Abstract
Disulfide bond formation drives protein import of most proteins of the mitochondrial intermembrane space (IMS). The main components of this disulfide relay machinery are the oxidoreductase Mia40 and the sulfhydryl oxidase Erv1/ALR. Their precise functions have been elucidated in molecular detail for the yeast and human enzymes in vitro and in intact cells. However, we still lack knowledge on how Mia40 and Erv1/ALR impact cellular and organism physiology and whether they have functions beyond their role in disulfide bond formation. Here we summarize the principles of oxidation-dependent protein import mediated by the mitochondrial disulfide relay. We proceed by discussing recently described functions of Mia40 in the hypoxia response and of ALR in influencing mitochondrial morphology and its importance for tissue development and embryogenesis. We also include a discussion of the still mysterious function of Erv1/ALR in liver regeneration.
1. Introduction
Because almost all proteins in eukaryotic cells are synthesized by cytosolic ribosomes, protein translocation across membranes is critical for organelle biogenesis. The invention of organelle-specific targeting systems in the cytosol was instrumental to facilitate correct translocation events and to avoid mistargeting. These pathways are usually complemented by machineries in the organelle lumen which provide driving force and ensure directionality. For example, in the endoplasmic reticulum (ER) and the mitochondrial matrix members of the Hsp70 family of chaperones bind incoming substrates and thereby prevent their backsliding (ratchet-like mechanism) [1]. A similar mechanism is employed for protein import into the mitochondrial intermembrane space (IMS). Here formation of inter- and intramolecular disulfide bonds by the essential mitochondrial disulfide relay is critical for translocation across the mitochondrial outer membrane [2–6]. In this review, we will discuss the disulfide relay and its components, compare and contrast the machineries in yeast and human cells, and discuss additional potentially nonoxidative functions of disulfide relay components in human cells.
2. Substrates of the Mitochondrial Disulfide Relay
Most proteins that are imported into mitochondria contain either a mitochondrial targeting signal (MTS) or internal targeting sequences [4, 7, 8]. They are thereby targeted into the mitochondrial matrix or to the two mitochondrial membranes. In contrast, only few of the precursors of IMS proteins carry the so-called bipartite presequences consisting of an MTS and a hydrophobic sorting region [8, 9]. The import of the majority of soluble IMS proteins is facilitated by the mitochondrial disulfide relay system in a process that is linked to the oxidative folding of the proteins [3, 10] (Figure 1). Most of the so far identified disulfide relay substrates belong to the families of twin-CX3C proteins or twin-CX9C proteins (C, cysteine; X, any amino acid) (Figure 1(a)). The members of both families are small proteins with most of them having a size of around 10 kDa. They share a common simple core structure that consists of two antiparallel alpha helices arranged in a helix-loop-helix motif [11]. Each helix contains two cysteines that are separated by either three or nine amino acids for members of the twin-CX3C or twin-CX9C families, respectively [11–16]. Twin-CX3C or twin-CX9C proteins fulfill diverse functions within the IMS. They serve as chaperones for newly imported proteins, are involved in metal transfer and insertion during respiratory chain biogenesis, or are part of mature respiratory chain complexes [13, 17–22]. In human and yeast cells exist a total of five proteins that belong to the twin-CX3C family. Conversely, the twin-CX9C family appears to be significantly larger in mammalian cells, and in addition numerous proteins exist that do not adhere exactly to the nine amino acid-wide spacing (and instead have, e.g., CX8C or CX10C motifs). So far more than 30 twin-CX9C family members have been identified in human cells, and some of them have been confirmed to be disulfide relay substrates [23, 24].
Figure 1.
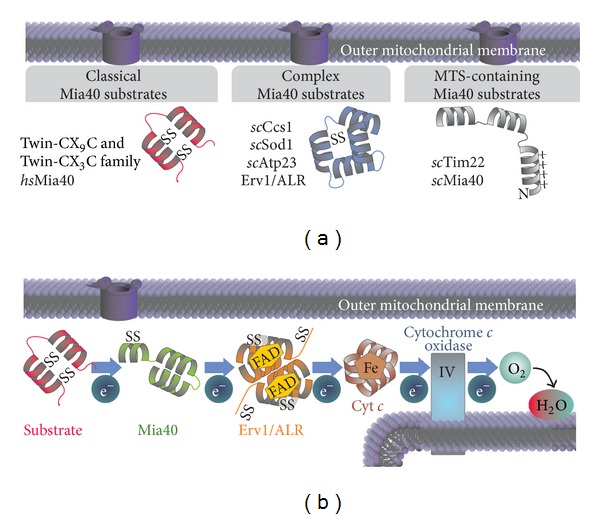
Substrates and general outline of the mitochondrial disulfide relay. (a) Mia40 substrates can be classified into three groups: (1) members of the twin-CX9C and twin-CX3C family, respectively. Members of both families rely on four cysteines localized within two α-helices for proper import. (2) The proteins scCcs1, scSod1, scAtp23, and Erv1/ALR form a second group of substrates with more complex folds and disulfide patterns. So far no common signal for the interaction with Mia40 has been identified in these proteins. (3) The two MTS-containing Mia40 substrates Tim22 and scMia40 are imported in a membrane potential-dependent manner and require Mia40 for proper folding only. (b) General outline of oxidative folding in the IMS. During substrate oxidation electrons are transferred from the substrate to Mia40. To reoxidize Mia40 electrons are transferred further via ALR to cytochrome c (Cyt c) and then to cytochrome c oxidase. Molecular oxygen (O2) is used as final electron acceptor to finally yield water (H2O).
In addition to twin-CX3C and twin-CX9C proteins several more complex substrates exist that rely on the mitochondrial disulfide relay for oxidation (Figure 1(a)). In yeast the import of the dually localized copper chaperone for superoxide dismutase 1 (Ccs1) and in part also that of superoxide dismutase 1 (Sod1) depends on the mitochondrial disulfide relay [25–27]. Likewise, import and oxidation of the sulfhydryl oxidase Erv1 which itself is part of the mitochondrial disulfide relay (see below) are driven by the disulfide relay system [28]. Further substrates are the mitochondrial protease Atp23 and the inner membrane protein Tim22 [29, 30]. The latter protein contains a bipartite presequence and thus requires oxidation only for folding but not for mitochondrial import. Because so far a systematic identification of interaction partners and substrates of the mitochondrial disulfide relay system is lacking in yeast and mammalian cells, we do not know how large the group of disulfide-containing IMS proteins is. It is likely that it will be significantly larger than previously anticipated as the recently solved partial IMS proteome contains numerous proteins that are dually localized between cytosol and IMS, but lack MTS, and might therefore be disulfide relay substrates [31].
3. The Mechanism of Oxidation-Dependent Protein Import by the Mitochondrial Disulfide Relay System
All proteins of the IMS are synthesized by cytosolic ribosomes [32] (Figure 2). However, only very few of them contain a classical MTS or internal targeting signals that guide them to mitochondria. Instead, most IMS proteins contain conserved cysteine patterns or other still ill-defined motifs that are recognized by the IMS-localized mitochondrial disulfide relay but likely also by so far not identified cytosolic factors [4, 10]. Such factors could ensure targeting of disulfide relay substrates to mitochondria for posttranslational import and maintain them in an import-competent unfolded state. In addition, disulfide relay substrates have to be kept in a reduced state in the cytosol. This is facilitated by cytosolic glutaredoxins in human cells and the thioredoxin system in yeast [33, 34] and potentially by the presence of zinc ions that can complex reduced cysteines [35, 36]. In yeast, the amounts of import-competent substrates are also controlled by the cytosolic proteasome system that degrades substantial amounts of newly synthesized disulfide relay system substrates before they can be imported [37]. At present it remains unclear whether such a degradation pathway is also found in mammalian cells and under which conditions it may serve in adapting amounts of imported IMS proteins.
Figure 2.
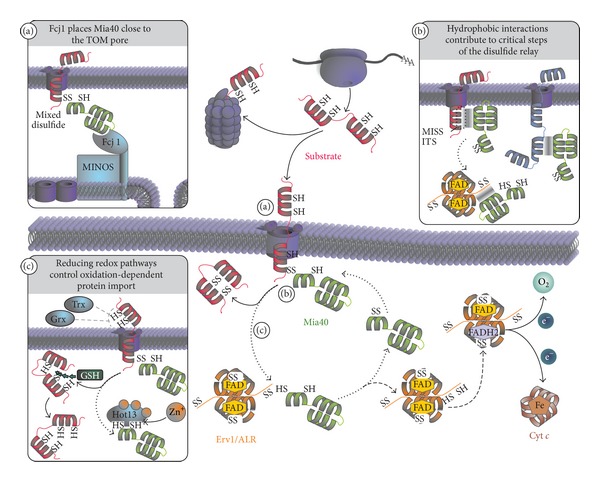
The mitochondrial disulfide relay system facilitates protein import and folding into the IMS. Classical substrates of the twin-CX3C and twin-CX9C families are translated on cytosolic ribosomes. In part, these proteins are degraded by the proteasome, while the majority becomes posttranslationally imported into the IMS through the TOM pore. Noncovalent and covalent interactions between Mia40 and the substrate are necessary for translocation and oxidative folding of the substrate. Immediately after the first cysteine of the substrate translocates a mixed disulfide between Mia40 and substrate is formed. The substrate becomes oxidized by resolving the mixed disulfide complex. Reduced Mia40 is then reoxidized by the flexible N-terminal domain of one subunit of Erv1/ALR, allowing another round of substrate oxidation. Within the Erv1/ALR homodimer electrons are transferred from the N-terminal cysteines of one subunit to the C-terminal cysteines of the other subunit from where they are shuttled to the prosthetic FAD molecule. Erv1/ALR then passes electrons onto cytochrome c—and further to cytochrome c oxidase and oxygen yielding H2O as product. Alternatively, electrons can be transferred from the FAD directly onto oxygen thus forming H2O2. (a) The MINOS complex is important for the organization of the IMS in yeast. Both the arrangement of the cristae and the close proximity of the TOM and TIM pore are mediated by MINOS. Fcj1 binds to the MINOS complex and also interacts with Mia40, thereby placing Mia40 close to the TOM pore. (b) Hydrophobic interactions between the hydrophobic groove of Mia40 and twin-CX3C and twin-CX9C proteins are necessary for substrate recognition by Mia40. The same hydrophobic patch on Mia40 also mediates its interaction with the N-terminal domain of ALR. Moreover, the hydrophobic groove of Mia40 also equips the protein with a holdase-like function that can serve in importing cysteine-less substrates. (c) Several redox control pathways facilitate efficient oxidative import and folding of substrates. In the cytosol substrate cysteines are maintained in their reduced state mainly by thioredoxins (Trx) and glutaredoxins (Grx) in yeast and human cells, respectively. During Mia40-dependent oxidation reduced glutathione (GSH) exhibits a proofreading function by reducing wrongly oxidized substrates and resolving trapped intermediates of substrate and Mia40. Upon becoming reduced, the cysteines of Mia40 are prone to bind zinc ions, thereby interfering with reoxidation. The zinc chelating protein Hot13 keeps Mia40 zinc free.
Translocation of IMS proteins takes place across the translocase of the outer membrane (TOM). Upon exposure of a recognition motif termed MISS or ITS (for mitochondrial intermembrane space sorting and IMS-targeting signal, resp.) disulfide relay substrates are recognized by the protein Mia40 (for mitochondrial IMS import and assembly; in mammalian cells also CHCHD4) [14, 38], which thereby serves both as import receptor and chaperone and oxidoreductase [23, 29, 39] (Figure 2, insets (a) and (b)). Mia40 consists of a structural helix-loop-helix motif with two stabilizing disulfide bonds that form hydrophobic substrate recognition and binding groove and a redox-active CPC motif that is positioned in a flexible helix which hovers over the substrate binding site [40, 41]. Yeast and human Mia40 share high homology, except for an N-terminal extension in yeast Mia40 that contains a bipartite presequence which is lacking in the human protein. Human Mia40 appears in two different splice variants (CHCHD4.1 and CHCHD4.2) [42, 43]. They are completely identical except for the very N-terminal part of the protein. The isoform 1 does contain an additional cysteine at position four; however, whether the isoforms exhibit different functionality or substrate specificity is not known. Like for ALR the import and folding of human Mia40 depend on the disulfide relay system [44]. In contrast, yeast Mia40 requires the disulfide relay system only for oxidative folding [45]. In yeast, Mia40 is positioned close to the trans-side of the TOM complex by its interaction with Fcj1 (for formation of cristae junction; in human cells mitofilin) [46] (Figure 2, inset (a)). Fcj1 is part of the MINOS complex which organizes the topology of the cristae in the inner mitochondrial membrane [46–50].
MISS/ITS motifs have been well defined for classical twin-CX3C and twin-CX9C proteins but their nature has to be clarified for the growing class of nonclassical substrates like Atp23. It contains hydrophobic residues, and in most cases, a single cysteine residue, that are positioned on the same side of an alpha helix [14, 38] (Figure 2, inset (b)). After recognition of the MISS/ITS signal by the hydrophobic binding groove of Mia40, the thiolate anion of a cysteine in the substrate performs a nucleophilic attack on the oxidized CPC motif of Mia40 which results in the formation of an intermolecular disulfide bond [2, 39]. This disulfide bond together with the hydrophobic interactions between substrate and Mia40 prevents the backsliding of the incompletely translocated substrate into the cytosol, thus coupling import to oxidative protein folding [51]. Consequently, mutation of critical cysteines in Mia40 substrates also results in very low amounts of these substrates in the IMS [2]. This indicates that hydrophobic interactions with Mia40 might be sufficient to drive IMS import at least of some proteins that neither contain classical MTS nor cysteines to interact with Mia40. Furthermore, Mia40 might also contribute to protein folding as it is capable of stabilizing cysteine-free unfolded proteins and prevents their aggregation [29] (Figure 2, insert (b)).
The intermolecular disulfide bond between substrate and Mia40 is resolved by another nucleophilic attack of a thiolate anion in the substrate leaving an oxidized substrate molecule and a reduced Mia40 molecule [39]. Thus, for this import mechanism to work Mia40 substrates have to contain at least two cysteines. For the introduction of more than one disulfide bond, multiple oxidized Mia40 molecules or molecular oxygen are necessary. It has been suggested that Mia40 can act more efficiently in the introduction of multiple disulfide bonds by forming a ternary complex with its substrate and the essential protein Erv1 (in mammalian cells augmenter of liver regeneration (ALR), growth factor erv1-like (Gfer1), hepatopoietin or hsErv1) [52, 53]. The very rapid introduction of disulfide bonds after the formation of the initial intermolecular disulfide bond is supported by the fact that in vivo no semioxidized intermediates of substrate proteins can be observed [23].
The sulfhydryl oxidase Erv1/ALR acts to reoxidize the CPC motif in Mia40 [39]. It is a homodimeric protein in which each subunit consists of two domains [54]. The first—N-terminal domain—contains a redox-active CXXC motif and serves as a “shuttle arm” that mediates the electron transfer from Mia40 to a CXXC motif in the C-terminal core domain of Erv1/ALR [39, 55]. To this end, this mainly unstructured domain interacts with the substrate-binding groove of Mia40 [56, 57]. Consequently, overexpression of Erv1/ALR in intact cells delays oxidative protein folding and IMS import because the N-terminal arm blocks substrate binding to Mia40 [23]. Both Mia40 and Erv1/ALR are perfectly adapted to this critical interaction of shuttle arm and hydrophobic groove. In a heterologous yeast system human ALR or human Mia40 when expressed individually could to a large part complement their yeast counterparts. However, only if human Mia40 and human ALR were concomitantly used to substitute the respective yeast proteins full complementation was ensured [44]. After Mia40 reoxidation the shuttle arm of Erv1/ALR swings over to the core domain of the second subunit of Erv1/ALR (intersubunit electron transfer) and becomes reoxidized by the core CXXC motif [55]. This core CXXC motif is reoxidized by the redox cofactor of Erv1/ALR-flavin adenine dinucleotide (FAD) by the formation of a charge-transfer complex [58]. The FAD is held in place by the very compact four-helix bundle structure of Erv1/ALR [54, 59, 60]. The dimer of Erv1/ALR is stabilized by hydrophobic interactions and in mammalian cells additionally also by disulfide bonds [39, 61]. Finally, the reduced FAD cofactor is reoxidized by either transferring electrons directly onto molecular oxygen which gives rise to the production of hydrogen peroxide or alternatively by transferring electrons to cytochrome c [39, 62, 63]. To which extent both pathways are utilized in intact cells remains unclear although in vitro cytochrome c appears to be the preferred electron acceptor [39, 58, 62, 64, 65]. At least in yeast cells Erv1 can transfer electrons also onto an anaerobic electron acceptor [66]. The identity of this acceptor and whether it is conserved in mammalian cells remains unclear.
In addition to Mia40 and Erv1/ALR further factors modulate oxidative folding in the IMS—the protein helper of Tim protein (Hot13, in mammalian cells RCHY1) and the local glutathione pool (Figure 2, insert (c)). Hot13 is a cysteine-rich protein that is capable of chelating zinc ions [67]. Although low amounts of zinc ions can facilitate mitochondrial import in vitro, too high amounts hamper substrate oxidation and Mia40 reoxidation by binding to reduced cysteines [36, 67, 68]. It has thus been proposed that Hot13 keeps the CPC motif of Mia40 in a zinc-free state thereby accelerating oxidation-dependent protein import. In vitro substrate oxidation appears to yield side products with nonnative disulfides or substrates that are trapped in their mixed disulfide complex with Mia40 [39]. Formation of these products is avoided by the presence of reduced glutathione. Also in intact cells reduced glutathione seems to be beneficial for oxidation-dependent protein import: on the one hand by contributing to the reduced redox state of Mia40 substrates in the mammalian cytosol, and on the other hand by accelerating oxidative protein folding by a still unresolved mechanism [23]. Like zinc ions glutathione might be a two-edged sword. The IMS glutathione pool in yeast and mammalian cells has been measured to be as reducing as the one in the cytosol [23, 69]. The IMS glutathione redox potential is thereby in the range of the redox potential of Mia40 substrates, raising the question of how these substrates can be oxidized and maintained in an oxidized state [35, 65, 69–73]. Although this point has not been addressed experimentally, it is likely that the thermodynamically feasible reduction of Mia40 substrates is kinetically prevented, for example, hampering the equilibration between protein thiols and glutathione. In principle glutathione can affect IMS proteins in vivo as the CPC motif of Mia40 is affected by glutathione in intact cells [23, 69]. Consequently, Mia40 is maintained in a partially reduced state in yeast cells [69]. The reduced part of molecules might well be involved in either isomerisation or reduction reactions like oxidoreductases in other systems that facilitate oxidative protein folding. However, such a novel role of Mia40 has not been shown.
Besides their function in oxidative protein folding in mitochondria Mia40 and Erv1/ALR also function in potentially unrelated (nonmitochondrial) pathways. Mia40 was shown to be critical for mitochondrial dynamics and the hypoxia response [43] (Figure 3). For Erv1/ALR, a plethora of different cellular and physiological functions were described (Figure 4). Erv1/ALR influences fusion and fission processes of mitochondria [74–77]; it is important for the development of certain organs during embryogenesis [78, 79] and functions as mitogen to enhance regenerative capacities of liver tissue [80–82]. These functions will be discussed in the following.
Figure 3.
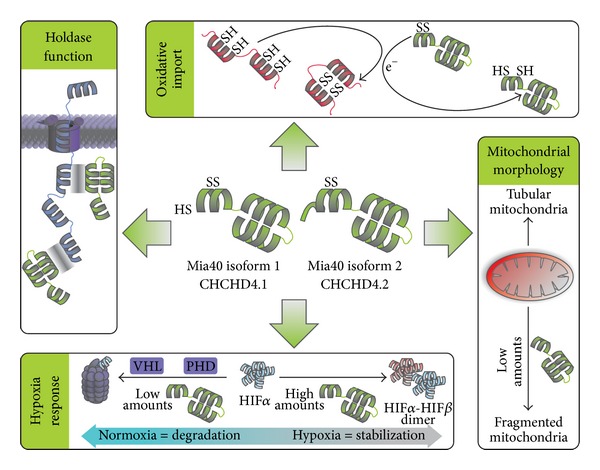
Functions of human Mia40. The protein exists in two different isoforms. Differences in function between the isoforms are not known. Oxidative Import. Mia40 is the main receptor for import and oxidation of twin-CX9C and twin-CX3C proteins in the IMS. Holdase Function. Mia40 shields hydrophobic patches on proteins imported into the IMS, thus allowing proper folding of these proteins. Mitochondrial Morphology. Reduced amounts of Mia40 lead to increased mitochondrial fission and the formation of a more fragmented mitochondrial network. Hypoxia Response. Under normoxic conditions the protein HIF1α is constantly degraded. This degradation is mediated by hydroxylation by prolyl hydroxylase domain enzymes (PHD) and ubiquitination by the Hippel-Lindau E3 ligase (VHL). Upon hypoxia oxygen as substrate of PHD is missing resulting in impaired HIF1α degradation and increased stability. If not degraded HIF1α can dimerize with constitutively expressed HIF1β and induce the hypoxia response. Silencing Mia40 using RNAi prevents stabilization of HIF1α under hypoxic conditions. In contrast increased amounts of Mia40 as can also be found in certain tumors result in increased stabilization of HIF1α.
Figure 4.
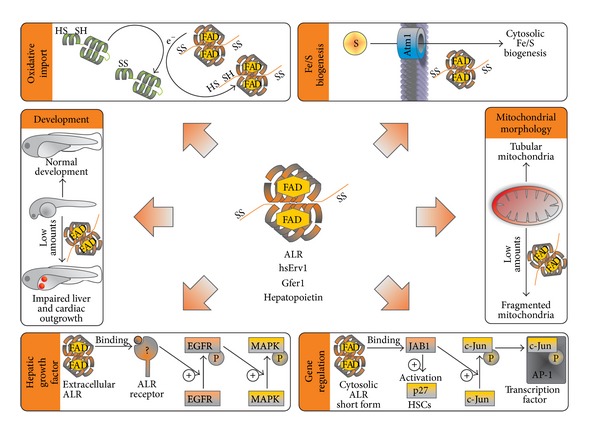
Functions of Erv1/ALR. Oxidative Import. Erv1/ALR reoxidizes Mia40. Fe/S Biogenesis. In yeast Erv1/ALR is required for the biogenesis of cytosolic Fe/S proteins but not for mitochondrial Fe/S proteins. Development. Erv1/ALR is expressed during development. Knockdown or chemical inhibition of Erv1/ALR leads to impaired development of organs such as liver and hamper cardiac outgrowth. Mitochondrial Morphology. Erv1/ALR is important for mitochondrial morphology in undifferentiated cells. Knockdown of Erv1/ALR in mouse embryonic stem cells leads to mitochondrial fragmentation and increased levels of Drp1 (dynamin-related protein 1). Hepatic Growth Factor. Extracellular Erv1/ALR increases regenerative capacities of liver tissue. Extracellular Erv1/ALR can bind to a so far unknown receptor. Upon binding tyrosine-phosphorylation of the epidermal growth factor receptor (EGFR) is enhanced which promotes phosphorylation and activation of mitogen activated protein kinase (MAPK). Gene Regulation. A truncated cytosolic form of Erv1/ALR can bind to Jun activating binding protein 1 (JAB1). Binding to JAB1 increases JAB1-mediated phosphorylation of c-Jun. Upon phosphorylation c-Jun can form a complex with AP-1 complex (activator protein 1). In hematopoietic stem cells (HSC) sequestering of JAB1 by ALR prevents binding of JAB1 to p27(kip).
4. Physiological Impact of Mia40 and Erv1/ALR
4.1. A Function of Mia40 in Hypoxia
Mia40 is not only necessary for proper assembly of the respiratory chain but is also involved in the stabilization of hypoxia inducing factor 1α (HIF1α) [43, 74] (Figure 3). In the presence of high amounts of oxygen HIF1α is continuously degraded by the proteasome after hydroxylation by oxygen-dependent prolyl hydroxylase domain (PHD) enzymes and subsequent ubiquitinylation by the E3 ligase VHL (von Hippel-Lindau) [83–85]. Under low oxygen conditions PHDs lack oxygen and fail to completely hydroxylate HIF1α. Moreover, reactive oxygen species take part in the stabilization process by further inhibiting PHD [86, 87]. The stabilization of HIF1α by low oxygen concentrations can be mimicked by incubating cells with iron chelators as PHD activity depends on an iron cofactor [88–90].
The modulation of Mia40 levels affects HIF1α stabilization at low oxygen concentration but not by treatment with iron chelators [43]. Upon depletion of Mia40 using siRNA-mediated knockdown HIF1α failed to accumulate under low oxygen conditions, while Mia40 overexpression enhanced HIF-1α stabilization under hypoxic conditions. Since the hypoxia response is critical for tumor growth Mia40 depletion effectively inhibited tumor growth and angiogenesis in vivo [43]. In line with these findings in human cancer, increased Mia40 expression was found to correlate with the signature of hypoxia gene expression [43]. Whether the described effect of Mia40 on the stabilization of HIF1α arises from a direct interaction or is indirectly mediated by an impaired respiratory chain remains unclear and is an exciting question for future research.
4.2. Physiological Functions of Erv1/ALR—in Mitochondria and the Cytosol?
Human patients with a homozygous mutation in Erv1/ALR exhibit respiratory-chain deficiency, myopathy, congenital cataract, sensorineural hearing loss, and delayed development [59, 91] (Figure 4). In zebrafish the formation of heart and liver is impaired upon chemical inhibition or silencing of Erv1/ALR [78, 79]. In addition, chemical inhibition of Erv1/ALR induces apoptosis in human embryonic stem cells [79]. Likewise, silencing of Erv1/ALR in mouse embryonic stem cells results in caspase-induced apoptosis as well as in excessive fragmentation of mitochondria and elimination of damaged mitochondria through mitophagy [76, 77]. Taken together these data underline the importance of Erv1/ALR for mitochondrial functionality especially during development.
Most of those physiological effects of Erv1/ALR likely derive directly or indirectly from its role in the mitochondrial disulfide relay (Figures 2 and 4). However, they might also be linked to a role in the biogenesis of cytosolic iron sulfur proteins which has been described for yeast Erv1 [92]. Moreover, they may derive from a so far unappreciated function of Erv1/ALR in the cytosol where overexpressed and tagged Erv1/ALR could be detected in some studies [92]. Unfortunately, overexpression of IMS proteins without bipartite MTS frequently results in mislocalization to the cytosol [23]. It thus remains unclear whether endogenous Erv1/ALR also is dually localized. Besides full length Erv1/ALR a shorter isoform consisting only of the C-terminal core domain has been described to exist in the cytosol and nucleus of mammalian cells and to be secreted as a growth factor [93, 94]. The existence of this isoform has been confirmed by immunoblotting of human cell lysate against endogenous ALR although specificity controls using siRNA-mediated knockdown were lacking in these studies.
4.3. A Role for Erv1/ALR in Liver Regeneration
Erv1/ALR has been described to enhance the regenerative capacities of liver tissue [95–97]. For this role of Erv1/ALR two different mechanisms were proposed. In one model Erv1/ALR acts extracellularly as mitogen [81]. Several studies describe the regeneration-enhancing abilities of Erv1/ALR on damaged liver tissue after application of the purified C-terminal domain of human or rat Erv1/ALR [80]. The C-terminal domain can be cross-linked to a 60 kDa protein which is probably located at the cellular surface [81]. The putative Erv1/ALR receptor does not interact with other mitogenic factors such as epidermal growth factor (EGF), transforming growth factor α (TGF-α), or insulin [81]. Binding of Erv1/ALR to its receptor triggers EGF-receptor phosphorylation which then results in activation of the mitogen-activated protein kinase (MAPK) signaling cascade [82].
In a second model cytosolic Erv1/ALR acts independently of the MAPK pathway. Cytosolic Erv1/ALR thereby interacts with Jun-activating domain-binding protein 1 (JAB1) which promotes phosphorylation of c-Jun and therefore formation of the c-Jun/activator protein-1 (AP-1) transcription factor complex [98]. C-Jun is part of the cytosolic COP9 signalosome that has also been shown to interact with Erv1/ALR [99]. The interaction between Erv1/ALR and JAB1 depends on the presence of the CXXC motif in the C-terminal core domain of Erv1/ALR because mutation of the motif to CXXS prevented phosphorylation of c-Jun [100]. The studies addressing the cytosolic function of Erv1/ALR were all either performed in vitro using recombinant proteins or by overexpressing Erv1/ALR. As already stated above overexpression of IMS proteins leads to cytosolic or nuclear mislocalization [23]. This is especially true for Erv1/ALR which becomes imported and folded more slowly than classical substrates of the disulfide relay.
The interaction of Erv1/ALR with JAB1 is not limited to liver cells. Knockdown of Erv1/ALR in hematopoietic stem cells leads to an increased inhibition of the cyclin-dependent kinase inhibitor p27(kip) by JAB1 while overexpression of ALR leads to a decreased inhibition of p27(kip), probably because JAB1 is sequestered by ALR [101]. Furthermore, it was shown that the quiescence promoting properties of ALR in HSC are dependent on Camk4 (Ca2+/calmodulin-dependent protein kinase 4). HSCs isolated from Camk4−/− mice possessed reduced levels of ALR and p27(kip) and were deficient in proliferation, which could be restored by ectopic expression of ALR [102].
A complementing explanation for the enhancement of liver regeneration is that Erv1/ALR treatment decreases cytotoxicity of natural killer cells and decreases IFN-γ (interferon-gamma) levels [103, 104]. Alternatively, it has been proposed that extracellularly administrated Erv1/ALR enhances liver regeneration by inducing anti apoptotic gene expression, thereby improving cell survival [105]. However, this anti apoptotic effect does not seem to be limited only to hepatocytes because in human lymphocytes recombinant Erv1/ALR also inhibited apoptosis [106]. Recently, it was shown that in primary hepatocytes the increased expression and synthesis of ALR after liver damage is regulated by the transcription factor Nrf2 [107]. This indicates that the regenerative abilities of ALR are not only achieved by extracellular treatment of damaged cells but might constitute physiological relevant cellular survival mechanisms.
4.4. The Disulfide Relay System—Open Questions
Mia40 and Erv1/ALR are well characterized regarding their functions as oxidoreductase and sulfhydryl oxidase of the mitochondrial disulfide relay, respectively. Still several open questions remain (Figure 5): first, despite the identification of many human twin-CX9C proteins by in silico approaches, a concise identification of disulfide relay substrates is still lacking. This becomes especially important because in recent years several proteins with complex structures have been identified as Mia40 substrates. Since these substrates do not adhere to classical cysteine patterns, they cannot be predicted by in silico approaches. This might indicate that the substrate range of the disulfide relay is much wider than previously anticipated. It might also include targets for thiol-dependent redox regulation that cycle between oxidized and reduced states and consequently adapt their activities.
Figure 5.
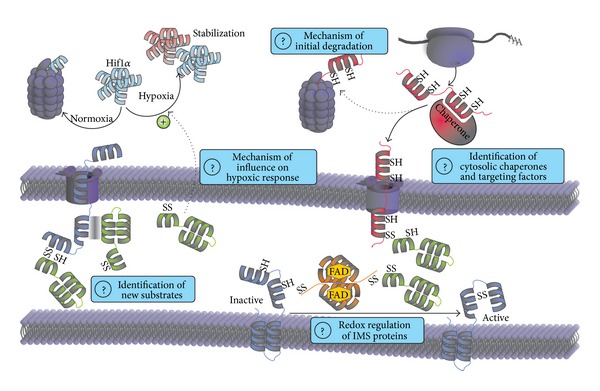
Open questions. Hypoxia. Why Mia40 is required for the stabilization of HIF1α under hypoxic conditions is unknown. It will be interesting to reveal the underlying mechanism and if the influence of Mia40 is direct or indirect. New Substrates. During the last years proteins were found to be dependent on Mia40 that do not share the same motifs as the classical twin-CX9C and twin-CX3C substrates. They are either dependent on the function of Mia40 as oxidoreductase or holdase. Initial Degradation and Cytosolic Chaperones/Targeting Factors. In yeast a portion of IMS proteins is directly degraded after translation. However, it is not known if this takes place in other organisms, how it is regulated, and how it works at the molecular level. Moreover, it remains unclear how proteins after translation are kept import competent and targeted to mitochondria. Redox Regulation. The mitochondrial disulfide relay is known to introduce structural disulfide bonds which are required for protein stability. However, it is not known if Mia40 or Erv1/ALR can regulate the activity of IMS proteins by reversible oxidation or reduction.
Secondly, while we understand oxidative protein folding in the IMS in detail, little is known about the cytosolic processes that take place before translocation across the outer membrane. In previous studies, cytosolic factors were identified which facilitate the import of MTS-containing proteins. However, most disulfide relay substrates lack such targeting information and thus appear like cytosolic proteins. It will therefore be exciting to identify factors that interact with IMS proteins after their translation and guide them to mitochondria or mediate their degradation.
Thirdly, the role of Mia40 and Erv1/ALR in processes that appear not directly linked to mitochondria such as hypoxia and liver regeneration is still mechanistically ill-defined. It especially remains unclear whether the functions of both proteins in each case are connected to their function in the disulfide relay or if they operate by completely different mechanisms. We think that it will be exciting to establish in detail the molecular mechanisms that underlie these potentially extra-mitochondrial functions and thus link the biochemistry of thiol oxidation with its physiological impact.
Acknowledgments
The authors thank Trine Kjaersgaard for critical reading of the paper and Johannes Herrmann for continuous support. Work in the Riemer laboratory is funded by the Deutsche Forschungsgemeinschaft, the Landesschwerpunkt Membrantransport, and the Fritz-Thyssen-Stiftung.
References
- 1.Tomkiewicz D, Nouwen N, Driessen AJM. Pushing, pulling and trapping—modes of motor protein supported protein translocation. The FEBS Letters. 2007;581(15):2820–2828. doi: 10.1016/j.febslet.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Mesecke N, Terziyska N, Kozany C, et al. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell. 2005;121(7):1059–1069. doi: 10.1016/j.cell.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Riemer J, Bulleid N, Herrmann JM. Disulfide formation in the ER and mitochondria: two solutions to a common process. Science. 2009;324(5932):1284–1287. doi: 10.1126/science.1170653. [DOI] [PubMed] [Google Scholar]
- 4.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell. 2009;138(4):628–644. doi: 10.1016/j.cell.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatzi A, Tokatlidis K. The mitochondrial intermembrane space: a hub for oxidative folding linked to protein biogenesis. Antioxidants & Redox Signaling. 2013;19(1):54–62. doi: 10.1089/ars.2012.4855. [DOI] [PubMed] [Google Scholar]
- 6.Endo T, Yamano K, Kawano S. Structural insight into the mitochondrial protein import system. Biochimica et Biophysica Acta. 2011;1808(3):955–970. doi: 10.1016/j.bbamem.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annual Review of Biochemistry. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann JM, Riemer J. The intermembrane space of mitochondria. Antioxidants and Redox Signaling. 2010;13(9):1341–1358. doi: 10.1089/ars.2009.3063. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann JM, Longen S, Weckbecker D, Depuydt M. Biogenesis of mitochondrial proteins. Advances in Experimental Medicine and Biology. 2012;748:41–64. doi: 10.1007/978-1-4614-3573-0_3. [DOI] [PubMed] [Google Scholar]
- 10.Riemer J, Fischer M, Herrmann JM. Oxidation-driven protein import into mitochondria: insights and blind spots. Biochimica et Biophysica Acta. 2011;1808(3):981–989. doi: 10.1016/j.bbamem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Banci L, Bertini I, Ciofi-Baffoni S, et al. A structural-dynamical characterization of human Cox17. Journal of Biological Chemistry. 2008;283(12):7912–7920. doi: 10.1074/jbc.M708016200. [DOI] [PubMed] [Google Scholar]
- 12.Banci L, Bertinia I, Ciofi-Baffonia S, et al. Structural characterization of CHCHD5 and CHCHD7: two atypical human twin CX9C proteins. Journal of Structural Biology. 2012;180(1):190–200. doi: 10.1016/j.jsb.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Longen S, Bien M, Bihlmaier K, et al. Systematic analysis of the twin Cx9C protein family. Journal of Molecular Biology. 2009;393(2):356–368. doi: 10.1016/j.jmb.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Milenkovic D, Ramming T, Müller JM, et al. Identification of the signal directing Tim9 and Tim10 into the intermembrane space of mitochondria. Molecular Biology of the Cell. 2009;20(10):2530–2539. doi: 10.1091/mbc.E08-11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb CT, Gorman MA, Lazarou M, Ryan MT, Gulbis JM. Crystal structure of the mitochondrial chaperone TIM9•10 reveals a six-bladed α-propeller. Molecular Cell. 2006;21(1):123–133. doi: 10.1016/j.molcel.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel K, Milenkovic D, Chacinska A, et al. Novel mitochondrial intermembrane space proteins as substrates of the MIA import pathway. Journal of Molecular Biology. 2007;365(3):612–620. doi: 10.1016/j.jmb.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 17.Horn D, Zhou W, Trevisson E, et al. The conserved mitochondrial twin Cx9C Protein Cmc2 is a Cmc1 homologue essential for cytochrome c oxidase biogenesis. Journal of Biological Chemistry. 2010;285(20):15088–15099. doi: 10.1074/jbc.M110.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oswald C, Krause-Buchholz U, Rödel G. Knockdown of human COX17 affects assembly and supramolecular organization of cytochrome c oxidase. Journal of Molecular Biology. 2009;389(3):470–479. doi: 10.1016/j.jmb.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Rigby K, Zhang L, Cobine PA, George GN, Winge DR. Characterization of the cytochrome c oxidase assembly factor Cox19 of Saccharomyces cerevisiae. Journal of Biological Chemistry. 2007;282(14):10233–10242. doi: 10.1074/jbc.M610082200. [DOI] [PubMed] [Google Scholar]
- 20.Milenkovic D, Gabriel K, Guiard B, Schulze-Specking A, Pfanner N, Chacinska A. Biogenesis of the essential Tim9-Tim10 chaperone complex of mitochondria: site-specific recognition of cysteine residues by the intermembrane space receptor Mia40. Journal of Biological Chemistry. 2007;282(31):22472–22480. doi: 10.1074/jbc.M703294200. [DOI] [PubMed] [Google Scholar]
- 21.Vial S, Lu H, Allen S, et al. Assembly of TIM9 and TIM10 into a functional chaperone. Journal of Biological Chemistry. 2002;277(39):36100–36108. doi: 10.1074/jbc.M202310200. [DOI] [PubMed] [Google Scholar]
- 22.Paschen SA, Rothbauer U, Káldi K, Bauer MF, Neupert W, Brunner M. The role of the TIM8-13 complex in the import of Tim23 into mitochondria. The EMBO Journal. 2000;19(23):6392–6400. doi: 10.1093/emboj/19.23.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer M, Horn S, Belkacemi A, et al. Protein import and oxidative folding in the mitochondrial intermembrane space of intact mammalian cells. Molecular Biology of the Cell. 2013;24(14):2160–2170. doi: 10.1091/mbc.E12-12-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavallaro G. Genome-wide analysis of eukaryotic twin CX9C proteins. Molecular BioSystems. 2010;6(12):2459–2470. doi: 10.1039/c0mb00058b. [DOI] [PubMed] [Google Scholar]
- 25.Varabyova A, Topf U, Kwiatkowska P, Wrobel L, Kaus-Drobek M, Chacinska A. Mia40 and MINOS act in parallel with Ccs1 in the biogenesis of mitochondrial Sod1. The FEBS Journal. 2013;280(20):4943–4959. doi: 10.1111/febs.12409. [DOI] [PubMed] [Google Scholar]
- 26.Klöppela C, Suzuki Y, Kojer K, et al. Mia40-dependent oxidation of cysteines in domain I of Ccs1 controls its distribution between mitochondria and the cytosol. Molecular Biology of the Cell. 2011;22(20):3749–3757. doi: 10.1091/mbc.E11-04-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groß DP, Burgard CA, Reddehase S, Leitch JM, Culotta VC, Hell K. Mitochondrial Ccs1 contains a structural disulfide bond crucial for the import of this unconventional substrate by the disulfide relay system. Molecular Biology of the Cell. 2011;22(20):3758–3767. doi: 10.1091/mbc.E11-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallergi E, Andreadaki M, Kritsiligkou P, et al. Targeting and maturation of Erv1/ALR in the mitochondrial intermembrane space. ACS Chemical Biology. 2012;7(4):707–714. doi: 10.1021/cb200485b. [DOI] [PubMed] [Google Scholar]
- 29.Weckbecker D, Longen S, Riemer J, Herrmann JM. Atp23 biogenesis reveals a chaperone-like folding activity of Mia40 in the IMS of mitochondria. The EMBO Journal. 2012;31(22):4348–4358. doi: 10.1038/emboj.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wrobel L, Trojanowska A, Sztolsztener ME, Chacinska A. Mitochondrial protein import: Mia40 facilitates Tim22 translocation into the inner membrane of mitochondria. Molecular Biology of the Cell. 2013;24(5):543–554. doi: 10.1091/mbc.E12-09-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogtle FN, Burkhart JM, Rao S, et al. Intermembrane space proteome of yeast mitochondria. Molecular & Cellular Proteomics. 2012;11(12):1840–1852. doi: 10.1074/mcp.M112.021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghaemmaghami S, Huh W, Bower K, et al. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 33.Banci L, Barbieri L, Luchinat E, Secci E. Visualization of redox-controlled protein fold in living cells. Chemistry & Biology. 2013;20(6):747–752. doi: 10.1016/j.chembiol.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Durigon R, Wang Q, Ceh Pavia E, Grant CM, Lu H. Cytosolic thioredoxin system facilitates the import of mitochondrial small Tim proteins. EMBO Reports. 2012;13(10):916–922. doi: 10.1038/embor.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan B, Lu H. Oxidative folding competes with mitochondrial import of the small Tim proteins. Biochemical Journal. 2008;411(1):115–122. doi: 10.1042/BJ20071476. [DOI] [PubMed] [Google Scholar]
- 36.Morgan B, Kim S, Yan G, Lu H. Zinc can play chaperone-like and inhibitor roles during import of mitochondrial small tim proteins. Journal of Biological Chemistry. 2009;284(11):6818–6825. doi: 10.1074/jbc.M808691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bragoszewski P, Gornicka A, Sztolsztener ME, Chacinska A. The ubiquitin-proteasome system regulates mitochondrial intermembrane space proteins. Molecular and Cellular Biology. 2013;33(11):2136–2148. doi: 10.1128/MCB.01579-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sideris DP, Petrakis N, Katrakili N, et al. A novel intermembrane space-targeting signal docks cysteines onto Mia40 during mitochondrial oxidative folding. Journal of Cell Biology. 2009;187(7):1007–1022. doi: 10.1083/jcb.200905134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bien M, Longen S, Wagener N, Chwalla I, Herrmann JM, Riemer J. Mitochondrial disulfide bond formation is driven by intersubunit electron transfer in Erv1 and proofread by glutathione. Molecular Cell. 2010;37(4):516–528. doi: 10.1016/j.molcel.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Banci L, Bertini I, Cefaro C, et al. MIA40 is an oxidoreductase that catalyzes oxidative protein folding in mitochondria. Nature Structural and Molecular Biology. 2009;16(2):198–206. doi: 10.1038/nsmb.1553. [DOI] [PubMed] [Google Scholar]
- 41.Kawano S, Yamano K, Naoé M, et al. Structural basis of yeast Tim40/Mia40 as an oxidative translocator in the mitochondrial intermembrane space. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14403–14407. doi: 10.1073/pnas.0901793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofmann S, Rothbauer U, Mühlenbein N, Baiker K, Hell K, Bauer MF. Functional and mutational characterization of human MIA40 acting during import into the mitochondrial intermembrane space. Journal of Molecular Biology. 2005;353(3):517–528. doi: 10.1016/j.jmb.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Staples O, Thomas LW, et al. Human CHCHD4 mitochondrial proteins regulate cellular oxygen consumption rate and metabolism and provide a critical role in hypoxia signaling and tumor progression. Journal of Clinical Investigation. 2012;122(2):600–611. doi: 10.1172/JCI58780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sztolsztener ME, Brewinska A, Guiard B, Chacinska A. Disulfide bond formation: sulfhydryl oxidase ALR controls mitochondrial biogenesis of human MIA40. Traffic. 2013;14(3):309–320. doi: 10.1111/tra.12030. [DOI] [PubMed] [Google Scholar]
- 45.Chatzi A, Sideris DP, Katrakili N, Pozidis C, Tokatlidis K. Biogenesis of yeast Mia40-uncoupling folding from import and atypical recognition features. The FEBS Journal. 2013;280(20):4960–4969. doi: 10.1111/febs.12482. [DOI] [PubMed] [Google Scholar]
- 46.von der Malsburg K, Müller JM, Bohnert M, et al. Dual role of mitofilin in mitochondrial membrane organization and protein biogenesis. Developmental Cell. 2011;21(4):694–707. doi: 10.1016/j.devcel.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 47.Bohnert M, Wenz L-S, Zerbes RM, et al. Role of mitochondrial inner membrane organizing system in protein biogenesis of the mitochondrial outer membrane. Molecular Biology of the Cell. 2012;23(20):3948–3956. doi: 10.1091/mbc.E12-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zerbes RM, Bohnert M, Stroud DA, et al. Role of MINOS in mitochondrial membrane architecture: cristae morphology and outer membrane interactions differentially depend on mitofilin domains. Journal of Molecular Biology. 2012;422(2):183–191. doi: 10.1016/j.jmb.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Harner M, Körner C, Walther D, et al. The mitochondrial contact site complex, a determinant of mitochondrial architecture. The EMBO Journal. 2011;30(21):4356–4370. doi: 10.1038/emboj.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoppins S, Collins SR, Cassidy-Stone A, et al. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. Journal of Cell Biology. 2011;195(2):323–340. doi: 10.1083/jcb.201107053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banci L, Bertini I, Cefaro C, et al. Molecular chaperone function of Mia40 triggers consecutive induced folding steps of the substrate in mitochondrial protein import. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20190–20195. doi: 10.1073/pnas.1010095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bottinger L, Gornicka A, Czerwik T, et al. In vivo evidence for cooperation of Mia40 and Erv1 in the oxidation of mitochondrial proteins. Molecular Biology of the Cell. 2012;23(20):3957–3969. doi: 10.1091/mbc.E12-05-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stojanovski D, Milenkovic D, Müller JM, et al. Mitochondrial protein import: precursor oxidation in a ternary complex with disulfide carrier and sulfhydryl oxidase. Journal of Cell Biology. 2008;183(2):195–202. doi: 10.1083/jcb.200804095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu CK, Dailey TA, Dailey HA, Wang B, Rose JP. The crystal structure of augmenter of liver regeneration: a mammalian FAD-dependent sulfhydryl oxidase. Protein Science. 2003;12(5):1109–1118. doi: 10.1110/ps.0238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daithankar VN, Farrell SR, Thorpe C. Augmenter of liver regeneration: substrate specificity of a flavin-dependent oxidoreductase from the mitochondrial intermembrane space. Biochemistry. 2009;48(22):4828–4837. doi: 10.1021/bi900347v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Banci L, Bertini I, Calderone V, et al. Molecular recognition and substrate mimicry drive the electron-transfer process between MIA40 and ALR. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):4811–4816. doi: 10.1073/pnas.1014542108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banci L, Bertini I, Calderone V, et al. An electron-transfer path through an extended disulfide relay system: the case of the redox protein ALR. Journal of the American Chemical Society. 2012;134(3):1442–1445. doi: 10.1021/ja209881f. [DOI] [PubMed] [Google Scholar]
- 58.Farrell SR, Thorpe C. Augmenter of liver regeneration: a flavin-dependent sulfhydryl oxidase with cytochrome c reductase activity. Biochemistry. 2005;44(5):1532–1541. doi: 10.1021/bi0479555. [DOI] [PubMed] [Google Scholar]
- 59.Daithankar VN, Schaefer SA, Dong M, Bahnson BJ, Thorpe C. Structure of the human sulfhydryl oxidase augmenter of liver regeneration and characterization of a human mutation causing an autosomal recessive myopathy. Biochemistry. 2010;49(31):6737–6745. doi: 10.1021/bi100912m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vitu E, Bentzur M, Lisowsky T, Kaiser CA, Fass D. Gain of function in an ERV/ALR sulfhydryl oxidase by molecular engineering of the shuttle disulfide. Journal of Molecular Biology. 2006;362(1):89–101. doi: 10.1016/j.jmb.2006.06.070. [DOI] [PubMed] [Google Scholar]
- 61.Ang SK, Lu H. Deciphering structural and functional roles of individual disulfide bonds of the mitochondrial sulfhydryl oxidase Erv1p. Journal of Biological Chemistry. 2009;284(42):28754–28761. doi: 10.1074/jbc.M109.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bihlmaier K, Mesecke N, Terziyska N, Bien M, Hell K, Herrmann JM. The disulfide relay system of mitochondria is connected to the respiratory chain. Journal of Cell Biology. 2007;179(3):389–395. doi: 10.1083/jcb.200707123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kay CW, Elsässer C, Bittl R, Farrell SR, Thorpe C. Determination of the distance between the two neutral flavin radicals in augmenter of liver regeneration by pulsed ELDOR. Journal of the American Chemical Society. 2006;128(1):76–77. doi: 10.1021/ja057308g. [DOI] [PubMed] [Google Scholar]
- 64.Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K. Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. Journal of Molecular Biology. 2005;353(5):937–944. doi: 10.1016/j.jmb.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 65.Tienson HL, Dabir DV, Neal SE, et al. Reconstitution of the Mia40-Erv1 oxidative folding pathway for the small tim proteins. Molecular Biology of the Cell. 2009;20(15):3481–3490. doi: 10.1091/mbc.E08-10-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dabir DV, Leverich EP, Kim S, et al. A role for cytochrome c and cytochrome c peroxidase in electron shuttling from Erv1. The EMBO Journal. 2007;26(23):4801–4811. doi: 10.1038/sj.emboj.7601909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mesecke N, Bihlmaier K, Grumbt B, et al. The zinc-binding protein Hot13 promotes oxidation of the mitochondrial import receptor Mia40. EMBO Reports. 2008;9(11):1107–1113. doi: 10.1038/embor.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curran SP, Leuenberger D, Leverich EP, Hwang DK, Beverly KN, Koehler CM. The role of Hot13p and redox chemistry in the mitochondrial TIM22 import pathway. Journal of Biological Chemistry. 2004;279(42):43744–43751. doi: 10.1074/jbc.M404878200. [DOI] [PubMed] [Google Scholar]
- 69.Kojer K, Bien M, Gangel H, Morgan B, Dick T, Riemer J. Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state. The EMBO Journal. 2012;31(14):3169–3182. doi: 10.1038/emboj.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. Journal of Biological Chemistry. 2008;283(43):29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu H, Woodburn J. Zinc binding stabilizes mitochondrial Tim10 in a reduced and import-competent state kinetically. Journal of Molecular Biology. 2005;353(4):897–910. doi: 10.1016/j.jmb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Banci L, Bertini I, Ciofi-Baffoni S, Hadjiloi T, Martinelli M, Palumaa P. Mitochondrial copper(I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):6803–6808. doi: 10.1073/pnas.0800019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voronova A, Meyer-Klaucke W, Meyer T, et al. Oxidative switches in functioning of mammalian copper chaperone Cox17. Biochemical Journal. 2007;408(1):139–148. doi: 10.1042/BJ20070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Napoli E, Wong S, Hung C, et al. Defective mitochondrial disulfide relay system, altered mitochondrial morphology and function in Huntington's disease. Human Molecular Genetics. 2013;22(5):989–1004. doi: 10.1093/hmg/dds503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Todd LR, Gomathinayagam R, Sankar U. A novel Gfer-Drp1 link in preserving mitochondrial dynamics and function in pluripotent stem cells. Autophagy. 2010;6(6):821–822. doi: 10.1091/mbc.E09-11-0937. [DOI] [PubMed] [Google Scholar]
- 76.Todd LR, Damin MN, Gomathinayagam R, Horn SR, Means AR, Sankar U. Growth factor ero1-like modulates Drp1 to preserve mitochondrial dynamics and function in mouse embryonic stem cells. Molecular Biology of the Cell. 2010;21(7):1225–1236. doi: 10.1091/mbc.E09-11-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilkerson DC, Sankar U. Mitochondria: a sulfhydryl oxidase and fission GTPase connect mitochondrial dynamics with pluripotency in embryonic stem cells. International Journal of Biochemistry and Cell Biology. 2011;43(9):1252–1256. doi: 10.1016/j.biocel.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, Farooq M, Sheng D, et al. Augmenter of liver regeneration (alr) promotes liver outgrowth during zebrafish hepatogenesis. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0030835.e30835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dabir DV, Hasson SA, Setoguchi K, et al. A small molecule inhibitor of redox-regulated protein translocation into mitochondria. Developmental Cell. 2013;25(1):81–92. doi: 10.1016/j.devcel.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang XM, Hu Z, Xie L, Wu Z, He F. In vitro stimulation of HTC hepatoma cell growth by recombinant human augmenter of liver regeneration (ALR) Acta Physiologica Sinica. 1997;49(5):557–561. [PubMed] [Google Scholar]
- 81.Wang G, Yang X, Zhang Y, et al. Identification and characterization of receptor for mammalian hepatopoietin that is homologous to yeast ERV1. Journal of Biological Chemistry. 1999;274(17):11469–11472. doi: 10.1074/jbc.274.17.11469. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Li M, Xing G, et al. Stimulation of the mitogen-activated protein kinase cascade tyrosine phosphorylation of the epidermal growth factor receptor by hepatopoietin. Journal of Biological Chemistry. 2000;275(48):37443–37447. doi: 10.1074/jbc.M004373200. [DOI] [PubMed] [Google Scholar]
- 83.Ivan M, Kondo K, Yang H, et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 84.Jaakkola P, Mole DR, Tian Y-M, et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 85.Maxwell PH, Wlesener MS, Chang G, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 86.Pan Y, Mansfield KD, Bertozzi CC, et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro . Molecular and Cellular Biology. 2007;27(3):912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial Complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. Journal of Biological Chemistry. 2000;275(33):25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 88.Myllyharju J, Kivirikko KI. Characterization of the iron- and 2-oxoglutavate binding sites of human prolyl 4-hydroxylase. The EMBO Journal. 1997;16(6):1173–1180. doi: 10.1093/emboj/16.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Callapina M, Zhou J, Schnitzer S, et al. Nitric oxide reverses desferrioxamine- and hypoxia-evoked HIF-1α accumulation-implications for prolyl hydroxylase activity and iron. Experimental Cell Research. 2005;306(1):274–284. doi: 10.1016/j.yexcr.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 90.Flagg SC, Martin CB, Taabazuing CY, Holmes BE, Knapp MJ. Screening chelating inhibitors of HIF-prolyl hydroxylase domain 2 (PHD2) and factor inhibiting HIF (FIH) Journal of Inorganic Biochemistry. 2012;113:25–30. doi: 10.1016/j.jinorgbio.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Fonzo A, Ronchi D, Lodi T, et al. The mitochondrial disulfide relay system protein GFER is mutated in autosomal-recessive myopathy with cataract and combined respiratory-chain deficiency. American Journal of Human Genetics. 2009;84(5):594–604. doi: 10.1016/j.ajhg.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lange H, Lisowsky T, Gerber J, Mühlenhoff U, Kispal G, Lill R. An essential function of the mitochondrial sulfhydryl oxidase Erv1p/ALR in the maturation of cytosolic Fe/S proteins. EMBO Reports. 2001;2(8):715–720. doi: 10.1093/embo-reports/kve161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Y, Wei K, Lu C, et al. Identification of hepatopoietin dimerization, its interacting regions and alternative splicing of its transcription. European Journal of Biochemistry. 2002;269(16):3888–3893. doi: 10.1046/j.1432-1033.2002.03054.x. [DOI] [PubMed] [Google Scholar]
- 94.Lu J, Xu W, Zhan Y, et al. Identification and characterization of a novel isoform of hepatopoietin. World Journal of Gastroenterology. 2002;8(2):353–356. doi: 10.3748/wjg.v8.i2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao CF, Zhou FG, Wang H, Huang Y-F, Ji Q, Chen J. Genetic recombinant expression and characterization of human augmenter of liver regeneration. Digestive Diseases and Sciences. 2009;54(3):530–537. doi: 10.1007/s10620-008-0372-1. [DOI] [PubMed] [Google Scholar]
- 96.Ilowski M, Kleespies A, de Toni EN, et al. Augmenter of liver regeneration (ALR) protects human hepatocytes against apoptosis. Biochemical and Biophysical Research Communications. 2011;404(1):148–152. doi: 10.1016/j.bbrc.2010.11.083. [DOI] [PubMed] [Google Scholar]
- 97.Gandhi CR. Augmenter of liver regeneration. Fibrogenesis Tissue Repair. 2012;5(1):p. 10. doi: 10.1186/1755-1536-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu C, Li Y, Zhao Y, et al. Intracrine hepatopoietin potentiates AP-1 activity through JAB1 independent of MAPK pathway. The FASEB Journal. 2002;16(1):90–92. doi: 10.1096/fj.01-0506fje. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Lu C, Wei H, et al. Hepatopoietin interacts directly with COP9 signalosome and regulates AP-1 activity. FEBS Letters. 2004;572(1–3):85–91. doi: 10.1016/j.febslet.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 100.Chen X, Li Y, Wei K, et al. The potentiation role of hepatopoietin on activator protein-1 is dependent on its sulfhydryl oxidase activity. The Journal of Biological Chemistry. 2003;278(49):49022–49030. doi: 10.1074/jbc.M304057200. [DOI] [PubMed] [Google Scholar]
- 101.Teng EC, Todd LR, Ribar TJ, et al. Gfer inhibits Jab1-mediated degradation of p27kip1 to restrict proliferation of hematopoietic stem cells. Molecular Biology of the Cell. 2011;22(8):1312–1320. doi: 10.1091/mbc.E10-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sankar U, Means AR. Gfer is a critical regulator of HSC proliferation. Cell Cycle. 2011;10(14):2263–2268. doi: 10.4161/cc.10.14.15919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Francavilla A, Vujanovic NL, Polimeno L, et al. The in vivo effect of hepatotrophic factors augmenter of liver regeneration, hepatocyte growth factor, and insulin-like growth factor-II on liver natural killer cell functions. Hepatology. 1997;25(2):411–415. doi: 10.1002/hep.510250225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Polimeno L, Margiotta M, Marangi L, et al. Molecular mechanisms of augmenter of liver regeneration as immunoregulator: its effect on interferon-γ expression in rat liver. Digestive and Liver Disease. 2000;32(3):217–225. doi: 10.1016/s1590-8658(00)80824-5. [DOI] [PubMed] [Google Scholar]
- 105.Polimeno L, Pesetti B, Annoscia E, et al. Alrp, a survival factor that controls the apoptotic process of regenerating liver after partial hepatectomy in rats. Free Radical Research. 2011;45(5):534–549. doi: 10.3109/10715762.2011.555482. [DOI] [PubMed] [Google Scholar]
- 106.Wang N, Sun H, Shen Y, et al. Augmenter of liver regeneration inhibits apoptosis of activated human peripheral blood lymphocytes in vitro . Immunopharmacology and Immunotoxicology. 2013;35(2):257–263. doi: 10.3109/08923973.2013.764502. [DOI] [PubMed] [Google Scholar]
- 107.Dayoub R, Vogel A, Schuett J, et al. Nrf2 activates augmenter of liver regeneration (ALR) via antioxidant response element and links oxidative stress to liver regeneration. Molecular Medicine. 2013;19(1):237–244. doi: 10.2119/molmed.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]


