Abstract
Mutations in the WNK4 gene cause pseudohypoaldosteronism type II (PHAII), an autosomal-dominant disorder of hyperkalemia and hypertension. The target molecules of this putative kinase and the molecular mechanisms by which the mutations cause the phenotypes are currently unknown. Although recent reports found that expression of WNK4 in Xenopus oocytes causes inhibition of the thiazide-sensitive NaCl cotransporter and the renal K channel ROMK, there may be additional targets of WNK4. For example, an increase in paracellular chloride permeability has been postulated to be a mediator of PHAII pathogenesis, a possibility supported by the localization of WNK4 at tight junctions in vivo. To determine the validity of this hypothesis, we measured transepithelial Na and Cl permeability in Madin-Darby canine kidney II cells stably expressing wild-type or a pathogenic mutant of WNK4. We found that transepithelial paracellular Cl permeability was increased in cells expressing a disease-causing mutant WNK4 (D564A) but that Na permeability was decreased slightly. Furthermore, WNK4 bound and phosphorylated claudins 1-4, major tight-junction membrane proteins known to be involved in the regulation of paracellular ion permeability. The increases in phosphorylation of claudins were greater in cells expressing the mutant WNK4 than in cells expressing wild-type protein. These results clearly indicate that the pathogenic WNK4 mutant possesses a gain-of-function activity and that the claudins may be important molecular targets of WNK4 kinase. The increased paracellular “chloride shunt” caused by the mutant WNK4 could be the pathogenic mechanism of PHAII.
Pseudohypoaldosteronism type II (PHAII) [OMIM (online Mendelian inheritance in man) database no. 145260] is a rare Mendelian form of hypertension. Recently, mutations in two homologous protein kinase genes, WNK1 and WNK4, were linked to PHAII by positional cloning (1). Determination of the pathogenesis of rare Mendelian forms of high blood pressure may provide important clues to the pathways underlying more common hypertension, termed “essential” hypertension, and may help identify new therapeutic targets. PHAII-causing mutations in the WNK1 gene are large deletions in the first intron that appear to increase WNK1 expression. On the other hand, mutations in the WNK4 gene are missense mutations that cluster within a span of four amino acids distal to the first putative coil domain (1). However, the consequence of these missense mutations for WNK4 function is poorly understood.
WNK1 and WNK4 are expressed in the distal nephron, suggesting that both kinases are involved in a previously unrecognized signaling pathway that regulates NaCl resorption in the distal nephron. However, the upstream regulators and the downstream molecular targets of these kinases are currently unknown. Based on clinical observations, two hypotheses have been proposed to explain the pathogenesis of PHAII. First, the mutations may cause an increase in distal nephron chloride permeability, also known as a “chloride shunt” (2). This increased chloride permeability would increase Na resorption and depolarize the transepithelial voltage, leading to hypertension and hyperkalemia, respectively. The second possibility is that the mutations increase the activity of the Na-dependent Cl cotransporter (TSC). This possibility is supported by the fact that PHAII can be treated with thiazide diuretics (3).
Recently, inhibition of TSC activity by wild-type WNK4, but not by the disease-causing mutant, was demonstrated in Xenopus oocytes (4, 5). Very recently, inhibition of the surface expression of ROMK channel by wild-type WNK4 was also reported. Interestingly, the disease-causing mutant further inhibited the surface expression of ROMK (6). However, these functional assays were performed only in Xenopus oocytes, and, to our knowledge, there have not been reports on the substrates of WNK4. TSC and ROMK are localized in the apical plasma membranes of distal nephron segments (7-9), whereas WNK4 is present in the tight-junction complexes in the cortical collecting ducts as well as in DCT (1). This finding suggests that there may be other molecular targets of WNK4, possibly including tight-junction proteins.
In this study, we first examined whether the expression of the wild-type or the disease-causing mutant WNK4 affects transepithelial paracellular ion permeability. To this end, we generated stable Madin-Darby canine kidney II (MDCK II) cell lines expressing the wild-type and one of the human disease-causing mutants (D564A) of WNK4 by using a tetracycline-inducible system. We found that transepithelial paracellular Cl permeability was increased in cells expressing the mutant WNK4. Accordingly, we searched for the downstream molecular target of WNK4 in tight-junction proteins.
Materials and Methods
Generation of WNK4-Expressing Cell Lines. Human WNK4 cDNA was isolated by RT-PCR using human kidney mRNA as a template. The cDNA was cloned into the pTRE2-hyg vector (Clontech) and a C-terminal hemagglutinin (HA) tag was added. We also generated a WNK4 construct with an N-terminal Flag-tag. The disease-causing mutation (D564A) was introduced with the QuikChange site-directed mutagenesis kit (Stratagene). Stable cell lines were isolated by using Tet-off MDCK II cells (Clontech) as a host cell line with selection by using hygromycin B (200 μg/ml). Cell lines were screened by immunoblotting. Rat anti-HA mAb (9Y10) (Roche Diagnostics) and mouse M2 mAb or rabbit anti-Flag Ab (Sigma) were used to detect HA-tagged and Flag-tagged proteins, respectively. Endogenous claudins and occludin were detected with specific Abs (Zymed).
Measurement of Paracellular Na and Cl Permeability. Cells were seeded into a Millicell-HA filter (12-mm diameter; Millipore). When the cells were confluent, expression of transgenes was induced for 4 days by the removal of doxycycline. Tracer-flux experiments were performed in a solution containing 150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1.5 mM MgCl2, and 10 mM Hepes·NaOH (pH 7.4). We added 1 μCi/ml (1 Ci = 37 GBq) 22Na or 5 μCi/ml 36Cl to the medium on one side of the membrane, and the appearance of radioactivity in the medium on the other side was measured at various times. The composition of medium on both sides of the membrane was identical. When 36Cl was used, cold NaCl was added to the medium on the other side of the membrane because the concentration of NaCl in 36Cl tracer was high (≈1.6 M). We measured 22Na and 36Cl activity with a gamma counter and a liquid scintillation counter, respectively. Permeabilities for 22Na and 36Cl were calculated by using the following equation: JCl = CCl × PCl × A, where JCl is transepithelial Cl flux (mol/s), CCl is concentration (mol/liter), A is surface area (cm2), and P is permeability. Statistical comparison of multiple groups was assessed by ANOVA. Chloride permeabilities in the WNK4 expressing cells between uninduced and induced states were compared by using a paired t test. Error bars are represent mean ± SEM. Statistical significance was defined at p < 0.05.
Phosphorylation of Claudins. Mammalian expression vectors (3× Flag CMV14; Sigma) encoding Flag-tagged claudins and occludin were generated by RT-PCR. Both wild-type and mutant WNK4 expression vectors and claudin expression vectors were cotransfected into COS7 and MDCK II cells with Lipofectamine 2000 (Invitrogen). At 24 h after transfection, cells were incubated for 2 h in phosphate-free DMEM with 0.5% BSA and 10 mM Hepes (pH 7.4). The cells were incubated further for 2 h in the same medium containing [32P]Pi (1 mCi/ml). After incubation, the cells were washed with phosphate-free DMEM, harvested, and lysed for 1 h on ice in 150 mM NaCl/15 mM Tris·HCl,/25 mM NaF/1 mM Na3VO4 (pH 8.0). Proteins were immunoprecipitated with anti-Flag M2 Ab. Western blot analyses of the immunoprecipitates were performed also by using an anti-Flag polyclonal Ab to confirm the expression of claudins. To detect the phosphorylation of endogenous claudins, immunoprecipitation of claudins in the WNK4-expressing MDCK II cells was performed by using anti-claudin 1 and 4 Abs (Zymed). Immunoprecipitated proteins were separated by SDS/PAGE, and phosphorylated proteins were visualized by autoradiography.
For in vitro kinase assays, a GST-fusion protein containing the kinase domain of WNK4 was generated by subcloning the SalI-MstI fragment of WNK4 into pGEX6P-1 (Amersham Biosciences). The cytoplasmic region of claudin 4 was also generated as a GST-fusion protein for uses as a substrate of WNK4. Protein kinase reactions were carried out at 30°C for 15 min in kinase buffer (10 mM Tris, pH 7.4/150 mM NaCl/10 mM MgCl2/0.5 mM DTT). Reaction mixtures were resolved by SDS/PAGE, and phosphorylated proteins were visualized by autoradiography.
Coimmunoprecipitation of WNK4 and Claudins. HA-tagged WNK4 and Flag-tagged claudins expressed in COS7 and MDCK II cells were immunoprecipitated with anti-Flag M2 Ab, and coprecipitation was examined by Western blotting with an anti-HA Ab. The interaction of HA-WNK4 and endogenous tight-junction proteins in the WNK4-expressing MDCK II cells was examined by immunoprecipitation by using an anti-HA Ab, followed by Western blotting using Abs to tight-junction proteins (Zymed).
Results
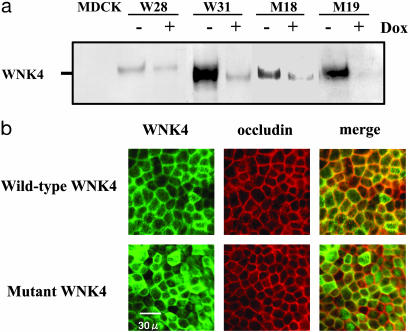
WNK4 Increases Paracellular Cl Permeability in MDCK Cells. We generated stable MDCK II cell lines expressing the wild-type and one of the human disease-causing mutants (D564A) of WNK4 by using a tetracycline-inducible system. We measured the transepithelial paracellular 22Na and 36Cl permeability of these cells grown on a permeable support. Before measuring the Na and Cl permeability, expression of the wild-type and the mutant WNK4 were confirmed by Western blot analysis and immunofluorescence microscopy. As shown in Fig. 1a, stable cell lines expressing the wild-type and the mutant WNK4 were isolated. In all cell lines, some expression, even in the presence of doxycycline, was observed. Accordingly, we performed the initial experiments in induced cells (doxycycline-negative). Intracellular localization of the wild-type and the mutant WNK4 colocalized with the tight-junction protein occludin but did not appear to be different between the two cell lines (Fig. 1b). This localization of WNK4 in tight junctions is consistent with the reported (1) association of wild-type WNK4 with tight junctions in vivo. Flag-tagged WNK4 and HA-tagged WNK4 did not show the difference in terms of their cellular localization (data not shown).
Fig. 1.
Generation of wild-type and mutant WNK4-expressing MDCK II cell lines. (a) Expression of wild-type and mutant WNK4 in MDCK II cells. HA-tagged WNK4 proteins were detected by using an anti-HA (9Y10) Ab. Expression of WNK4 was induced by removal of doxycycline from the medium for 4 days. W28 and W31 are wild-type WNK4-expressing cell lines, and M18 and M19 are mutant WNK4-expressing cell lines. (b) Immunofluorescence of wild-type and mutant WNK4 expressed in isolated stable cell lines. Cells were grown on a permeable support, and the expression of WNK4 was induced for 4 days. WNK4 was detected by using an anti-HA Ab in conjunction with an Alexa 546-conjugated anti-rat IgG Ab. Cells were stained for occludin by using an anti-occludin Ab (Zymed) in conjunction with an Alexa 488-conjugated anti-rabbit IgG Ab.
We isolated several transformants after transfection with the empty vector, as well as the wild-type and mutant WNK4 cDNAs. We chose the four cell lines with the highest expression of the transgenes. Each cell line was cultured on a permeable support and was grown to confluence. Expression of WNK4 was induced for 4 days by removal of doxycycline from the medium. Transepithelial Na and Cl permeability were determined by assessing transepithelial 22Na and 36Cl flux across the epithelium. In these assays, the medium composition on both sides of the membrane was identical. Radioisotope was added to the basal side of the membrane, and the appearance of radioisotope in the apical medium was measured over time. Our preliminary experiments revealed that Na and Cl flux was linear for 45 min. Therefore, we adopted 30 min as an incubation time for subsequent experiments. The calculated basal to apical permeability was identical to the apical to basal permeability. Addition of 1 mM ouabain/0.1 mM bumetanide/0.1 mM amiloride/0.1 mM hydrochlorothiazide/1 mM 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid/1 mM 4-acetamido-4′-isothiocyanatostilbene- 2,2′-disulfonic acid had no effect on Na or Cl flux. In addition, Na and Cl fluxes measured in this assay appeared not to saturate as a function of NaCl concentration up to 200 mM. These results (see Figs. 6 and 7, which are published as supporting information on the PNAS web site) suggest that our measurements represent paracellular Na and Cl permeability of the epithelium. Our permeability calculations [P (Na) = 25.8 ± 0.9 cm/s (mean ± SEM, n = 7), P (Cl) = 2.0 ± 0.3 cm/s (mean ± SEM, n = 11) in the host cells] also agreed with calculations made previously by using other methods (10). These calculated permeabilities suggest that our simplified assay is valid for measuring transepithelial Na and Cl permeability.
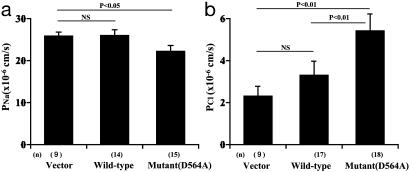
As shown in Fig. 2a, Na permeability was not changed by the expression of the wild-type WNK4 compared with the vector transfection. However, the mutant WNK4 causes a statistically significant decrease in Na permeability (p < 0.05). The Cl permeability in the wild-type WNK4-expressing cell lines tended to be increased over the control (empty-vector transfectants), but the difference was not statistically significant. In the two wild-type WNK4-transfected cell lines that showed the least leaky WNK4 expression in the presence of doxycycline, chloride permeabilities under induced and noninduced conditions were not statistically different. On the other hand, the mutant WNK4 caused an apparent increase in Cl permeability (p < 0.01) over the control and also over the wild type (p < 0.01). This increase of chloride permeability was not due to a general increase of permeability of the epithelia because Na permeability was reduced. To further confirm that the increase in chloride permeability in the mutant WNK4-expressing cells correlated with the expression of the mutant WNK4, we chose the two mutant WNK4-transfected cell lines that showed the least leaky WNK4 expression. In those cells, chloride permeability under the induced and noninduced conditions was 5.8 ± 0.4 and 2.8 ± 0.2 (×10-6 cm/s), respectively, which were statistically significant (mean ± SEM, n = 6, p < 0.01).
Fig. 2.
Transepithelial 22Na (a) and 36Cl (b) permeability in MDCKII cells expressing wild-type and mutant WNK4. Each bar represents experiments using at least three cell lines. The numbers of assays are given in parentheses. At least three assays were performed with each cell line.
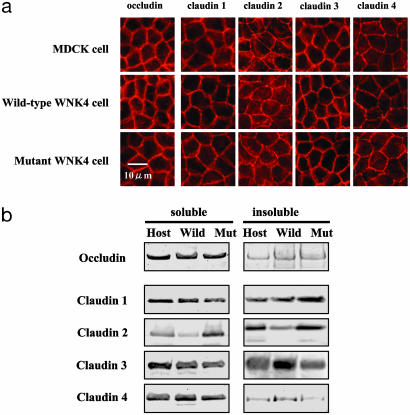
Effect of WNK4 Expression on Cellular Localization of Tight-Junction Proteins. We suspected that WNK4 expression enhanced Cl permeability by modifying proteins in the tight junction. We first performed immunofluoresence and Western blot analyses of tight-junction proteins to determine whether there was any difference in expression or localization. Fig. 3a shows the immunofluorescence staining for several claudins and occludin. The localization of these proteins in the tight junctions did not appear to be affected by wild-type or mutant WNK4 expression. Because the recruitment of some proteins to the tight junctions, including occludin, claudins, and ZO-1, are regulated by various signals (11), we examined their distribution in the Triton X-100-soluble (cytosol) and -insoluble (cytoskeleton and tight junctions) cellular fractions by Western blotting. As shown in Fig. 3b, the distribution of the claudins into Triton X-100-insoluble fraction was not significantly altered by the expression of wild-type or mutant WNK4, although two of four wild-type WNK4-expressing cell lines displayed decreased claudin 2 expression. A similar reduction in claudin 2 expression was reported (12) in cells overexpressing claudin 8, although that was thought to be an artifact of overexpression. We, therefore, concluded that the increased Cl permeability in WNK4-expressing cells was not caused by changes in the recruitment of tight-junction proteins.
Fig. 3.
Tight-junction proteins in WNK4-expressing cells. (a) Immunofluorescence of tight-junction proteins in the WNK4-expresing cells. Stable WNK4-expressing cell lines were grown on a permeable support. Cells were immunostained with anti-claudin 1-4 Abs or with an anti-occludin Ab. (b) Expression of claudins and occludin in Triton X-100-soluble and -insoluble fractions in WNK4-expressing cells. WNK4 expression was induced for 4 days, and cells were harvested in a buffer containing 1% Triton X-100. The Triton X-100-soluble and -insoluble fractions were resolved by SDS/PAGE, and the expression of claudins and occludin were determined by Western blot analysis.
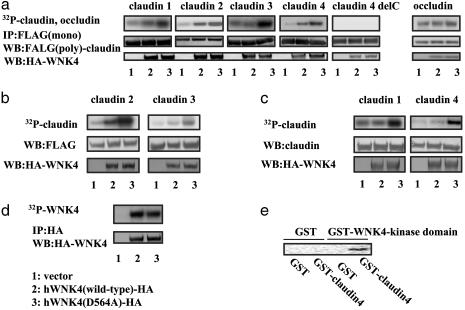
Increased Phosphorylation of Claudins Induced by WNK4 Overexpression. We next investigated whether the change in Cl permeability was associated with modification of the tight-junction proteins (i.e., phosphorylation). Because commercially available Abs for claudins are not always effective for immunoprecipitation, we initially examined the phosphorylation of Flag-tagged claudins. We first used COS7 cells to obtain consistent expression of exogenous claudins. Fig. 4a shows that all claudins tested (claudin 1-4) were phosphorylated in cells expressing WNK4. Cells expressing mutant WNK4 contained a higher level of phosphorylated claudin than cells expressing wild-type WNK4. Occludin, an endogenously phosphorylated tight-junction protein, was not phosphorylated further in cells expressing either the wild-type or the mutant WNK4. Less phosphorylation of claudins by the wild-type WNK4 than the mutant, and the lack of further phosphorylation of occludin by WNK4 suggested that the phosphorylation of claudins by the mutant WNK4 was not due to an artifact of overexpression. The same results were obtained in MDCK II cells (Fig. 4b). To further confirm these results, we also checked the phosphorylation of endogenous claudins in the WNK4-expressing MDCK cells. As shown in Fig. 4c, endogenous claudin 1 and claudin 4 were apparently phosphorylated in the mutant-expressing MDCK II cells, identifying a putative molecular target for this protein kinase. We also examined the level of WNK4 phosphorylation because WNK1 is known to autophosphorylate (13, 14). As shown in Fig. 4d, there was an equal level of phosphorylation on wild-type and mutant WNK4. Finally, to verify that claudins were direct substrates for WNK4, we performed an in vitro kinase assay by using recombinant GST-fusion proteins of the kinase domain of WNK4 and the cytoplasmic domain of claudin 4. As shown in Fig. 4e, WNK4 directly phosphorylated the cytoplasmic domain of claudin 4.
Fig. 4.
Phosphorylation of claudins by WNK4. (a) Phosphorylation of Flag-tagged claudins by WNK4 in COS7 cells. COS7 cells were transfected with HA-tagged WNK4 and Flag-tagged claudins or occuludin. Claudin 4 delC lacks the entire C-terminal cytosolic region of claudin4. Cells were labeled with [32P]Pi (1 mCi/ml), and proteins were immunoprecipitated with an anti-Flag Ab (M2). The immunoprecipitated Flag-claudins were separated by SDS/PAGE, electrophoretically transferred to nitrocellulose, and analyzed by Western blotting with an anti-Flag polyclonal Ab. After detecting the immunoprecipitated claudins, claudin phosphorylation was detected by autoradiography of the nitrocellulose membrane. (b) Phosphorylation of Flag-tagged claudins in the WNK4-expressing MDCK II cells. MDCK II cells stably expressing HA-tagged WNK4 were transfected with Flag-tagged claudin 2 and 3. Phosphorylated claudin 2 and 3 were detected, as described in a.(c) Phosphorylation of endogenous claudins in the mutant WNK4-expressing MDCK II cells. The WNK4-expressing cells were labeled with [32P]Pi, and the endogenous claudins were immunoprecipitated by using anti-claudin 1 and 4 Abs (Zymed). Phosphorylation of claudins was visualized by autoradiography. (d) Phosphorylation of wild-type and mutant WNK4. MDCK II cells were transfected with HA-tagged wild-type and mutant WNK4. Cells were labeled with [32P]Pi, and WNK4 proteins were immunoprecipitated with an anti-HA Ab. Immunoprecipitated proteins were resolved on SDS/PAGE, electrophoretically transferred to nitrocellulose, and analyzed by Western blotting with an anti-HA Ab. WNK4 phosphorylation was detected by autoradiography. (e) In vitro kinase assay with GST-WNK4. GST-WNK4 (kinase domain) and GST-claudin 4 (C-terminal cytoplasmic domain) were incubated at 37°C for 15 min in kinase buffer, and the phosphorylation of GST-claudin 4 was visualized by SDS/PAGE, followed by autoradiography.
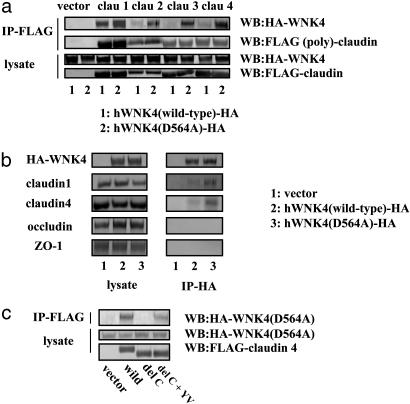
Protein-Protein Interaction of WNK4 and Claudins. Our results clearly show that WNK4, especially the mutant WNK4, can phosphorylate claudins. Moreover, it appears that the mutant WNK4 has an enhanced ability to phosphorylate these proteins. To investigate this possibility further, we first examined whether WNK4 associates with the claudins. As expected from the phosphorylation study, claudins and WNK4 coimmunoprecipitated (Fig. 5a). The mutant WNK4 showed a much higher association with claudins than the wild-type WNK4. Because our analyses of WNK4 phosphorylation suggested that the wild-type and mutant proteins possess the same level of autophosphorylation (Fig. 4d) and, therefore, the same kinase activities, it appears that the enhanced phosphorylation of claudins by mutant WNK4 is due to the increased claudin-WNK4 association. Interaction of the mutant WNK4 with the endogenous claudin 1 and 4 but not with ZO-1 and occludin was confirmed in the mutant-expressing MDCK II cells (Fig. 5b).
Fig. 5.
Analysis of WNK4-claudin interaction. (a) Coimmunoprecipitation of WNK4 and claudins. COS7 cells were transfected with HA-tagged WNK4 and Flag-tagged claudins. Proteins were immunoprecipitated with an anti-Flag M2 Ab, and the immunoprecipitates were analyzed by Western blot analysis by using anti-HA Ab. (b) Coimmunoprecipitation of WNK4 and endogenous tight-junction proteins. Proteins from MDCK II cells stably expressing the wild-type and mutant HA-WNK4 were immunoprecipitated with anti-HA Ab, and the immunoprecipitates were analyzed by Western blot analysis by using Abs to claudin 1, claudin 4, ZO-1, and occludin. (c) Binding of claudin 4 and its mutants by mutant WNK4. Claudin 4 binding was assessed by coimmunoprecipitation in lysates from MDCK II cells as described in a. Mutants examined include delC (the claudin 4 mutant lacking the entire C terminal cytoplasmic region) and del C plus YV (the del C mutant with Y and V added to the C terminus). Addition of YV residues restored the binding to WNK4.
In our phosphorylation studies (Fig. 4a), we found that deletion of the entire cytoplasmic C terminus of claudin 4 eliminated its phosphorylation, suggesting that the C terminus is the site of phosphorylation and/or binding by WNK4. Fig. 5c confirms that this C-terminal deletion mutant lost its ability to bind to the mutant WNK4, but that the addition of the C-terminal YV, a sequence highly conserved in all claudins (a binding site to PDZ domains of ZO-1, 2, and 3) (15), restored binding to the mutant WNK4. This finding indicates that the YV sequence is necessary for claudin binding and explains the fact that the mutant WNK4 binds all of the claudins tested. Phosphorylation of this mutant was not restored by the addition of YV motif (data not shown), suggesting that phosphorylation sites were present within the cytosolic C terminus.
Discussion
Two findings are presented in this article. First, we identified claudins as molecular targets of the protein kinase WNK4, especially the disease-causing mutant WNK4. Second, we show that paracellular ion permeability is regulated by the mutant WNK4. This study characterizes WNK4 expressed in polarized epithelial cells, and both data are quite consistent with the in vivo localization of WNK4 and the chloride shunt hypothesis in PHAII.
A growing body of evidence, including genetic (16) and cell biological (10, 12, 17-20) data, has suggested that paracellular transepithelial transport is important in vectorial ion and solute transport across the epithelia. Thus, it appears that, like channels, paracellular transport possesses selectivity for ions and solutes. Although several lines of evidence have suggested that claudins are the major determinants of paracellular ion selectivity (10, 12, 17-20), their phosphorylation has not, to our knowledge, been demonstrated. Our data showed a good correlation between increased chloride permeability and claudin phosphorylation induced by the mutant WNK4, suggesting that the two phenomena are related causally. However, whether the phosphorylation of claudins causes an increase in the chloride permeability directly remains to be determined. A mutagenesis study showed that the extracellular domain of claudin controls paracellular charge selectivity and resistance (10). However, it is not clear now whether the phosphorylation of the cytoplasmic domain affects chloride permeability. Currently, there is no good system, to our knowledge, for assaying the function of exogenously expressed claudins in mammalian cells because of the presence of numerous endogenous claudins. We can only speculate that the phosphorylation by WNK4 affects the conformation of claudins, increasing Cl ion pore permeability or affecting their interaction with intracellular regulators, such as cytoskeletal proteins.
We found in this study that the disease-causing mutation in WNK4 has a gain-of-function effect, causing increased chloride permeability and hyperphosphorylation of claudins. This finding provides a good explanation for the autosomal-dominant nature of inheritance in PHAII. Very recently, Kahle et al. (6) reported the inhibition of surface expression of the renal K+ channel ROMK by coexpression of wild-type WNK4 in Xenopus oocytes. Interestingly, a disease-causing mutation in WNK4 that relieves TSC inhibition (4, 5) markedly increases inhibition of ROMK. This difference in the action of the mutant WNK4 on TSC and ROMK indicates that WNK4 functions as a multifunctional regulator of diverse ion transporters. Identification of functions of WNK4 in this study further supports this notion. However, how this mutation increases WNK4 activity is not clear, as in the case of ROMK inhibition. In terms of claudin phosphorylation, one possible explanation for the enhanced effect of the mutant is change in the binding to claudins. In fact, Xu et al. (14) recently reported the presence of an autoinhibitory domain in WNK1 that resides near the coil domain. Interestingly, PHAII-causing mutations are concentrated near the putative autoinhibitory domain of WNK4. Therefore, it is possible that the disease-causing mutations in WNK4 somehow affect its interaction with its negative regulators including its own domains, releasing the active site, and allowing enhanced binding to claudins. Although we show that YV motif of claudins is important for the interaction with WNK4, further work will be required to define the whole mechanisms of their interaction.
In this article, we identified the claudins as targets of WNK4 kinase and showed that the disease-causing mutant WNK4 expression increases paracellular chloride permeability without increasing sodium permeability. A similar phenomenon may occur in the collecting ducts of PHAII patients. Therefore, increased chloride shunt caused by a gain-of-function activity of mutant WNK4 may underlie or contribute to the pathogenesis of PAHII.
Supplementary Material
Acknowledgments
We thank H. Ichijo (Tokyo University) for his excellent advice. This study was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Salt Science Research Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PHAII, pseudohypoaldosteronism type II; TSC, Na-dependent Cl cotransporter; MDCK II, Madin-Darby canine kidney II; HA, hemagglutinin.
References
- 1.Wilson, F. H., Disse-Nicodeme, S., Choate, K. A., Ishikawa, K., Nelson-Williams, C., Desitter, I., Gunel, M., Milford, D. V., Lipkin, G. W., Achard, J. M., et al. (2001) Science 293, 1107-1112. [DOI] [PubMed] [Google Scholar]
- 2.Schambelan, M., Sebastian, A. & Rector, F. C., Jr. (1981) Kidney Int. 19, 716-727. [DOI] [PubMed] [Google Scholar]
- 3.Farfel, Z., Iaina, A., Rosenthal, T., Waks, U., Shibolet, S. & Gafni, J. (1978) Arch. Intern. Med. (Moscow) 138, 1828-1832. [PubMed] [Google Scholar]
- 4.Wilson, F. H., Kahle, K. T., Sabath, E., Lalioti, M. D., Rapson, A. K., Hoover, R. S., Hebert, S. C., Gamba, G. & Lifton, R. P. (2003) Proc. Natl. Acad. Sci. USA 100, 680-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang, C. L., Angell, J., Mitchell, R. & Ellison, D. H. (2003) J. Clin. Invest. 111, 1039-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahle, K. T., Wilson, F. H., Leng, Q., Lalioti, M. D., O'Connell, A. D., Dong, K., Rapson, A. K., MacGregor, G. G., Giebisch, G., Hebert, S. C. & Lifton R. P. (2003) Nat. Genet. 35, 372-376. [DOI] [PubMed] [Google Scholar]
- 7.Schuster, V. L. & Stokes, J. B. (1987) Am. J. Physiol. 253, F203-F212. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin, M. D., Kaplan, M. R., Verlander, J. W., Lee, W. S., Brown, D., Poch, E., Gullans, S. R. & Hebert, S. C. (1996) Kidney Int. 50, 174-183. [DOI] [PubMed] [Google Scholar]
- 9.Xu, J. Z., Hall, A. E., Peterson, L. N., Bienkowski, M. J., Eessalu, T. E. & Hebert, S. C. (1997) Am. J. Physiol. 273, F739-F748. [DOI] [PubMed] [Google Scholar]
- 10.Colegio, O. R., Itallie, C. V., Rahner, C. & Anderson, J. M. (2003) Am. J. Physiol. 284, C1346-C1354. [DOI] [PubMed] [Google Scholar]
- 11.Nishiyama, R., Sakaguchi, T., Kinugasa, T., Gu, X., MacDermott, R. P., Podolsky, D. K. & Reinecker, H. C. (2001) J. Biol. Chem. 276, 35571-35580. [DOI] [PubMed] [Google Scholar]
- 12.Yu, A. S., Enck, A. H., Lencer, W. I. & Schneeberger, E. E. (2003) J. Biol. Chem. 278, 17350-17359. [DOI] [PubMed] [Google Scholar]
- 13.Xu, B., English, J. M., Wilsbacher, J. L., Stippec, S., Goldsmith, E. J. & Cobb, M. H. (2000) J. Biol. Chem. 275, 16795-16801. [DOI] [PubMed] [Google Scholar]
- 14.Xu, B. E., Min, X., Stippec, S., Lee, B. H., Goldsmith, E. J. & Cobb, M. H. (2002) J. Biol. Chem. 277, 48456-48462. [DOI] [PubMed] [Google Scholar]
- 15.Itoh, M., Furuse, M., Morita, K., Kubota, K., Saitou, M. & Tsukita, S. (1999) J. Cell Biol. 147, 1351-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon, D. B., Lu, Y., Choate, K. A., Velazquez, H., Al-Sabban, E., Praga, M., Casari, G., Bettinelli, A., Colussi, G., Rodriguez-Soriano, J., et al. (1999) Science 285, 103-106. [DOI] [PubMed] [Google Scholar]
- 17.Amasheh, S., Meiri, N., Gitter, A. H., Schoneberg, T., Mankertz, J., Schulzke, J. D. & Fromm, M. (2002) J. Cell Sci. 115, 4969-4976. [DOI] [PubMed] [Google Scholar]
- 18.Colegio, O. R., Van Itallie, C. M., McCrea, H. J., Rahner, C. & Anderson, J. M. (2002) Am. J. Physiol. 283, C142-C147. [DOI] [PubMed] [Google Scholar]
- 19.Furuse, M., Furuse, K., Sasaki, H. & Tsukita, S. (2001) J. Cell Biol. 153, 263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Itallie, C., Rahner, C. & Anderson, J. M. (2001) J. Clin. Invest. 107, 1319-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.