, K., Clemons, S., Busby, W. & Handwerger, S. (1990) J. Clin. Invest. 86, 878-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart, D. R., Erikson, M. S., Erikson, M. E., Nakajima, S. T., Overstreet, J. W., Lasley, B. L., Amento, E. P. & Seppala, M. (1997) J. Clin. Endocrinol. Metab. 82, 839-846. [DOI] [PubMed] [Google Scholar]
- 27.Unemori, E. N., Erikson, M. E., Rocco, S. E., Sutherland, K. M., Parsell, D. A., Mak, J. & Grove, B. H. (1999) Hum. Reprod. 14, 800-806. [DOI] [PubMed] [Google Scholar]
- 28.Iams, J. D., Goldsmith, L. T. & Weiss, G. (2001) J. Soc. Gynecol. Investig. 8, 39-42. [PubMed] [Google Scholar]
- 29.Palejwala, S., Stein, D. E., Weiss, G., Monia, B. P., Tortoriello, D. & Goldsmith, L. T. (2001) Endocrinology 142, 3405-3413. [DOI] [PubMed] [Google Scholar]
- 30.Palejwala, S., Stein, D. E., Wotjczuk, A., Weiss, G. & Goldsmith, L. T. (1998) Endocrinology 139, 1208-1212. [DOI] [PubMed] [Google Scholar]
- 31.Press, M., Spaulding, B., Groshen, S., Kaminsky, D., Hagerty, M., Sherman, L., Christensen, K. & Edwards, D. P. (2002) Steroids 67, 799-813. [DOI] [PubMed] [Google Scholar]
- 32.Dallenbach-Hellweg, G., Dawson, A. B. & Hisaw, F. L. (1966) Am. J. Anat. 119, 61-78. [Google Scholar]
- 33.Hisaw, F. L., Hisaw, F. L., Jr., & Dawson, A. B. (1967) Endocrinology 81, 375-385. [DOI] [PubMed] [Google Scholar]
- 34.Siebold, J. R., Korn, J. H., Simms, R., Clements, P. J., Moreland, L. W., Mayes, M. D., Furst, D. E., Rothfield, N., Steen, V., Weisman, M., et al. (2000) Ann. Intern. Med. 132, 871-879. [DOI] [PubMed] [Google Scholar]
- 35.Critchley, H. O. D., Brenner, R. M., Henderson, T. A., Williams, K., Nayak, N. R., Slayden, O. D., Millar, M. R. & Saunders, P. T. K. (2001) J. Clin. Endocrinol. Metab. 86, 1370-1378. [DOI] [PubMed] [Google Scholar]
- 36.Mote, P. A., Balleine, R. L., McGowan, E. M. & Clarke, C. L. (1999) J. Clin. Endocrinol. Metab. 84, 2963-2971. [DOI] [PubMed] [Google Scholar]
- 37.Unemori, E. N. & Amento, E. P. (1990) J. Biol. Chem. 265, 10681-10685. [PubMed] [Google Scholar]
- 38.Salamonsen, L. A., Butt, A. R., Hammond, F. R., Garcia, S. & Zhang, J. (1997) J. Clin. Endocrinol. Metab. 82, 1409-1415. [DOI] [PubMed] [Google Scholar]
- 39.Singer, C. F., Marbaix, E., Kokorine, I., Lemoine, P., Donnez, J., Eeckhout, Y. & Courtoy, P. J. (1997) Proc. Natl. Acad. Sci. USA 94, 10341-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marbaix, E., Kokorine, I., Moulin, P., Donnez, J., Eeckhout, Y. & Courtoy, P. J. (1996) Proc. Natl. Acad. Sci. USA 93, 9120-9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer, C. F., Marbaix, E., Lemoine, P., Donnez, J., Courtoy, P. J. & Eeckhout, Y. (1999) Eur. J. Biochem. 259, 40-45. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, J. & Salamonsen, L. A. (2002) J. Clin. Endocrinol. Metab. 87, 2346-2351. [DOI] [PubMed] [Google Scholar]
- 43.Hall, J. A., Cantley, T. C., Day, B. N. & Anthony, R. V. (1990) Biol. Reprod. 42, 769-774. [DOI] [PubMed] [Google Scholar]
- 44.Min, G., Hartzog, M. G., Jennings, R. L., Winn, R. J. & Sherwood, O. D. (1997) Endocrinology 138, 560-565. [DOI] [PubMed] [Google Scholar]